Volume 26 Number 1
The fundamental goal of wound prevention: recent best evidence
Emily Haesler, Nicoletta Frescos and Robyn Rayner
Keywords skin tears, pressure injury, Wound prevention, venous leg ulcers, foot ulcers.
Abstract
Preventing wounds is a fundamental goal for all wound professionals and is enshrined within the Australian national wound management standards. The paper presents an overview of the recent evidence in the prevention of venous leg ulcers, pressure injuries, diabetes-related foot ulcers and skin tears. Recent literature searches identified a paucity of high-quality evidence for most wound prevention interventions. The paper presents evidence available to support current best practice. Despite the lack of strong scientific evidence, fundamental care interventions, including promoting healthy skin integrity and off-loading pressure, continue to be mainstay in preventing wounds of all aetiology.
Introduction
“An ounce of prevention is worth a pound of cure.”
— Benjamin Franklin1
Famously advising the city of Philadelphia on fire management, US Founding Forefather and inventor Benjamin Franklin not only highlighted the importance of initiating precautions to prevent catastrophe, but also captured the essence of excellence in wound practice.
Chronic and acute wounds are a significant burden on health care systems and have an appreciable impact on the lives of people who experience them. Prevalence of wounds is variable by aetiology, clinical setting and population. Prevalence of venous leg ulcers (VLUs), which also increase in older adults, is reported at between 0.05% and 1% in the general community, rising to 2.5% in aged care settings2. Diabetes-related foot ulcers (DRFUs) have a prevalence of 0.02% to 10% in the general community. In one study that focused on hospitalised people with diabetes, as many as 20% had a current DFU2. Pressure injuries (PIs), which occur across all age groups, have prevalence rates varying from 1% to 27% in acute care settings, 7% to 53% in aged care, and 6% to 29% in the community2. Skin tears (STs), which comprise a substantial proportion of all wounds found amongst older adults3-7, have a prevalence in older adults of 41% to 59% in Australia, 14% to 22% in North America and 4% to 14% in Japan3,5,8-11.
These prevalence rates suggest that, in combination, wounds of any aetiology are implicated as a significant burden on health, particularly that of older people. Demographic data showed that in 2015 around 15% of all Australians were aged over 65 years, with population projections indicating that by 2056 this age group will grow to 22%12,13. The increase in longevity and the concomitant number of older adults with age-related skin changes suggests a potentially large and increasing burden of wound care on health budgets and resources. Although acute and chronic pain, lower health-related quality of life, loss of self-esteem and reduced engagement in social activities are reported in the wound-related literature14-18 at the present time, there is a paucity of research on the full extent of the human and economic impact of chronic wounds19.
Preventing wounding and maximising the healing potential of every individual are enshrined in the Australian Standards for Wound Prevention and Management as the essential goals of wound practice and a priority for all wound professionals20.
Identifying the risk of a wound
As will be discussed in the next section, many preventive wound care strategies are based on avoiding specific types of wounds. Different wound aetiologies require very particular preventive strategies that address underlying pathophysiology. Implementing the full gambit of wound preventive strategies for every individual is generally unnecessary and indisputably costly. Therefore, a significant component of preventive wound care is establishing an individual’s risks for a wound.
Early identification of individuals at risk of a wound permits timely and targeted implementation of preventive strategies to reduce wound incidence, optimising quality of life and minimising health care expenditure. Risk identification involves recognising underlying disease, other individual factors and environmental risks that can contribute to development of a wound. Maintaining contemporary knowledge on the aetiology of wounds and understanding the risk factors for different types of wound is key to making an initial evaluation of the risk assessments most appropriate to any particular person. In many organisations, a suite of risk-assessment tools that are broadly applicable to the clinical setting and individuals receiving care within it are used as standard clinical practice.
There is a wide range of assessment tools designed to assess the risk of specific types of wounds. Identifying factors placing a person at risk of a wound informs the appropriateness of developing a wound prevention plan and helps in selecting appropriate strategies that a prevention plan should include. Assessment tools should be selected for the demographic of the individual being assessed (for example, age and clinical setting), ensuring the tool has strong psychometric properties for that population20. Using a reliable and valid tool ensures that the assessment is accurately measuring the characteristics being targeted21.
In individuals who are diagnosed with venous disease, both clinical classification and severity of disease are indicators of the risk of a VLU. The CEAP (clinical, aetiological, anatomical and pathophysiology) scale, which is recognised internationally, consists of seven classifications that describe the severity of the patient’s venous disease22,23. Higher classifications are indicative of the higher risk of experiencing a VLU. In addition, a number of venous disease severity scales are reliable and valid for evaluating the severity of disease (Table 1)23,24.
Table 1: Tools associated with assessing wound risk/disease severity
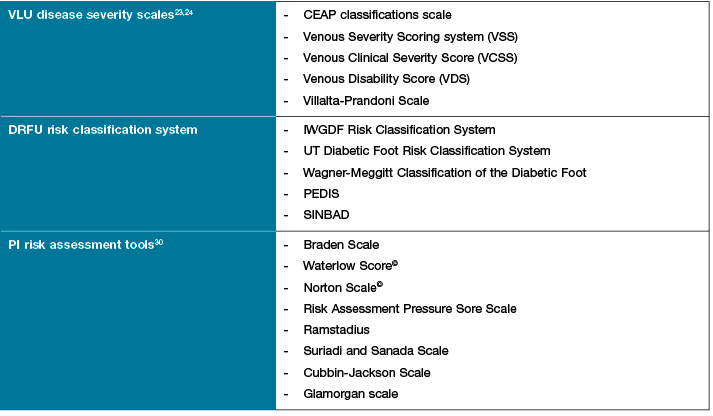
There are several diabetes foot risk classification systems, with most focusing on DFRU clinical measures of grade, category and prediction of ulcer outcome or risk of lower extremity amputation. The University of Texas and the International Working Group for Diabetes Foot (IWGDF) have classification systems that categorise the risk category of the foot and are widely used by podiatrists to inform clinical decision making. Although there is no gold standard for a prediction tool for amputation, most classification systems have high sensitivity, specificity and positive predictive value25,26.
Significant work has been done in developing and refining PI risk assessment tools that provide a structured approach to assessment. Although evaluation of their effectiveness compared to clinical judgement in identifying people who have a higher risk of a PI shows mixed findings27-29, PI risk assessment tools offer health care professionals a structure framework; reminders of risk factors to consider; and reliable measurement of specific risk factors30. Some of the more commonly used tools are listed in Table 1.
There is minimal literature on risk assessment for STs. A recent systematic review identified only 17 studies exploring the topic31. Numerous factors are identified as playing a role in increasing the risk of STs, including age-related skin changes, hydration and nutrition, sensory changes, impairments to mobility, medication use and mechanical factors related to care of the skin31. However, there is currently no validated risk assessment tool for STs.
Best evidence on wound prevention
The most appropriate interventions for wound prevention are informed by the risk assessment and the person’s needs and preferences. The following section provides an overview of the best evidence on preventive strategies specific to VLUs, DRFUs, STs and PIs. The section is based on searches of the literature published from January 1980 conducted in the following electronic databases: PubMed, Medline, CINAHL, Embase, Scopus, Evidence Based and Medicine Reviews (EBM). Searches were refined according to wound type and used search terms specific to each wound aetiology. The searches covered literature published up to February 2016 (VLUs), December 2017 (PIs) and February 2018 (DRFUs and STs).
Prevention of venous leg ulcers
The underlying pathophysiology responsible for venous ulceration is venous hypertension, commonly occurring due to venous reflux and/or obstruction in either the superficial or deep venous system or, in more progressed disease, both32. The complex pathophysiology involves dilatation of the lower limb veins, leading to valvular incompetence and subsequent increased venous pressure, which is exacerbated during exercise33. Less frequently, venous hypertension may develop subsequent to thrombotic syndrome in which deep vein thrombosis (DVT), inflammation, recanalisation and reflux (with or without venous obstruction) lead to increased venous pressure34,35. In the later stages of venous disease, VLUs can occur.
Management of underlying venous disease is essential in preventing progression to ulceration. Traditionally, the focus of preventive strategies has been on lifestyle change and medical management. The most recent evidence indicates there also is a role for management of venous disease with surgery or minimally invasive procedures in order to prevent progression to (or recurrence of) a VLU. To promote early intervention, people with symptomatic disease or who have already experienced a VLU should be evaluated by a specialist for candidacy for surgical/minimal invasive procedure36-39.
Conventional surgical procedures such as vein ligation and stripping, phlebectomy and venous valvular repair are associated with reductions in incidence of VLUs. Some studies show that conventional surgery on superficial veins can reduce recurrence of a VLU by 25% more than conventional preventive strategies40,41. The ESCHAR trial42,43, conducted with 500 individuals with superficial venous disease and either healed or active VLUs, compared venous stripping with gold standard medical management (compression therapy). Recurrence rates for venous ulcers were significantly lower for individuals who underwent the surgical procedure (24% versus 52%, p=0.044)42-44. Varicose vein surgery has increasingly been used to manage venous disease and reduce progression to VLU since the ESCHAT trial results.
Minimally invasive surgical procedures, specifically endovenous ablation and sclerotherapy, have been gaining popularity in the past 20 years. Endovenous ablation is a process in which a laser or radioactive fibre is applied to the vein to produce vein collapse, sclerosis and cauterisation. A Cochrane review that included 13 randomised controlled trials (RCTs)45 comparing endovenous ablation to conventional surgery reported no significant difference in VLU recurrence between endovenous ablation and open surgery (odds ratio [OR] 0.72, 95% confidence interval [CI] 0.43 to 1.22; p=0.22)45. In the sclerotherapy procedure a chemical substance (detergent, alcohol agents or osmotic agent) is applied inside the vein to destroy the endothelium and occlude the vein with clotting46. In a Cochrane review45 that pooled results of three RCTs, effect on VLU incidence was not reported; however, there was no significant difference between sclerotherapy and conventional surgery for recurrence of varicose veins (OR 1.74, 95% CI 0.97 to 3.12, p=0.06).
Selection of the most appropriate surgical/minimally invasive procedure is based on individual risks and benefits. Minimally invasive procedures can be conducted under local anaesthetic and may have lower risks47,48, especially for older people and those with co-morbidities.
Although surgery and minimally invasive procedures are commonly used to prevent VLUs, the gold standard for VLU prevention is compression therapy. In combination with preventive lifestyle strategies, compression therapy (usually compression stockings) is highly recommended for people who have experienced a VLU and should be considered by all people who have symptomatic venous disease. Compression therapy, which aims to promote venous return, reduce venous pressure and prevent stasis, is shown to significantly reduce the risk of VLUs, compared to no compression (RR 0.46, 95% CI 0.27 to 0.76. p=0.003)49. Studies on the level of compression therapy necessary for reducing VLU risk have mixed results. While some RCTs show no difference between higher and more moderate strengths of compression50,51, others suggest that the risk of a VLU decreases with higher strength compression therapy. Relative risks (RR) are reported from RR 0.57 (95% CI 0.39 to 0.81, p=0.002) to 0.82 (95% CI 0.61 to 1.12, p=0.02)49. In general, selection of the type and strength of compression therapy is individualised according to what can be tolerated.
Preventive lifestyle interventions are essential for all people at risk of a VLU, in conjunction with gold standard compression therapy and/or surgical interventions. The goal of lifestyle change is to reduce avoidable risk factors for VLU. Strategies include reducing excess body weight, avoiding prolonged standing and heavy lifting, elevating the legs regularly52,53 and engaging in exercise that promotes the calf muscle pump function52. There is limited evidence on the effectiveness of lifestyle change. One recent analysis reported a significant reduction in VLUs associated with elevating the legs for at least 30 minutes each day (hazard ratio [HR] 0.33, 95% CI 0.19 to 0.56, p<0.001 compared to no elevation) and with walking for at least 3 hours a day (HR 0.66, 95% CI 0.45 to 0.98, p=0.04 compared to no walking)52. Regular skin moisturising is recommended54, although there is no strong evidence on efficacy.
Prevention of diabetic-related foot ulcers
Foot ulceration is a common lower extremity complication, which may arise due to the presence of diabetes mellitus or other medical conditions that place an individual at increased risk. The prevention of DRFU is challenging because of the multifactorial aetiology involving both intrinsic and extrinsic factors. Once an ulcer has healed, the risk of recurrence is between 30% and 40% within the first year55. However, many DRFUs remain in a prolonged and stagnant inflammatory stage of healing and have increased risk of infection and amputation56.
Early diagnosis of underlying pathophysiology that increase risk is the first step in preventing or reducing the adverse effects of foot problems in diabetes and ensuring healthy maintenance of the lower limb57. The key risk factors that interact and subsequently result in ulceration are a combination of neuropathy, with or without peripheral vascular disease and foot deformity, with or without trauma58. Approximately 20% of individuals with a DRFU will primarily have inadequate arterial blood flow, 50% will primarily have neuropathy and 80% will have both conditions59.
Best practice management to prevent DFRU includes:
- regular monitoring of neurovascular status;
- regular monitoring of skin integrity;
- regular debridement of hyperkeratotic lesions and corns; and
- offloading of elevated pressure areas due to biomechanical abnormalities55.
Neuropathy is a pivotal risk factor for both ulceration and amputation caused by the loss of protective sensation (LOPS) due to peripheral sensory neuropathy. The 10 g Semmes-Weinstein monofilament (SWF) and vibratory perception threshold (VPT) examinations are considered simple and effective screening instruments to identify individuals at risk of ulceration. Although both these tools are claimed to identify risk of ulceration, there is no consensus in the literature about the best method to identify LOPS. Comparative studies have generally found differences in SWF having a high level of specificity but a low sensitivity than a VPT ≥ 25 V and that prevalence of peripheral neuropathy was two times more frequent using VPT ≥ 25 V as diagnostic criteria than SWF60. Furthermore, studies suggest that the biothesiometer identifies far more individuals with impaired peripheral sensation than the SWF, and that the VPT measurement may identify people at an earlier stage of impairment than SWF testing61. From a practical perspective, the SWF is very simple, inexpensive and is quicker and easier to apply compared with the VPT. Nevertheless, the IWGDF recommends using either of these tests for screening individuals62.
In people with diabetes, the risk of developing peripheral arterial disease (PAD) is fourfold in comparison to the general population. In diabetes, PAD tends to affect the distal arteries, which increases the risk of ulceration, infection and amputation. Detection and appropriate management of PAD is important to ensure effective wound healing in optimised. Even asymptomatic individuals with mild PAD have the same risk as individuals with symptoms63. Assessment of physical signs that may indicate vascular disease include colour of the limb, skin and nail changes, limb temperature and palpation of pedal pulses.
The palpation of pedal pulses alone is not considered reliable in people with diabetes63. To obtain a more quantitative evaluation of arterial status, the ankle brachial pressure index (ABPI), the toe brachial pressure index (TBPI) and continuous wave Doppler (CWD) ultrasound are frequently used to diagnose and estimate disease severity64. Although widely used, the ABPI has significant limitations in the presence of medial arterial calcification (MAC), a common condition associated with diabetes that results in a falsely elevated ABPI of >1.3 (n.b. an ABPI >0.9 is considered normal and an ABPI <0.8 is associated with claudication)57. A recent study demonstrated that the specificity of the ABPI was high (92.6%) in participants with and without diabetes, but the sensitivity was poor in individuals with (45%) and without diabetes (47%)64.
Based on the assumption that the arteries in the toe are less susceptible to MAC, it is advocated that a TBPI is a better indicator of PAD for individuals with diabetes. The sensitivity of the TBPI for detecting PAD was lower in people with diabetes (63%) than those without diabetes (83%), and the specificity was higher in both those with and without diabetes64. However, a study investigating the reliability of the TBPI found that intra-rater reliability was 0.75 (95% limits of agreement (LOA) –0.22 to 0.28) and inter-rater reliability 0.77 (95% LOA –22.91 to 29.17), indicating that the reliability is questionable65.
However, the most sensitive test for people with or without diabetes was CWD because this test was more likely to detect significant PAD when comparing to both TBI and ABPI assessments. CWD is a low-cost screening tool and is quick and easy to use. However, the interpretation of the waveform can be subjective and is based on the knowledge and understanding of the operator64. Automated systems that incorporate the ABPI, TBPI and pulse waveforms offer a simpler method for the calculation; however, further research is required to determine the accuracy of these systems.
Most DRFU occur at areas of increased pressure. Approximately 90% of plantar wounds are directly attributed to pressure, and non-plantar foot ulcers are caused by both pressure and shear forces, generally associated with ill-fitting footwear. Therefore, pressure offloading is essential in the prevention and management of DRFU66. Increases in pressure and shear forces are related to foot structure, limited joint mobility and biomechanical abnormalities. The elevated level of mechanical pressure, in combination with LOPS, contributes to the development of callous and eventual tissue damage. If the pressure on the anatomical site is not effectively offloaded, and the callous not debrided, resulting tissue damage will lead to ulceration67. Such a course is often a precursor to lower extremity amputation67. A comparison of offloading-customised orthotics devices with traditional podiatric treatment consisting of paring of plantar hyperkeratotic skin, moisturising and padding reported that rigid orthotic devices were associated with a greater reduction in callous grade (p<0.02)68.
Preventive interventions to offload high-pressure areas include felt padding adhered to the foot, padded insoles, customised orthotic devices, therapeutic footwear and shoe modifications. A systematic review on the effectiveness of offloading interventions specifically for primary ulcer prevention in people with diabetes found limited research on the topic69. Several studies have demonstrated that prescribed therapeutic footwear have greater positive effect over standard footwear in pressure reduction, although one RCT showed no effect69. Another study found therapeutic shoes plus customised insoles might be useful in reducing plantar pressures when used for more than six months but increased after 12 months, which may suggest replacement of the insoles and footwear is required after 12-month wear. Overall, there is weak evidence to support the use of therapeutic footwear, although recent studies on pressure mapping and therapeutic footwear are showing some promising results70. Irrespective of the lack of strong evidence, offloading the area of high pressure has been the mainstay to heal DRFUs and prevent recurrence of foot ulceration. The IWGDF recommends that when a foot deformity or pre-ulcerative sign is present, prescription of therapeutic footwear, custom-made insoles or orthotic devices should be prescribed67.
In cases of severe foot deformity together with a history of chronic wounds, conservative pressure offloading may not suffice, and surgical procedures such as Achilles tendon lengthening, tenotomise and arthroplasties may improve healing and reduce the risk of recurrence66. However, the current evidence based on surgical interventions for preventing DRFUs is also weak.
Prevention of pressure injuries
PIs essentially occur due to sustained pressure load on the skin and tissues. Pressure load causes a deformation of the skin and tissues and a resulting reduction in oxygen and nutrient supplied to the tissues, leading to ischaemia (a PI) at the point of pressure loading30. Other extrinsic factors such as shear and moisture increase the risk of a PI developing, as do intrinsic factors related to the individual’s background and health status71.
Best practice interventions for preventing PIs focus on interventions that reduce the risk associated with sustained pressure, shear and moisture. Promoting mobility and/or regularly repositioning people who are immobile reduces sustained interface pressure at a specific anatomical point (usually bony prominences). While ethical considerations mean there are few studies comparing repositioning to not repositioning individuals, a seminal RCT established that turning an individual at least every four hours was associated with reduction in the risk of Stage II or greater PIs (OR 0.12; 95% CI 0.03 to 0.48)72. The most recent research has focused on the most effective positions and frequencies of repositioning; however, there are insufficient high-quality studies in this field to definitively recommend specific repositioning regimens for preventing PIs73. Current international consensus supports individualising the frequency of repositioning based on assessments of tissue tolerance, skin condition, comfort and the person’s overall health status and level of mobility30.
Implemented in conjunction with repositioning, selection of an appropriate pressure redistribution support surface (mattress or seat) can dramatically reduce the risk of a PI. A Cochrane review pooled the findings from five RCTs on support mattresses for PIs in people who were assessed as having a high risk. The meta-analysis showed significant risk reduction associated with constant low-pressure, high-specification foam mattresses compared with standard hospital foam mattresses (RR 0.40, 95% CI 0.21 to 0.74, p=0.004)74. For people who have a very high risk of experiencing a PI, an alternating pressure surface (for example, low air loss) could be used. Alternating support surfaces work through inflation and deflation cycles of the air-filled cells that comprise the mattress, overlay or cushion. There is limited high-quality evidence efficacy of alternating support surfaces30,74; pooled of findings from nine moderate and lower quality RCTs showed no significant difference in PI incidence between alternating support surfaces and constant low-pressure surfaces (RR 0.85, 95% CI 0.64 to 1.13, p=not significant)74. An alternating support surface might be most appropriate when a person who has a high risk of PIs is unable to be repositioned regularly30.
Repositioning and a pressure redistribution support surface are just as important to address for people who are seated out of bed, including people who use a wheelchair. A wide range of pressure redistribution seating cushions (air, fluid, gel and foam designs) are available, and although individual RCTs report effectiveness of most seating cushions in reducing PI risk, the evidence on which might be the best type of cushion to prevent PIs use is inconclusive74. Using any high-specification pressure redistribution cushion, in conjunction with redistributing the person’s weight regularly is considered best practice in preventing development of a PI when a person is seated. Limiting the time spent sitting out of bed or, for individuals with sufficient upper body strength, teaching the individual to perform pressure relief manoeuvres are strategies that are supported by limited evidence75 and international consensus30,76.
Emerging best practice now includes application of preventive dressings for individuals at higher risk of developing a PI. A multilayered polyurethane foam dressing has been shown to reduce pressure, shear and friction77-80, thereby decreasing the risk of a PI developing. Pooling of eight trials that compared a polyurethane foam dressing to no dressing showed a significant reduction in PIs (RR 0.17, 95% CI 0.12 to 0.26) can be achieved by applying a preventive dressing to bony prominences (particularly heels and the sacrum).
Maintaining skin integrity is recommended as best practice for PI prevention. Skin moisture is a recognised PI risk factor71; keeping the skin clean and dry, which includes continence management, can protect the skin30. General principles of promoting healthy skin, including washing the skin with a pH-neutral cleanser and promoting skin hydration by regularly moisturising are also recommended for preventing PIs81,82.
Prevention of skin tears
To date, strategies to prevent STs are largely experiential and based on clinical expertise and collective experiences rather than structured clinical research. The primary focus of these strategies has been on identifying and utilising measures to avoid the risk of these injuries. This omission is not surprising, given the ethical difficulties of older individuals participating in clinical research in terms of obtaining informed consent and the need to minimise the risk of harm or discomfort to participants83-85.
ST prevention strategies include application of moisturisers, limb protectors, adequate nutrition and hydration, provision of safe environments, use of safe equipment, individual education, and appropriate training of health providers in the provision of care86-88. Five significant primary articles that used structured clinical research to evaluate ST prevention strategies present the best evidence on their prevention11,89-91. The focus of all these studies was aimed at maintaining skin integrity.
A four-month quasi-experiment study of 43 aged care residents by Mason (1997) evaluated the effectiveness of emollient antibacterial soap compared to non-emollient antibacterial soap to improve skin quality and reduce STs92. The incidence of STs was reported to be 34.8% lower in residents using emollient soap compared to non-emollient soap.
Groom et al. conducted a skin care intervention study to evaluate a nutrient-based skin care product, compared to products without nutrients with the aim to measure the number of ST-free days90. Individuals receiving a daily nutrient-based product had more ST-free days, compared to people receiving non-nutrient-based products (179.7 versus 154.6 days).
A six-month cluster-RCT of 984 resident conducted by Carville et al.93 also evaluated the effectiveness of twice-daily moisturising to the extremities compared to ‘usual’ skin care for reducing ST incidence in older adults. In this study, application of moisturiser twice-daily was associated with a nearly 50% reduction in the incidence of STs, compared with ad-hoc or no standardised skin-moisturising regimen (5.76 versus 10.57 per 1000 occupied bed days)93.
Edwards et al. undertook a six-month pre–post study to evaluate the effectiveness of a multifaceted Champions for Skin Integrity (CSI) model to transfer evidence into practice for wound management in residential aged care91. The model involved using local champions, education and skills development workshops, creation of multidisciplinary networks, audit and feedback cycles, development of a comprehensive educational resource kit and awareness raising activities. Following the implementation of the CSI model and using the STAR Skin Tear Classification System, a significant reduction in Category 3 STs was reported (11% pre-implementation versus 4.5% post-implementation, p=0.02)6,91. However, there was no significant reduction in the prevalence of Category 1 and Category 2 STs.
Powell et al. conducted a pilot study of 90 participants at risk of STs of the lower extremities from a United Kingdom county care homes and primary care service89. Participants were randomised over a 16-week period to receive either knee-length protective socks (n=44) or usual care (n=46). Of the 79 participants (88%) who completed the trial 61% (n=27) were in the intervention arm of the study. In total, 18.2% (n=8) of the participants who used protective socks sustained an ST, compared to 21.4% (n=10) who received usual care. Participants in the usual care group were reported to have sustained more STs, repeated STs, and more severe injuries89.
DISCUSSION
The evidence presented in the previous section outlines some of the more significant interventions for preventing a variety of wound types. The available research is generally of low quality, and many well-recognised interventions continue to be underpinned by a limited formal evidence base. However, many of the interventions discussed above, including offloading pressure and maintaining skin integrity, are fundamental nursing skills and intrinsic to the philosophy of wound prevention20.
Maintaining skin integrity, through appropriate cleansing, drying, moisturising and protection, is a universal preventive strategy. The importance of maintaining the skin in a healthy, well-hydrated and well-nourished state is a key strategy for preventing VLUS, PIs, DRFUs and STs. The process of providing daily care also provides the opportunity to engage in regular skin inspection, whether it be to identify erythema in an individual on bed rest, dry skin on an older adult at risk of STs or calloused feet in a person with diabetes.
Other basic principles of health care provision are relevant in preventing all types of wounds. Promoting patient autonomy through education is an important component of preventing wounds. Teaching individuals at risk of a wound strategies to assess their own skin and ways in which they can adopt preventive skills into their everyday life is important, particularly for people who will continue to be at risk of wounds throughout disease or age progression. When people understand the purpose of interventions, their self-implementation of wound-preventive strategies may increase. This is of particular importance when interventions are considered uncomfortable (for example, compression stockings), unattractive (for example, orthotic shoes) or expensive (for example, a high-quality support surface).
CONCLUSION
This literature review has outlined identification of individuals at risk of a wound as the first stage in wound prevention. Regular and comprehensively conducted relevant risk assessments inform the development of a wound prevention plan. The current body of evidence for preventing VLUs, PIs, DRFUs and STs is generally quite limited. There is a need for more structured research to better understand measures to prevent wounds of all types, as well as investigation into the relative effectiveness of these measures. A combination of structured clinical research, experiential knowledge and clinical judgement is needed to identify and integrate best evidence to better guide clinical decision making in wound care. Health professionals who uphold the core wound care goal of wound prevention embrace the wisdom of Thomas Fuller who stated that “[h]e who cures a disease may be the skillfullest, but he that prevents it is the safest physician”94.
Author(s)
Emily Haesler*
PhD, PGradDipAdvNurs (Gerontics), BN
Adjunct Associate Professor, Western Australia Centre for Evidence Informed Healthcare Practice, Wound Healing and Management Node, Curtin University, WA, Australia
Honorary Senior Lecturer, The Australian National University, ANU Medical School, ACT, Australia
Honorary Associate, Australian Centre for Evidence Based Aged Care, School of Nursing and Midwifery La Trobe University, Bundoora, Vic, Australia
Email Emily.Haesler@anu.edu.au
Nicoletta Frescos
BApplSci(Podiatry), MPH
Discipline of Podiatry, School of Allied Health,
La Trobe University, Bundoora, Vic, Australia
Robyn Rayner
BSc (Nursing), Post Grad (Health Admin),
Master Wound Care, PhD, RN
* Corresponding author
References
- Attributed to Benjamin Franklin. Letters section, Pennsylvania Gazette, 4 February 1735.
- Graves N, Zheng H. The prevalence and incidence of chronic wounds: a literature review. Wound Practice & Research 2014;22(1):4–19.
- Everett S, Powell T. Skin tears — the underestimated wound. Primary Intention 1994;2:28–31.
- Malone ML, Rozario N, Gavinski M, Goodwin J. The epidemiology of skin tears in the institutionalized elderly. J Am Ger Soc 1991;39(6):591–595.
- White MW, Karam S, Cowell B. Skin tears in frail elders: A practical approach to prevention. Ger Nurs 1994;15:95–9.
- Carville K, Lewin G, Newall N et al. STAR: A consensus for skin tear classification. Primary Intention 2007;15:18–28.
- Ratliff C, Fletcher KR. Skin tears: A review of the evidence to support prevention and treatment. Ostomy Wound Manage 2007;53:32–42.
- LeBlanc KA, Christensen D, Cook J, Culhane B, Gutierrez O. Prevalence of skin tears in a long-term care facility. J Wound Ostomy Continence Nurs 2013;40(6):1–5.
- Sanada H, Nakagami G, Koyano Y, Iizaka S, Sugama J. Incidence of skin tears in the extremities among elderly patients at a long-term medical facility in Japan: A prospective cohort study. Geriatr Gerontol Int 2015;15(8):1058–1063.
- Koyano Y, Nakagami G, Iizaka S, Sugama J, Sanada H. Skin property can predict the development of skin tears among elderly patients: A prospective cohort study. Int Wound J 2017;14(4):691–697.
- Carville K, Leslie G, Osseiran-Moisson R, Newall N, Lewin G. The effectiveness of a twice-daily skin-moisturising regimen for reducing the incidence of skin tears. Int Wound J 2014;11(4):446–453.
- Australian Bureau of Statistics. Cat No. 4430.0 — Disability, ageing and carers, Australia: Summary of findings. 2015.
- Australian Bureau of Statistics. Cat No. 3222.0 — Population projections, Australia: 2012 (base) to 2101. 2013.
- Degenholtz H, Rosen J, Castle N, Mittal V, Liu D. The association between changes in health status and nursing home resident quality of life. Gerontologist 2008;48(5):584–584.
- Galhardo VAC, Magalhaes MG, Blanes L, Juliano Y, Ferreira LM. Health-related quality of life and depression in older patients with pressure ulcers. Wounds 2010;22(1):20–26.
- Briggs M, Collinson M, Wilson L et al. The prevalence of pain at pressure areas and pressure ulcers in hospitalised patients. BMC Nurs 2013;12(1).
- Green J, Jester R, McKinley R, Pooler A. The impact of chronic venous leg ulcers: A systematic review. J Wound Care 2014;23(12):601–12.
- Upton D, Solowiej K. Pain and stress as contributors to delayed wound healing. Wound Practice & Research 2010;18(3):114–22.
- Järbrink K, Ni G, Sönnergren H et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Systematic Reviews, 2017; 6: 15.
- Wounds Australia, Standards for Wound Prevention and Management. 3rd edition. Osborne Park, WA: Cambridge Media, 2016.
- DeVon HA, Block ME, Moyle-Wright P et al. A psychometric toolbox for testing validity and reliability. J Nurs Scholarsh 2007;39(2):155–64.
- Eklöf B, Rutherford R, Bergan J et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40(6):1248–52.
- Anonymous. Classification, severity scoring systems and terminology of chronic venous disorders. Int Angiol 2014;33(2):104–110.
- Writing Committee, Wittens C, Davies AH, Baekgaard N et al., Guidelines Committee. Editor’s choice — Management of chronic venous disease: Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2015;49(6):678–737.
- Jeon BJ, Choi HJ, Kang JS, Tak MS, Park ES. Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int Wound J 2017;14(3):537–545.
- Peters EJG, Lavery LA. Effectiveness of the Diabetic Foot Risk Classification System of the International Working Group on the Diabetic Foot. Diabetes Care 2001;24(8).
- Moore Z, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev 2014; Issue 2. Art. No.: CD006471. DOI: 10.1002/14651858.CD006471.pub3.
- Reddy M, Gill SS. Systematic review: The effectiveness of pressure ulcer risk assessment instruments and associated intervention protocols remains uncertain. Evid Based Med 2014;19(3):93.
- García-Fernández F, Pancorbo-Hidalgo P, Soldevilla J. Predictive capacity of risk assessment scales and clinical judgment for pressure ulcers. J Wound Ostomy Continence Nurs 2014;41(1):1–11.
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance, Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. E Haesler (Ed). Osborne Park, Western Australia: Cambridge Media, 2014.
- Serra R, Ielapi N, Barbetta A, de Franciscis S. Skin tears and risk factors assessment: a systematic review on evidence-based medicine. Int Wound J 2018;15(1):38–42.
- Linton RR. The post-thrombotic ulceration of the lower extremity: its etiology and surgical treatment. Ann Surg 1953;138:415–33.
- Alexander CJ. The theoretical basis of varicose vein formation. Med J Aust 1972;1:258–61.
- Johnson BF, Manzo RA, Bergelin RO, Strandness JDE. Relationship between changes in the deep venous system and the development of the post-thrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg 1995;21:307–12.
- Johnson BF, Manzo RA, Bergelin RO, Strandness JDE. The site of residual abnormalities in the leg veins in long-term follow-up after deep vein thrombosis and their relationship to the development of the post-thrombotic syndrome. Int Angiol 1996;15:14–9.
- Pannier F, Rabe E. Results from RCTs in Sclerotherapy: European Guidelines for Sclerotherapy in Chronic Venous Disorders. Phlebology 2014;29(S1):39–44.
- Rabe E, Breu FX, Cavezzi A et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology 2014;29(6):338–354.
- Rabe E, Pannier F. Indications, contraindications and performance: European Guidelines for Sclerotherapy in Chronic Venous Disorders. Phlebology 2014;29(S1):26–33.
- O’Donnell TF, Passman MA, Marston WA et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg 2014;60:3S–59S.
- Malas MB, Qazi U, Lazarus G et al. Comparative effectiveness of surgical interventions aimed at treating underlying venous pathology in patients with chronic venous ulcer. J Vasc Surg 2014;2(2):212–225.
- Mauck KF, Asi N, Undavalli C et al. Systematic review and meta-analysis of surgical interventions versus conservative therapy for venous ulcers. J Vasc Surg 2014;60(2 Suppl):60s–70s.e2.
- Gohel MS, Barwell JR, Taylor M et al. Long-term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ 2007;335:83.
- Barwell JR, Davies CE, Deacon J et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet 2004;363:1854–9.
- Gohel MS, Barwell JR, Taylor M et al. Long-term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): Randomised controlled trial. BMJ 2007;335:83.
- Nesbitt C, Bedenis R, Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus conventional surgery for great saphenous vein varices. Cochrane Database Syst Rev 2014;7.
- Geroulakos G. Foam sclerotherapy for the management of varicose veins: a critical reappraisal. Phlebolymphology 2006;13(4):202–6.
- Siribumrungwong B, Noorit P, Wilasrusmee C, Attia J, Thakkinstian A. A systematic review and meta-analysis of randomised controlled trials comparing endovenous ablation and surgical intervention in patients with varicose vein. Eur J Vasc Endovasc Surg 2012;44:214–223.
- Rueda CA, Bittenbinder EN, Buckley CJ, Bohannon WT, Atkins MD, Bush RL. The management of chronic venous insufficiency with ulceration: The role of minimally invasive perforator interruption. Ann Vasc Surg 2013;27(1):89–95.
- Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2012;8.
- Clarke-Moloney M, Keane N, O’Connor V et al. Randomised controlled trial comparing European standard class 1 to class 2 compression stockings for ulcer recurrence and patient compliance. Int Wound J 2014;11(4):404–408.
- Kapp S, Miller C, Donohue L. The clinical effectiveness of two compression stocking treatments on venous leg ulcer recurrence: A randomized controlled trial. Int J Low Extrem Wounds 2013;12(3):189–198.
- Finlayson K, Wu ML, Edwards HE. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: A longitudinal study. Int J Nurs Stud 2015;52(6):1042–51.
- Finlayson K, Edwards H, Courtney M. Relationships between preventive activities, psychosocial factors and recurrence of venous leg ulcers: A prospective study. J Adv Nurs 2011;67(10):2180–2190.
- Australian Wound Management Association Inc (AWMA), New Zealand Wound Care Society Inc (NZWCS), Australia and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers. Australia: AWMA, 2011.
- Bus SA, Netten JJ, Lavery LA et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev 2016;32:16–24.
- Jeffcoate WJ, Price P, Harding KG. Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev 2004;20(S1):S78–S89.
- Boulton AJ, Armstrong DG, Albert SF et al. Comprehensive foot examination and risk assessment: A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31(8):167–1685.
- Lazzarini PA, Hurn SE, Kuys SS et al. Direct inpatient burden caused by foot-related conditions: a multi-site point-prevalence study. BMJ Open 2016;6:e010811.
- Singh N, Armstrong DG, Lipsky BA, Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217–228.
- Kärvestedt L, Mårtensson E, Grill V et al. The prevalence of peripheral neuropathy in a population-based study of patients with type 2 diabetes in Sweden. J Diabetes Complications 2011;25(2):97–106.
- Richard J-L, Reilhes L, Buvry S, Goletto M, Faillie J-L. Screening patients at risk for diabetic foot ulceration: a comparison between measurement of vibration perception threshold and 10-g monofilament test. Int Wound J 2014;11(2):147–151.
- Apelqvist J, Bakker K, van Houtum WH, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007): prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev 2008;24(S1):S181–7.
- Vowden K, Vowden P. The importance of detecting lower limb ischaemia. Wounds Int 2016;7(4):6–9.
- Tehan PE, Bray A, Chuter VH. Non-invasive vascular assessment in the foot with diabetes: sensitivity and specificity of the ankle brachial index, toe brachial index and continuous wave Doppler for detecting peripheral arterial disease. J Diabetes Complications 2016;30(1):155–160.
- Romanos MT, Raspovic A. PBM. The reliability of toe systolic pressure and the toe brachial index in patients with diabetes. J Foot Ankle Res 2010;3:31.
- Bergin SM, Gurr J, Allard BP et al. Australian Diabetes Foot Network: management of diabetes-related foot ulceration — a clinical update. Med J Aust 2012;197:226–229.
- Bus SA, Armstrong DG, Deursen RW et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab Res Rev 2016;32:25–36.
- Colagiuri S, Marsden LL, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Res Clin Pract 1995;28(1):29–34.
- Heuch L, Streak Gomersall J. Effectiveness of offloading methods in preventing primary diabetic foot ulcers in adults with diabetes: a systematic review. JBI Database System Rev Implement Rep 2016;14(7):236–265.
- Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Vasc Surg 2010;52(3 Suppl):37S–43S.
- Coleman S, Gorecki C, Nelson A et al. Patient risk factors for pressure ulcer development: Systematic review. Int J Nurs Stud 2013;50(7):974–1003.
- Defloor T, De Bacquer D, Grypdonck MHF. The effect of various combinations of turning and pressure reducing devices on the incidence of pressure ulcers. Int J Nurs Stud 2005;42(1):37–46.
- Gillespie BM, Chaboyer WP, McInnes E, Kent B, Whitty JA, Thalib L. Repositioning for pressure ulcer prevention in adults. Cochrane Database Syst Rev 2014;4:CD009958.
- McInnes E, Jammali-Blasi A, Bell-Syer SEM, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2015;9(CD001735).
- Sonenblum SE, Vonk TE, Janssen TW, Sprigle SH. Effects of wheelchair cushions and pressure relief maneuvers on ischial interface pressure and blood flow in people with spinal cord injury. Arch Phys Med Rehabil 2014;95(7):1350–1357.
- Houghton PE, Campbell KE, CPG Panel. Canadian Best Practice Guidelines for the Prevention and Management of Pressure Ulcers in People with Spinal Cord Injury. A resource handbook for clinicians. Ontario Neurotrauma Foundation, 2013. http://www.onf.org.
- Call C, Pedersen J, Bill B, Oberg C, Ferguson-Pell M. Microclimate impact of prophylactic dressings using in vitro body analog method. Wounds, 2013; 25(4): 94–103.
- Call E, Pedersen J, Bill B et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J 2015;12:408–13.
- Huang L, Woo KY, Liu LB, Wen RJ, Hu AL, Shi CG. Dressings for preventing pressure ulcers: A Meta-analysis. Adv Skin Wound Care 2015;28(6):267–273.
- Levy A, Frank MBO, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability 2015;24(1):1–11.
- Shannon RJ, Coombs M, Chakravarthy D. Reducing hospital-acquired pressure ulcers with a silicone-based dermal nourishing emollient-associated skincare regimen. Adv Skin Wound Care 2009;22(10):461–467.
- Bou J, Segovia G, Verdu S, Nolasco B, Rueda L, Perejamo M. The effectiveness of a hyperoxygenated fatty acid compound in preventing pressure ulcers. J Wound Care 2005;14(3):117–21.
- Rosin AJ, van Dijk Y. Subtle ethical dilemmas in geriatric management and clinical research. J Med Ethics 2005;31(6):355–359.
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310(20):2191–2194.
- National Health Medical Research Council. National statement on ethical conduct in human research. Canberra: National Health and Medical Research Council, 2007:1–107.
- Beechey R, Priest L, Peters M, Moloney C. An evidence-based approach to the prevention and initial management of skin tears within the aged community setting: A best practice implementation project. JBI Database System Rev Implement Rep 2015;13(5):421–443.
- LeBlanc KA, Baranoski S, Holloway S, Langemo D, Regan M. A descriptive cross-sectional international study to explore current practices in the assessment, prevention and treatment of skin tears. Int Wound J 2014;11(4):424–430.
- Baranoski S, LeBlanc K, Gloeckner M. Preventing, assessing, and managing skin tears: A clinical review. Am J Nurs 2016;116(11):24–30.
- Powell RJ, Hayward CJ, Snelgrove CL et al. Pilot parallel randomised controlled trial of protective socks against usual care to reduce skin tears in high risk people: ‘STOPCUTS’. Pilot Feasibility Stud 2017;3:43.
- Groom M, Shannon RJ, Chakravarthy D, Fleck CA. An evaluation of costs and effects of a nutrient-based skin care program as a component of prevention of skin tears in an extended convalescent center. J Wound Ostomy Continence Nurs 2010;37(1):46–51.
- Edwards HE, Chang AM, Gibb M et al. Reduced prevalence and severity of wounds following implementation of the Champions for Skin Integrity model to facilitate uptake of evidence-based practice in aged care. J Clin Nurs 2017;26(23–24):4276–4285.
- Mason S. Type of soap and the incidence of skin tears among residents of a long-term care facility. Ostomy Wound Manage 1997;43(8):26–30.
- Carville K, Leslie G, Osseiran-Moisson R, Newall N, Lewin G. The effectiveness of a twice-daily skin-moisturising regimen for reducing the incidence of skin tears. Int Wound J 2014;11(4):446–453.
- Fuller T. Practical Spelling: A Text Book For Use in Commercial Schools. Practical Textbook Company, 1902:34.



