Volume 25 Number 4
Evidence summary: Pressure injuries: preventing medical device related pressure injuries
Emily Haesler
August 2017
Clinical question
What is the best available evidence on prophylactic dressings to prevent medical device related pressure injuries (MDRPI)?
Summary
Medical device related pressure injuries occur from the use of devices designed and applied to the body for diagnostic purposes or for the delivery of treatment. The MDRPI occurs as a result of ongoing pressure on the skin from the device or from fixations used to secure the device.1 Individuals in intensive care setting2-6 and children/neonates7-12 are at particular risk of developing a MDRPI (Levels 1, 2, 3 and 4 evidence). Interventions designed to reduce interface pressure and protect the skin, such as regularly repositioning the device (Level 5b evidence), alternating devices (Level 1c evidence), moisturising the skin (Level 1c evidence), and applying a prophylactic dressing (Levels 1, 2 and 4 evidence), are effective in reducing the risk of MDRPI.
Clinical practice recommendations
- Select a correctly fitted and sized medical device made from the least damaging materials. (Grade B)
- Conduct regular skin assessments under and around medical devices. (Grade A)
- Regularly moisturise the skin underneath a MDRPI. (Grade B)
- Reposition medical devices on a regular basis whenever possible. (Grade A)
- Apply a prophylactic dressing underneath a medical device. (Grade B)
Considerations when using prophylactic dressings underneath medical devices
Consider the following when using prophylactic dressings under a medical device:
- Continue to conduct regular skin inspections (at least twice daily).13 Some prophylactic dressings are designed to be easily removed and reapplied to facilitate skin inspection without causing medical adhesive related skin injury13, 14 (Level 5b evidence).
- Consider the effect of the chosen prophylactic dressing on skin microclimate. A cohort study with children wearing different types of prophylactic dressings under oxygen facial masks demonstrated high levels of skin hydration with hydrogel dressings (p<0.001) and silicon foam dressings (p=0.005) compared to no dressing11 (Level 3c evidence). Ability to absorb moisture could contribute to the efficacy of a prophylactic dressing14, 15 (Level 5c evidence).
- There is minimal evidence on appropriate thickness of a prophylactic dressing underneath a medical device. Prophylactic dressings with multiple layers may be more effective in reducing the impact of pressure, shear and friction forces15, 16) (Level 5c evidence); however, excessively thick or layered prophylactic dressings may increase pressure at the skin-medical when used under a medical device13 (Level 5b evidence).
Evidence
Skin assessment and preventive care
Regularly inspecting the skin underneath a medical device identifies areas that are being exposed to detrimental pressure or shear forces. The process of inspecting the skin also provides an opportunity to repositioning and rotate the device. Clinical guidelines recommend inspecting the skin underneath and around medical devices on a regular (at least twice daily) basis13 (Level 5b evidence). For individuals who are vulnerable to localised or generalised oedema, skin should be assessed more frequently.13 Health care professionals can educate individuals and their caregivers to perform skin inspections under medical devices,13 including demonstrating how to safely move a medical device for skin visualisation and providing information about skin and tissue changes that require medical attention and intervention (Level 5b evidence).
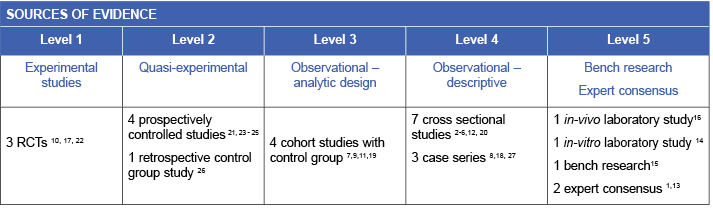
A randomised controlled trial (RCT) conducted in an ICU demonstrated efficacy of a hyper-oxygenated fatty acid moisturising regimen used under plastic facial masks. Skin assessments were conducted every four to six hours and moisturiser was reapplied according to skin hydration status. The moisturising intervention was associated with lower rates of MDRPI than no intervention (p=0.05), a thin prophylactic dressing (p=0.03) or a foam dressing (p<0.001)17 (Level 1c evidence).
Selecting, applying and positioning medical devices to prevent MDRPI
Medical device related injuries increase when devices are incorrectly sized or fitted. If the device does not fit correctly, there can be higher interface pressure (pressure between the device and the skin) and/or increased shear1, 13 (Level 5b evidence). Correctly sizing or adapting the medical device and its securing tapes is shown to decrease MDRPI in children (n=68) wearing halo vests.18 (Level 4c evidence) In a study conducted in healthy volunteers, securing a ventilation masks using straps with tighter tension was associated with higher interface pressure on the bridge of the nose19 (Level 3c evidence).
Selecting devices that are made of softer and more flexible materials, particularly at the point the device that interfaces with the skin, could reduce the risk of a MDRPI. Reduction in the rates of MDRI was seen in a trauma centre when ET tubes of less rigid material were introduced to the facility20 (Level 4.b evidence). When a cloth nasal mask was used instead of a plastic facial mask with a prophylactic dressing, lower rates of facial PIs were observed in children receiving oxygen therapy (Level 3c evidence). If there is an option, rotating the type of medical device used can reduce risk of MDRPI. A study in neonates demonstrated lower rates of nasal PIs when oxygen delivery system was rotated between nasal prongs and masks compared to using only one type of oxygen delivery device10 (Level 1c evidence).
The type of tape used to secure devices may also reduce the risk of PUs. In one non-randomised, non-blinded study, a commercial holder for nasogastric tubes (NGTs) was associated with fewer nasal PIs than a particular method of using regular adhesive tape to secure the NGT21 (Level 2c evidence).
Because MDRPI occur due to prolonged pressure on the skin, relieving pressure by repositioning or rotating the device regularly is likely to decreases the risk of MDRPIs. In a RCT conducted with neonates, alternating between nasal prongs and a facial mask for delivering oxygen therapy resulted in fewer Stage 1 MDRPIs than using either nasal prongs or a facial mask continuously (p<0.001)10 (Level 1c evidence).
Prophylactic dressings to prevent MDRPI
A number of studies (details below) support the use of a prophylactic dressing underneath nasal prongs,22 oxygen face masks,23 endotracheal/tracheostomy (ET) tube ties,24, 25 casts applied over a bony prominence,26 and nasotracheal tubes.27The available evidence provides support for a range of different prophylactic dressings compared to no dressing, but there is no evidence to indicate if a particular prophylactic dressing is more effective for reducing MDRPI than other dressing types.
Two studies have demonstrated efficacy of silicone pressure-reducing strips underneath ET tube twill ties in individuals with facial burns25 and under nasal prongs used for delivering oxygen therapy for preterm infants. In both studies, the silicone strips were associated with significant reduction in MDRPIs compared to no prophylactic dressing22, 25 (Level 1.c and level 2d evidence). Odds of a MDRPI was 3.43 times higher for preterm infants (p<0.05) using nasal prongs without a prophylactic dressing22 (Level 1c evidence).
A soft silicone foam dressing was associated with a significant reduction in the risk of tracheostomy site MDRPI in children compared to no prophylactic dressing (0% versus 11.8%, p=0.02)24(Level 2d evidence).
A polyurethane foam pad was effective underneath leg/foot casts in reducing MDPRI compared to no intervention. The relative risk of developing a heel pressure ulcer when a prophylactic polyurethane foam dressing was applied was 0.08 (95% CI 0.02 to 0.33)26 (Level 2d evidence).
A hydrocolloid dressing applied to the nasal bridge when a facial mask was applied for non-invasive ventilation was associated with an absolute risk reduction of MDRPI of more than 50%.23 (Level 2c evidence).
A case series demonstrated reduction in nasal PIs associated with using a foam packing dressing in the nostril to protect the skin from pressure from nasotracheal tube for individuals having maxillofacial surgery27 (Level 4c evidence).
Methodology
This evidence summary is based on a structured database search combining search terms that describe heel PIs with search terms related to prophylactic dressings. Searches were conducted in EMBASE, Pubmed, Medline, Scopus and the Cochrane Library. Evidence published up to June 2017 in English was considered for inclusion. Retrieved studies were appraised for relevance and rigour using Joanna Briggs Institute appraisal tools.28
Author(s)
Emily Haesler
References
- Black J, Alves P, Brindle CT, Dealey C, Santamaria N, Call E, et al. Use of wound dressings to enhance prevention of pressure ulcers caused by medical devices. Int Wound J. 2013: doi: 10.1111/iwj.12111.
- Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device related pressure ulcers in hospitalised patients. Int Wound J. 2010;7(5):358-65.
- Hanonu S, Karadag A. A Prospective, Descriptive Study to Determine the Rate and Characteristics of and Risk Factors for the Development of Medical Device-related Pressure Ulcers in Intensive Care Units. Ostomy Wound Manage. 2016;62(2):12-22.
- Yamaguti WP, Moderno EV, Yamashita SY, Gomes TG, Maida AL, Kondo CS, et al. Treatment-related risk factors for development of skin breakdown in subjects with acute respiratory failure undergoing noninvasive ventilation or CPAP. Respiratory care. 2014;59(10):1530-6.
- Hobson DB, Chang TY, Aboagye JK, Lau BD, Shihab HM, Fisher B, et al. Prevalence of graduated compression stocking-associated pressure injuries in surgical intensive care units. Journal of Critical Care. 2017;40:1-6.
- Coyer FM, Stotts NA, Blackman VS. A prospective window into medical device-related pressure ulcers in intensive care. Int Wound J. 2014;11(6):656-64.
- Schindler CA, Mikhailov TA, Kuhn EM, Christopher J, Conway P, Ridling D, et al. Protecting fragile skin: nursing interventions to decrease development of pressure ulcers in pediatric intensive care. American Journal of Critical Care. 2011;20(1):26-35.
- Schluer AB, Halfens RJ, Schols JGA. Pediatric pressure ulcer prevalence: A multicenter, cross-sectional, point prevalence study in Switzerland. Ostomy Wound Manage. 2012;58(7):18-31.
- Fujii K, Sugama J, Okuwa M, Sanada H, Mizokami Y. Incidence and risk factors of pressure ulcers in seven neonatal intensive care units in Japan: a multisite prospective cohort study. Int Wound J. 2010;7(5):323-8.
- Newnam KM, McGrath JM, Salyer J, Estes T, Jallo N, Bass WT. A comparative effectiveness study of continuous positive airway pressure-related skin breakdown when using different nasal interfaces in the extremely low birth weight neonate. Appl Nurs Res. 2015;28(1):36-41.
- Visscher MO, White CC, Jones JM, Cahill T, Jones DC, Pan BS. Face masks for noninvasive ventilation: Fit, excess skin hydration, and pressure ulcers. Respiratory Care. 2015;60(11):1536-47.
- Bonell-Pons L, García-Molina P, Balaguer-López E, Montal MÁ, Rodríguez MC. Neonatal facial pressure ulcers related to noninvasive ventilation: Incidence and risk factors. EWMA Journal. 2014;14(2):33-.
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Haesler E, editor. Osborne Park, Western Australia: Cambridge Media; 2014.
- Call E, Pedersen J, Bill B, Oberg C, Ferguson-Pell M. Microclimate impact of prophylactic dressings using in vitro body analog method. Wounds 2013;25(4):94-103.
- Call E, Pedersen J, Bill B, Black J, Alves P, Brindle CT, et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J. 2015;12:408-13.
- de Wert LA, Schoonhoven L, Stegen JHCH, Piatkowski AA, van der Hulst RR, Poeze M, et al. Improving the effect of shear on skin viability with wound dressings. J Mech Behav Biomed Mater. 2016;60:505-14.
- Otero DP, Dominguez DV, Fernandez LH, Magarino AS, Gonzalez VJ, Klepzing JV, et al. Preventing facial pressure ulcers in patients under non-invasive mechanical ventilation: a randomised control trial. J Wound Care. 2017;26(3):128-36.
- Limpaphayom N, Skaggs DL, McComb G, Krieger M, Tolo VT. Complications of halo use in children. Spine. 2009;34(8):779-84.
- Worsley PR, Prudden G, Gover G, Bader D. Investigating the effects of strap tension during non-invasive ventilation mask application: a combined biomechanical and biomarker approach. Medical Devices: Evidence and Research. 2016;9:409-16.
- Zaratkiewicz S, Whitney JD, Lowe JR, Taylor S, O’Donnell F, Minton-Foltz P. Development and implementation of a hospital-acquired pressure ulcer incidence tracking system and algorithm. Journal For Healthcare Quality. 2010;32(6):44-51.
- Ambutas S, Staffileno BA, Fogg L. Reducing nasal pressure ulcers with an alternative taping device. Medsurg Nursing. 2014;23(2):96-100.
- Günlemez A, Isken T, Gökalp A, Türker G, Arisoy E. Effect of silicon gel sheeting in nasal injury associated with nasal CPAP in preterm infants. Indian Pediatrics. 2010;47:265-7.
- Weng M. The effect of protective treatment in reducing pressure ulcers for non-invasive ventilation patients. Intensive & Critical Care Nursing. 2008;24(5):295-9.
- Kuo C, Wootten CT, Tylor D, Werkhaven J, Huffman K, Goudy S. Prevention of pressure ulcers after pediatric tracheostomy using a Mepilex Ag dressing. The Laryngoscope. 2013: doi:10.1002/lary.24094.
- Whitley AB, Nygaard RM, Endorf FW. Reduction of pressure-related complications with an improved method of securing endotracheal tubes in burn patients with facial burns. J Burn Care Res. 2017;31.
- Forni C, Loro L, Tremosini M, Mini S, Pignotti E, Bigoni O, et al. Use of polyurethane foam inside plaster casts to prevent the onset of heel sores in the population at risk. A controlled clinical study. Journal of Clinical Nursing. 2011;20(5/6):675-80.
- Singh R, Sood N, Kerai S, Puri A. Use of Merocel aids in prevention of nasal pressure ulcers following nasal intubation: Case series of 33 patients. Indian J Anaesth. 2017;61(6):513-5.
- The Joanna Briggs Collabortion. Handbook for Evidence Transfer Centers – Version 4. The Joanna Briggs Institute, Adelaide. 2013.




