Volume 26 Number 1
Low-frequency ultrasonic debridement and topical antimicrobial solution Polyhexamethylene biguanide for use in chronic wounds: a case series
Alison Vallejo, Marianne Wallis, Eleanor Horton and David McMillan
Keywords Chronic wounds, biofilms, ultrasonic debridement, polyhexamethylene, wound healing.
Abstract
Background: Chronic wounds can be difficult to heal, or sometimes never heal. Current evidence indicates that wound biofilm can interfere with healing. Combining two treatments targeting chronic wound biofilm may improve outcomes when standard care is unsuccessful. The aim of this study was to determine feasibility and acceptability of combining low-frequency ultrasonic debridement (LFUD) and the antiseptic polyhexamethylene biguanide (PHMB) post treatment.
Methods: This was an exploratory case study of four patients with non-healing wounds with suspected microbial involvement, who were subjected to a combination therapy approach. Data collection included retrospective health care record audit, wound observations and structured individual interviews. Interview data captured patient feedback about the acceptability of the therapy and provided information regarding feasibility of conducting larger trials.
Results: Standard care for the four participants had failed to assist with healing. The new combination therapy was acceptable and appeared to accelerate wound healing, with nil adverse effects.
Conclusions: Chronic wounds with suspected biofilm have the potential to heal if treatment is multifactorial. The combination of techniques used in this series was acceptable to patients and shows promise as an effective treatment, as it may have assisted the healing process. Further trials are needed to determine the efficacy.
Key points
What is known about the topic?
Chronic wounds affect almost half a million Australians1; it is not uncommon to see these wounds endure for months, years, decades, or never heal2,3. Many chronic wounds contain bacteria and microbes in a protected biofilm state that hinders healing4. Although methods for identification of major bacterial species present in chronic wounds are well advanced, it is much more difficult to diagnose the presence of a biofilm, with diagnostic methods currently being designed5. As a consequence, clinicians must use their expertise and judgement based on the patient history and wound characteristics, such as recalcitrance to treatment, and resistance to antimicrobials, to predict a biofilm presence and initiate targeted strategies.
Preliminary in vitro and animal models using low-frequency ultrasonic debridement (LFUD) reported to disrupt wound biofilms6,7. Polyhexamethylene biguanide (PHMB) is a surfactant antimicrobial solution demonstrated to kill, safely, a broad spectrum of microorganisms, with low cell toxicity, in an in vitro study8. The added surfactant is said to aid in the prevention of biofilm formation and assist in the eradication of mature biofilm, without interfering with healing9,10. Current wound infection and biofilm consensus guidelines suggest the application of multiple strategies that include debridement and topical antiseptics4,5,11. Although no formal evidence is available on the combination of LFUD and PHMB, this approach warrants exploration.
What does this paper add?
This paper explores, from a patient’s perspective, the experience of (1) receiving a combination treatment of ultrasonic debridement and (2) a topical application of PHMB post debridement, for chronic leg wounds. The study found that this dual therapy was well tolerated and appeared to promote healing in otherwise non-healing wounds.
What are the implications for practitioners?
Current standard of care varies from setting to setting, and sometimes does not include the use of adequate therapies to remove high levels of bioburden, including products that are listed as appropriate to aid in the prevention of biofilm reformation4,5. Wound practitioners should suspect wound biofilm when wounds do not respond to current care, and ensure their care practices have included a multi-strategy approach. Debridement tools such as LFUD and alternative modern antiseptics are options to be considered when wounds are stalled. The topical antiseptic PHMB is widely available to clinicians, is easy to use and appears as a safe and well-tolerated option10,12. Ultrasonic debridement machines, although costly to purchase, may be more cost-effective, if it increases the healing rate, with further studies required to investigate this.
Introduction
Chronic wounds can be defined as a dysregulation of normal wound healing, hindered by underlying physiological, pathological, mechanical and/or local factors13. These factors can reduce the supply of blood, oxygen and nutrients to the wound site; result in an unclean wound environment, or reduce the host’s response to infection; and interfere with the body’s normal wound healing processes3. As chronic wounds are open to the environment they are a portal of entry for microbes and an ideal environment for their growth, increasing the risk of infection, and delayed wound healing3,13,14. Depending on the site of the wound, the pathogenicity of the infectious organism, the total numbers of bacteria in the wound, and the host response, the severity of wound infection will significantly vary3.
Classic signs of acute infection can include pain, swelling, warmth, redness and the presence of purulent, malodorous discharge3. Microorganisms causing acute infection can also disseminate from the wound site, and in severe cases cause sepsis and death2,3,5. In contrast to acute infections, chronic infections can be more difficult to diagnose, and are presumptively identified on the basis of clinical signs which may include: persistent inflammation, increased exudate, slough, low-level erythema, hypergranulating and friable tissue, and an indolent wound, despite treatment4,5,15. A wound history of: antimicrobial failure, ongoing infection for more than 30 days, or failure of a wound to heal as expected are also indicative of chronic infection4. The use of topical antimicrobials is recommended treatment for localised infections, and systemic antimicrobials for disseminated infections5. Inadequate treatment for both acute and chronic wounds not only increases the risk of delayed healing, but is also an ongoing risk of mortality3.
The bacteria present in chronic wound infections are thought to exist in the biofilm state16. In fact, wound biofilms are now considered a causative factor for non-healing wounds, especially if underlying disease processes and co-morbidities have been addressed5. Bacteria in biofilms are sessile, and aggregate into complex, structured communities, covered by an extracellular polymeric substance, and express different genes to the same species in a planktonic, free-living state5. Accordingly, bacteria in biofilms have a different phenotype5,17. Relevant to treatment of chronic wounds, this allows the bacteria in biofilms to generally have a higher resistance to antibiotics, antiseptics, and host-immune molecules2,3,5,17,18.
While the presence of biofilm is invisible to the naked eyeI5,17, multiple bacterial species in high abundance, found in large clustered colonies is generally considered to be predictive of their presence18,19. Stalled healing and/or altered “wound behaviour”16 are also used as predictors of the presence of biofilm in the clinical setting. Further observational studies suggest that a rapidly returning, easily removed, translucent, gel-like layer is suggestive of biofilm20,21; and if suspected, should prompt initiation of removal and prevention of reformation — biofilm-based wound care (BBWC). As biofilms can re-establish in a matter of hours17,22 via dormant persister cells23, best-practice treatment must include additional strategies that take into account the effect of wound biofilm on healing, such as debridement therapies and antiseptic treatments.
For over 60 years, polyhexamethylene biguanide, polyhexanide, or PHMB has been used as a broad-spectrum biocide antiseptic and disinfectant against bacteria, yeasts and fungi9,12,24 and is commonly found in topical solutions for eye and mouth care, baby wipes, cosmetics, pool sanitisers, and more recently in wound products9,12,24-26. It is most commonly used as a topical cleansing solution with a combined surfactant betaine, and can be found in foams, gels, gauzes and biocellulose wound products. PHMB is bactericidal while remaining non-toxic to human skin cells and flora at the concentrations used in clinical settings10,24,27. PHMB is considered one of the safest, most effective and tolerated antiseptics in wound management10,12, with no reports of resistance, or systemic absorption; low sensitivity levels25 and an increased antimicrobial efficacy when used following physical biofilm disruption4.
Physical disruption of wound biofilm is commonly and rapidly achieved via sharp debridement38. More recently, LFUD has been suggested as an alternative debridement method, shown to disrupt biofilms in vitro6,7 and reduce bacterial burden in in-vivo studies9,28,29. LFUD has been reported to reduce infection rates, the need for ongoing antibiotic treatment30, stimulate faster healing31,32 and reduce pain during and after treatment9,28,30,33-36,40. It is a safe and selective method with a reduced risk of sharps injury29,37-39. LFUD provides additional biophysical effects of cellular stimulation, such as increased cell membrane permeability, collagen synthesis and fibroblast proliferation40. It is also reported to work more effectively with antiseptic agents to enhance killing of both planktonic and biofilm bacteria40.
An in-vitro study that utilised a dual LFUD/PHMB treatment7 reported that LFUD effectively disrupted biofilms, resulting in bacteria transitioning to a free-floating, planktonic form. In turn, this allowed the PHMB to penetrate and kill the bacteria more effectively than if the bacteria were in the biofilm state. While LFUD and PHMB have individually been established as safe and effective wound management therapies, the acceptability to patients and efficacy of the combination of these two therapies has not been established in vivo. The primary aim of this preliminary study was to investigate how acceptable this treatment was to patients. Secondary aims were to investigate wound response to this treatment, and determine the feasibility of a larger interventional study.
Methods
Research design
A retrospective, exploratory case study of four case histories was chosen to explore wound responses to this dual therapy and how acceptable it was to patients, that is to say, how well they tolerated the treatment and their self-reported acceptance of the therapy. The case study design gave the researcher time to capture the fine detail related to the treatment41,42.
Participant recruitment
The cases were purposefully selected for their similarities in wound characteristics and history. Patients were chosen to receive the combination therapy and participate in the study if they had attended the specialist wound clinic for their recalcitrant wounds and fitted the inclusion criteria. Inclusion criteria included: patients with lower extremity wounds with devitalised tissue; suitable for ultrasound; clinical signs of chronic infection; a history of non-healing for more than six weeks and a history of prior use of antimicrobial therapies, including antibiotics. An invitation letter was mailed out from a non-research team member to four participants who had previously undergone the LFUD/PHMB treatment. All those approached agreed to participate in the study.
Treatment received
The four participants received LFUD following a comprehensive assessment to ensure they had no contraindications for either element of the combination therapy (for example, allergic to PHMB). Prior to LFUD, a topical anaesthetic cream (LMX-4) was applied for 20–30 minutes to reduce any discomfort. After the ultrasound treatment, the application of PHMB solution was applied to the wound for 15 minutes on a soaked gauze pad. The primary wound dressing then consisted of various sustained antimicrobial dressings, such as silvers or alginogels. A secondary dressing of a superabsorbent pad and compression therapy was applied, to address all of the underlying factors. At each dressing change, the use of PHMB solution was applied according to the manufacturer’s instructions, consistently at each dressing change, including when care was performed outside the clinic.
Ethics
Human Research Ethics Committee approval was given from Uniting Care Queensland (UCQ) (UCQ/HREC/18916) and University of the Sunshine Coast (USC) (S/16/965). Participants gave written informed consent for the collection and use of data by structured, audio-recorded interviews and retrospective audit of clinical records.
Data collection
Retrospective data were collected from patient files and focused on: co-morbidities, age, sex, wound duration prior to commencement; previous wound treatments; history of antibiotic therapy; use of topical antimicrobials; confirmed bacterial identification; wound type; wound size; tissue type; wound-associated pain; and concurrent treatment. Wound healing data collected was by direct observation, serial photographs, and measurements via tracings. The semi-structured interviews were recorded to avoid loss of information and allow verbatim transcription. Questions were designed to explore how well the treatment was tolerated and how acceptable the participants found the treatment regime. These questions focused on: previous experiences with wound treatment; feelings around the new treatment (including concerns and thoughts about new and different techniques); feelings and sensations experienced during and after the procedure; and what happened when the treatment commenced.
Analysis
Retrospective community health records were audited. Quantitative data were recorded in a table to allow comparison between participants of pre-treatment characteristics and treatment acceptability. Qualitative data were recorded and transcribed verbatim. Data were read repeatedly, with the participants' comments organised into categories and recurring themes, using the pattern-matching strategy and replication logic42. Themes and patterns were labelled and identified from the specific guided questions and tabulated for clarity. Findings were used to illustrate the topic using credible sources, such as quotes. All treatment activities and data collection processes involved personal contact with the researcher, this, along with prior knowledge of the topic, assisted with the interpretation of the data42.
Results
Participants and wound characteristics
Three participants were female and had a mean age of 76. One participant was a 95-year-old male. The four participants had five wounds in total and all wounds were venous leg ulcers; two with associated lymphoedema. Table 1 summarises the characteristics of the participants. The four participants had lived with their non-healing wound for months to years (mean duration 8.2 years +/– 7.10). One participant had a wound that had healed and recurred three times over seven years, while for another participant, this was the second wound lasting three years. One female participant presented with bilateral leg wounds of 14–17 years' duration.
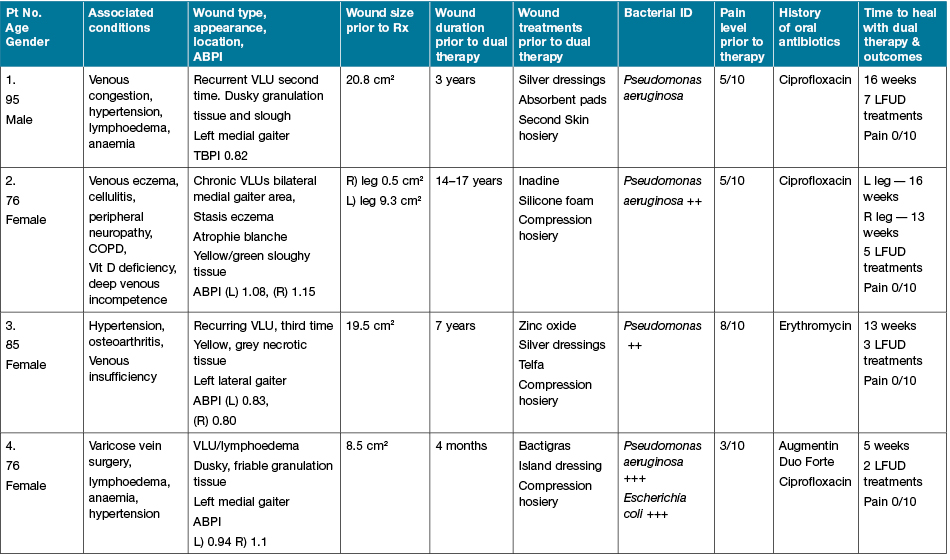
The participants had similar wound characteristics (Table 1) and previous wound care experiences prior to initial assessment (Table 2). Upon observation, all wounds contained unhealthy tissue of a yellowy/brown, slimy layer, with green-stained exudate, consistent with wound infection. All wounds also had a history of non-healing, despite antimicrobial dressings and compression therapy (see Figures 1–5 of initial presentation, prior to treatment). The wound history and wound behaviour were also consistent with chronic wound infection and the presence of biofilm, which were presumptively diagnosed by the clinician. These signs included: long duration of non-healing; failure of multiple doses of antibiotic treatment to improve wound outcomes; persistent inflammation; regular presence of exudate; and unhealthy, friable granulation tissue (Table 1). Pseudomonas aeruginosa was diagnosed in all wounds via a swab culture prior to attending the clinic for all four participants. Additionally, Escherichia coli was identified in the wound of one patient.
Table 1: Participant and wound characteristics
Care in the community prior to clinic admission
The wound care practices that the participants received prior to the clinic were similar, including: self-care; and a local doctor and/or a community nurse. The care they received consisted of: various antimicrobials and systemic antibiotics with compression therapy, that did not achieve healing. Despite non-healing, the standard treatment continued, for months to years. One patient requested that community nurses attend his wound in the home after he had attended the local doctor daily for 12 months. The second participant was referred to community nurses after a hospital episode for cellulitis. The third participant was seen by the practice nurse and local doctor on and off for seven years. The fourth patient had a similar experience: daily wound care at the general practice clinic for four months, with no healing progress.
Experience of the treatment with dual therapy
LFUD was applied weekly in the clinic until it was deemed unnecessary. This was determined when the wound bed demonstrated signs of red, healthy granulation, an epithelial wound edge advancement and wound size reduction. For the two participants with the longest duration of wounds, five to seven LFUD applications were performed (Table 1).
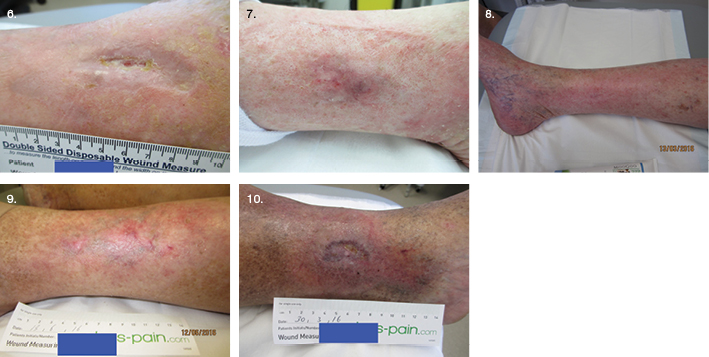
Qualitative data were collected from the four participants on their experience with the dual therapy, guided by the interview questions. All of the wounds healed within 16 weeks. Participants 1, 2 and 3 expressed great satisfaction at this achievement, after a long duration of non-healing (Figures 6–10 of post-treatment). Participant 4’s wound healed in five weeks, after four months of non-healing (Figure 10). The treatment was accepted, with no reports of discomfort during the LFUD procedure or application of antiseptic. Participant No.1 initially reported pain in the evening, following treatment, which was managed well with an oral opioid after a case conference with the local doctor, family and clinic staff. The other three reported no pain during or following treatment, very little, or they couldn’t remember enough to report. Ongoing pain levels using the Numerical Rating Scale (NRS) reduced to zero as the wound healing progressed.
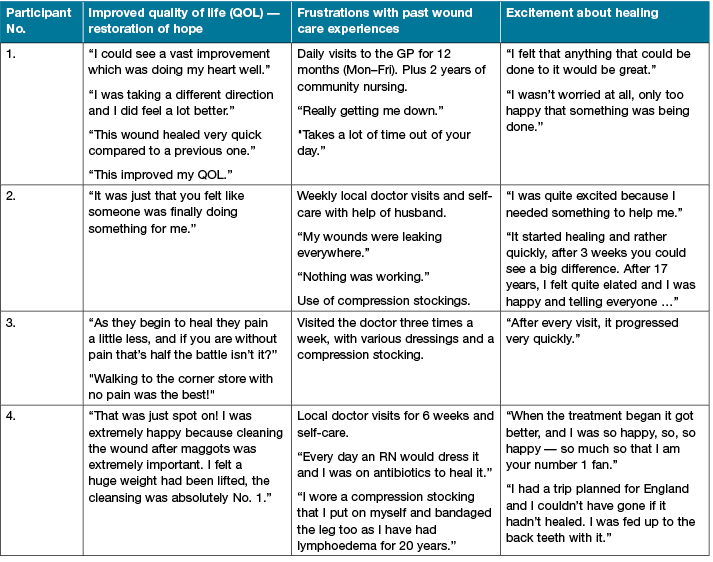
From the qualitative data (Table 2), the themes that emerged included: an improved QOL and restoration of hope; frustrations about previous wound care practices; and excitement about the rapid healing progress, with confidence in the cleansing effect. For example, one participant had a confirmed P. aeruginosa plus E. coli infection, and maggots (free range) prior to admission. This had distressed the participant immensely and the idea of ultrasound therapy and an antiseptic made them extremely happy — “to have the wound cleansed thoroughly instead of just adding stuff on top of it all the time, the cleansing of the wound to me was absolutely number one”. One participant was eager to accept new possibilities, hopeful of healing a long-term ulcer, stating: “I felt that anything that could be done to it was great because it was virtually at a stalemate”. One excited participant stated: “I needed something to help me as I had not had any help from the other treatments I was doing”. When asked how the LFUD felt, it was described as “soothing”, with “some tingling, but it wasn’t uncomfortable, it was just that you felt like someone was finally doing something for me”. For participant No. 2, with the longest duration of wounds, five LFUD treatments were applied (Table 1). In 13 weeks, the left leg ulcer closed, and in 16 weeks the right leg ulcer closed. After 17 years, this patient was elated: “I was happy and I was telling everyone”. “I had people around to look at my legs … just a few friends … and no one could get over it, how quickly it had started to come good” (Figures 7 and 8).
Table 2: Qualitative interview — themes and participants 1–4
Discussion
The results of this case study demonstrated that not only was the combination of LFUD and topical antiseptic PHMB safe, acceptable and improved the QOL for four patients, it appeared to heal their wounds within a short time frame (Table 1). The four case histories represented typical wound types and scenarios. They were older people with venous leg ulcers, a history of frequent systemic antibiotics and various topical antimicrobial dressings for the difficult-to-remove and commonly encountered biofilm forming organism — P. aeruginosa3,43. All four participants had previously received compression therapy to address the underlying aetiology, but none had received any form of mechanical wound debridement or the application of antiseptics, in combination. This highlights the difference between how “standard care” is defined and the inadequacies that are practised in clinical care settings.
Chronic wounds such as the ones discussed in this case study are a challenge for clinicians in everyday clinical practice. They are wounds that recur frequently, take a long time to heal, or do not heal44,45 due to complex microorganism reactions, unless disrupted43. As demonstrated, standard wound care practice does not always involve this bacterial disruption and often consists of significant evidence-based practice gaps46, including a lack of dedicated multidisciplinary teams shown to promote healing with hard-to-heal wounds45,47-49, or the availability of skilled clinicians able to provide expert advice and perform specific tasks46, such as wound debridement. Biofilm-based wound care (BBWC) is the emerging standard of care for managing chronic wounds and has been shown to improve wound healing22,50. The combination of debridement techniques and antimicrobial therapies are outlined as best-practice in current consensus guidelines4,11 and should be seen as standard care.
Because the wounds in this series had not received any wound bed preparation, the assumption is that other forms of debridement may also have assisted with the healing effect. While there are various forms of debridement, these four participants received LFUD because of its unique ability to selectively avoid healthy tissue, without inducing pain28,36 and provide additional benefits of cellular stimulation40. LFUD was chosen to disrupt the suspected biofilm, with reports of reaching biofilms at a deeper level29, understanding that these hard to reach microorganisms live in a quiescent state in the biofilm core, and are a challenge to remove51. When the biofilm structure is disrupted, antimicrobials are thought to be more effective, and kill the free-floating bacteria in its planktonic state7. When the antiseptic applied is non-toxic12 with high biocidal activity51, such as PHMB in Prontosan“ 52-56, it is anticipated that surface microbe counts are reduced and prevented from reforming into their protective state, while not interfering with normal cell proliferation.
In this series, there is preliminary evidence that not only was the dual treatment acceptable, reduced pain levels, and showed no adverse effects, the wounds healed, no antibiotics were required, and QOL was restored. And while these outcomes may have resulted from the act of debridement, the technique used, the antiseptic, or the combination; a multi-targeted strategy worked. To date, there are limited studies on this subject, on the patient experience. One randomised control trial (RCT) compared surgical debridement to LFUD for venous leg ulcers, and showed LFUD to significantly improve QOL, with a lower risk profile30.
It is well known that chronic wounds can impact significantly on a person and affect different aspects of their QOL46,57. Studies have shown that pain, restrictions and limitations can affect the wellbeing of those with chronic wounds58. Similar reports were received during the qualitative interviews as an emerging theme of frustrations about previous wound care experiences was heard. Other themes included: improved QOL with restoration of hope, and the excitement of the wound healing. Overall, the participants expressed satisfaction with the treatment (Table 2).
QOL improved as the wound improved. Signs of improvement included a reduction in wound exudate, pain, and product cost, with less frequent clinic visits. This restored a sense of hope to the patient, and developed feelings that someone was doing something for them, that was working. Because it was working, the treatment was not altered, the patients were excited to attend and developed a trust in the clinic staff. They commented that their wounds were clean, as the ultrasound treatment left an instant visible difference. Excitement about the rapid healing effect improved their wellbeing, that is sometimes altered when living with a chronic wound57. Restrictions were lifted as they could bathe without covering the area, or go out without worrying about their wounds leaking. For one participant, being able to proceed with a planned vacation overseas was a major relief; and for another walking to the corner store with no pain was the best.
Pain can be an early sign of infection59, and has been reported as one of the most commonly associated symptoms for those with chronic wounds60. These participants all presented with significant pain (Table 1), which reduced steadily with wound healing. This implies that bacterial levels and pain may be closely related. The participants considered their pain as part of having a chronic wound44,45, and despite this, were willing to try something very different. One RCT that compared LFUD to sharp wound debridement reported significantly lower pain levels with LFUD treatment32. Another RCT reported reduced wound pain shortly after treatment28, and a small pilot study and two observational studies reported LFUD as a painless experience33,30,36; suggesting this may be a result of bacterial reduction. Evidence suggests that Prontosan® also assists with pain reduction26,55,56. A topical anaesthetic cream LMX-4 applied prior to the therapy also assisted to reduce any discomfort. Pain reduced to zero upon wound closure, implying that the pain experienced was wound-related.
The frustrations about previous wound care experiences were similar among the four participants. These experiences are not uncommon for people who live with chronic wounds They mentioned frequent, ongoing visits to the local doctor, some daily; one received two years of community nursing visits. Comments were made about the time taken out of their day, with little results. Participant No. 4 made a point that previous care involved putting “stuff on top of stuff”. This was related to the antimicrobial dressing products that are often seen layered on wounds. It was interesting to note that these participants were advised not to wash their wounds, but squirted on a few drops of saline at each dressing change. This technique lacks interference with wound biofilm and foreign bodies, as the volume, pressure and type of solution used for irrigation are considered critical variables when thoroughly cleansing a wound to reduce infection53,61,62. Ultrasonic therapy delivers this thorough cleansing effect, with the topical antiseptic post procedure to potentially destroy remaining microbes and prevent their replication, as per current practice guidelines63.
This case study generates further questions as to whether these four wounds healed because they received a form of debridement that was seen to prepare the wound bed. Further investigations are required to test whether it was the consistent use of the PHMB solution, or the combination of both, that influenced the positive outcome seen in these four cases. This study has established that applying this combination therapy is acceptable and clinically feasible. Future larger interventional trials are required to determine efficacy.
Conclusion
The five wounds studied in this series all healed in 16 weeks. This was seen as a success, given that these wounds had been present for years. In this study of the patient experience, LFUD and PHMB was an acceptable combination and had a positive effect on the QOL and wounds for four patients. Wound care in the home or the local doctor’s surgery does not always involve best-practice, nor current standard care. “Standard care” needs to address all of the underlying reasons, including actions to remove and prevent pathogens from taking up wound residence. Future research using robust experimental methods are now required to determine the efficacy, effectiveness and cost savings of this combination therapy.
Disclosure of interests
There are no disclosures of interest.
Author(s)
Alison Vallejo*
RN, PostGradDipWound, PhD (C)
Wound Solutions Clinic Blue Care
The University of the Sunshine Coast
School of Nursing, Midwifery and Paramedicine
Locked Bag 4, Maroochydore, QLD 4558, Australia
Email Alison.Vallejo@research.usc.edu.au
Marianne Wallis
PhD, FACN, RN
School of Nursing, Midwifery and Paramedicine, The University of the Sunshine Coast, Maroochydore, QLD, Australia
Eleanor Horton
PhD, MHlthSc(Nsg), BHlthSc(Nsg), ADN, RN School of Nursing, Midwifery and Paramedicine, University of the Sunshine Coast, Maroochydore, QLD, Australia
David McMillan
PhD, BSc(Hons)
INFLAME Biomedical Research Cluster, School of Health and Sport Sciences, The University of the Sunshine Coast, Maroochydore, Australia
* Corresponding author
References
- Wounds Australia. Western Australia, 2017. Available from: www.woundsaustralia.com.au/pages/wac.php
- Edwards-Jones V. Microbiology: the basics. In: Edwards-Jones V (Eds). Essential microbiology for wound care. Oxford, UK: Oxford University Press, 2016:11–31.
- White R. Acute versus chronic wounds: microbiological differences. In: Edwards-Jones V (Eds). Essential microbiology for wound care. Oxford, UK: Oxford University Press, 2016:53–66.
- World Union of Wound Healing Societies. Position Document. Management of biofilm. Wounds Int 2016:1–26.
- International Wound Infection Institute. Wound infection in clinical practice. Principles of best practice. 2016:1–30.
- Seth AK, Nguyen KT, Geringar MR et al. Noncontact, low-frequency ultrasound as an effective therapy against Pseudomonas aeruginosa-infected biofilm wounds. Wound Repair Regen 2013;21:216–74.
- Crone S, Garde C, Bjarnsholt T, Alhede M. A novel in vitro wound biofilm model used to evaluate low-frequency ultrasonic-assisted wound debridement. J Wound Care 2015;24(2):64–72.
- Wiegand C, Abel M, Ruth P, Hipler U. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polyhexanide. Wound Repair Regen 2009;17:730–8.
- Butcher M. PHMB: an effective antimicrobial in wound bioburden management. Br J Nurs 2012;21(12):S16–S21.
- Daeschlein G. Antimicrobial and antiseptic strategies in wound management. Int Wound J 2013;10(Suppl 1):9–14.
- Shulze G, Bjarnsholt T, James GA. Consensus guidelines for the identification and treatment of biofilm in chronic non-healing wounds. Wound Repair Regen 2017; Accepted Article 1–50. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28960634
- Hubner NO, Kramer A. Review on the efficacy, safety, and clinical applications of polyhexanide, a modern wound antiseptic. Skin Pharmacol Physiol 2010;23(Suppl 1):17–27.
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89(3):219–29.
- Martin M. Physiology of Wound Healing. In: Flanagan M (Eds). Wound healing and skin integrity, principles and practice. Sussex, UK: John Wiley & Sons, Ltd, 2013:33–51.
- Percival SL, Thomas JG, Williams D. An introduction to the world of microbiology and biofilmology. In: Percival SL, Cutting KF (Eds). Microbiology of wounds. Florida, USA: CRC Press, 2010:1–59.
- Wolcott R. Biofilms cause chronic infections. J Wound Care 2017;26(8):423–5.
- Bjarnsholt T, Eberlein T, Malone M, Shultz GS. Management of wound biofilm made easy. Wounds Int 2017;8(2):1–6.
- Cooper D. Biofilms and wounds: much ado about nothing? Wounds UK 2010;6:84–90.
- Vyasks S, Wong LK. Detection of biofilms in wounds as an early indicator for risk of tissue infection and wound chronicity. Ann Plast Surg 2016;76(1):127–31.
- Hurlow J, Bowler PG. Potential implications of biofilm in chronic wounds: a case series. J Wound Care 2012;21(3):109–17.
- Keast D, Swanson T, Carville K, Fletcher J, Shultz GS, Black J. Top Ten Tips: Understanding and managing wound biofilm. Wounds Int 2014;5(20):1–4.
- Wolcott R. Understanding biofilm formation and biofilm-based wound care. Wounds Middle East 2014 2014;1(1):24–6.
- Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Environ Microbiol 2013;79(23):7116–21.
- Roberts C. Antimicrobial agents used in wound care. In: Edwards-Jones V (Eds). Essential microbiology for wound care. 1 ed. Oxford, UK: Oxford University Press, 2016:103–21.
- Cutting KF. Addressing the challenges of wound cleansing in the modern era. Br J Nurs 2010;Tissue Viability Suppl 19(11):S.
- Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new Polyhexamethylene biguanide antimicrobial foam dressing — clinical trial results. Adv Skin Wound Care 2011;24:78–84.
- Wounds UK. Consensus document: PHMB and its potential contribution to wound management. Wounds UK, Aberdeen, 2010. Available from: http://www.wounds-uk.com/journal-articles/phmb-and-its-potential-contribution-to-wound-management-2.
- Gehling ML, Samies JH. The effect of noncontact, low intensity, low frequency, therapeutic ultrasound on lower extremity chronic wound pain: A retrospective chart review. Ostomy Wound Manage 2007;53(3):44–50.
- Serena T, Lee K, Lam K, Attar P, Meneses P, Ennis W. The impact of noncontact, nonthermal, low-frequency ultrasound on bacterial counts in experimental and chronic wounds. Ostomy Wound Manage 2009;55(1):22–30.
- Breuing KH, Bayer L, Neuwalder J, Orgill DP. Early experience using low-frequency ultrasound in chronic wounds. Ann Plast Surg 2005;55(2):183–7.
- Madhok B, Vowden K, Vowden P. New techniques for wound debridement. Int Wound J 2013;10:247–51.
- Singh A. Usage of ultrasound in wound management. Comparison between ultrasonic wound debridement and sharp debridement in diabetic foot ulcers: a randomized clinical trial 2004. Malaysia, University of Malaya. Faculty of Medicine. 2006.
- Butcher G, Pinnuck L. Wound bed preparation: ultrasonic-assisted debridement. Br J Nurs 2013;22(6):S36–43.
- Herberger K, Franzke N, Blome C, Kirsten N, Augustin M. Efficacy, tolerability and patient benefit of ultrasound-assisted wound treatment versus surgical debridement: a randomized clinical study. Dermatology 2011;222(3):244–9.
- Kim PJ, Steinberg JS. Wound care: biofilm and its impact on the latest treatment modalities for ulcerations of the diabetic foot. Semin Vasc Surg 2012;25(2):70–4.
- Tan J, Abisi S, Smith A, Burnand KG. A painless method of ultrasonically assisted debridement of chronic leg ulcers: A Pilot Study. Eur J Vasc Endovasc Surg 2007;33(2):234–8.
- Medigroup Australia. SONOCA — 185: Ultrasonic debridement accelerated wound treatment. Melbourne, Victoria: Medigroup Australia, 2014:1–4.
- Strohal R, Dissemond J, O’Brien JJ et al. EWMA Document: Debridement An updated overview and clarification of the principle role of debridement. J Wound Care 2013;22(Supp. 1):S1–S52.
- Australian and New Zealand Horizon Scanning Network (ANZHSN). Horizon scanning technology prioritising summary low-frequency ultrasound debridement. Department of Health and Ageing. Royal Australasian College of Surgeons, 2007.
- Kloth LC, Niezgoda JA. Ultrasound for wound debridement and healing. In: McCullough JM, Kloth LC (Eds). Wound healing: Evidence-based management. 4th ed. Philadelphia, 2010:545–75.
- Merriam SB. Introduction to qualitative research. Jossey-Bass Publishers, 2002. Available from: https://stu.wesga.edu/~bthibau1MEDT8484-Baylen/introduction_to_qualitative_research/introduction_to_qualitative_research.pdf
- Yin RK. Case study research: Design and methods. 5th ed. CA, USA: SAGE Publications Inc, 2014.
- Edwards-Jones V. Wound pathogens. In: Edwards-Jones V (Ed). Essential Microbiology for wound care. Oxford, UK: Oxford University Press, 2016:67–101.
- Walker N, Rodgers A, Birchall N, Norton R, MacMahon S. Leg ulcers in New Zealand: age at onset, recurrence and provision of care in an urban population. N Z Med J 2002;115(1156):286–9.
- Edwards H. Chronic venous leg ulcers: effect of a community nursing intervention on pain and healing. Nurs Stand 2005;19(52):47–54.
- Australian Centre for Health Services Innovation. Issues paper: Chronic wounds in Australia. Australia: Australian Centre for Health Services Innovation, 2017:1–22.
- Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs 2009;18(11):1541–9.
- Moore Z, Butcher G, Corbett LQ, McGuiness W, Snyder R, J, van Acker K. Managing wounds as a team. J Wound Care 2014;23(5 Suppl):S1–S38.
- Medical Advisory Secretariat. Community-based care for chronic wound management: an evidence-based analysis. Health Quality Ontario, 2009.
- Metcalf D, Bowler P, Parsons D. Wound biofilm and therapeutic strategies. INTECH, 2016:272–94.
- Ortega-Pena S, Hidalgo-Gonzalez C, Robson MC, Krotzsch E. In vitro microbiocidal, anti-biofilm and cytotoxic effects of different commercial antiseptics. Int Wound J 2016.
- Kaehn K, Eberlein T. An in-vitro model for comparing the efficacy of wound rinsing solutions. Br J Nurs 2009;18(6):229–34.
- Seipp HM, Hofman S, Hack A, Strowronsky A, Hauri A. Efficacy of various wound irrigation solutions against biofilms. ZfW 2005;4:160–4. Available from: http://www.klinion.nl/files/file/Efficacy%20of%20Various%20Wound%20Irrigants%20against%20Biofilms_Seipp.pdf?phpMyAdmin=e45916cab41b966193627d0ad8837577
- Perez R, Davis C, Kaehn K. Effect of different wound rinsing solutions on MRSA biofilm in a porcine wound model. Hygiene + Medizin 2010;35(12):464–8.
- Valenzuela AR, Perucho NS. The effectiveness of 0.1% polyhexanide. Rev Enferm 2008;31(4):7–12.
- Romanelli M. Evaluation of the efficacy and tolerability of a solution containing Undecylenamidopropyl-Betaine and Polyhexanide (Prontosan) in controlling the bacterial burden of chronic wounds during wound bed preparation. Wound Healing Research Unit, Dept of Dermatology, University of Pisa, 2008. Available from: http://www.prontosan.co.uk/docs/clinical%20evidence/romanelli%20m.pdf
- Wounds International. International Consensus. Optimising wellbeing in people living with a wound. An expert working group review. London, 2012. Available from: http://www.woundsinternational.com/media/issues/554/files/content_10309.pdf
- Herber O, Schnepp W, Rieger M. A systematic review on the impact of leg ulceration on patients' quality of life. Health and Quality of Life Outcomes 2007;5(44):1–12.
- Edwards-Jones V, Flanagan M. Wound infection. In: Flanagan M (Eds). Wound healing and skin integrity. West Sussex, UK: John Wiley & Sons Ltd, 2013:87–102.
- Jones J, Barr W, Robinson J, Carlisle C. Depression in patients with chronic venous ulceration. Br J Nurs 2006;15(11):S17–23.
- Horrocks A. Prontosan wound irrigation and gel: management of chronic wounds. Br J Nurs 2006;22(S 1222):1224–8.
- Griffiths RF, Ussia C. The effectiveness of solutions and techniques and pressure in wound cleansing. Systematic Review. Australia: The Joanna Briggs Institute for Evidence Based Nursing and Midwifery, 2002, Report No. 20.
- Shultze G, Sibbald G, Falanga V et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(1):1–28.



