Volume 26 Number 1
How well do perioperative practitioners implement pressure injury prevention guidelines? An observational study
Isabel Wang, Rachel Walker and Brigid M Gillespie
Keywords pressure injury, pressure ulcer, operating room, perioperative, pressure injury prevention.
Abstract
Background: Perioperative pressure injury (PI) remains problematic. Five key pressure injury prevention (PIP) strategies were identified according to literature. However, little is known about current perioperative PIP practice in compliance to international guidelines.
Methods: This study describes perioperative health care practitioners’ implementation of five key PIP strategies in compliance with international PIP guidelines in a tertiary hospital. This was achieved via observation of the implementation of PIP strategies using a structured data collection tool and skin inspection on postoperative day 2.
Results: Seventy-three patients undergoing surgical procedures were recruited. Of these, 36 were identified as at-risk of developing PI based on the Waterlow risk assessment. The number of PIP strategies implemented across the sample ranged from 27 to 49 strategies; and increased according to risk, with high-risk patients receiving more PIP strategies than those patients at moderate-risk, although this finding was statistically non-significant. However, there were significant correlations between the number of PIP strategies used and length of surgery and number of medical devices and/or equipment used, with 4 Stage 1 PIs observed postoperative day 2 on patients’ ears, resulting from oxygen tubing.
Conclusions: These clinical and statistically significant results may inform the development of education regarding improvement of perioperative PIP practice.
What is already known on the topic:
- Perioperative PI remains problematic.
- Intrinsic and extrinsic risk factors lead to the development of perioperative PI, for example, patient’s co-morbidity and positioning aids used.
- Support surfaces and positioning aids are well-researched PIP strategies.
What this manuscript contributes:
- The first prospective observational study of healthcare professionals’ practice in relation to perioperative PIP.
- Five key PIP strategies have been identified based on modifiable risk factors of developing perioperative PI.
- Skin inspection and interprofessional communication are important PIP strategies.
- There is a lack of research on guiding how to use medical devices and/or equipment in relation to perioperative PIP.
Introduction
Pressure injury (PI) is an injury that can result in an occlusion of blood flow, which may ultimately affect the skin, soft tissue, muscle and bone, and lead to the development of localised ischaemia, tissue inflammation, tissue anoxia and necrosis. Although often preventable, a PI is recognised as one of the most costly and complicated conditions1,2. While hospitalised patients with restricted mobility have increased risk of developing a PI, anaesthetised patients undergoing surgery are at even greater risk3, yet little is known about the strategies that are used during anaesthesia and surgery to minimise this group’s risk of developing a PI in the postoperative period.
This article presents the results of an observational study, describing the compliance of current perioperative health care practitioners’ practice in relation to pressure injury prevention (PIP) with evidence-based, international PIP guidelines. A data collection tool for observation was developed based on the modifiable and non-modifiable risk factors identified in the literature, such as support surfaces or warming devices used, and patients’ co-morbidities1-4. The observation of practitioners’ practice was related to the five key PIP strategies identified from the literature, including skin inspection, support surfaces to positioning aids, thermal regulation, medical devices and/or equipment, and interprofessional communication.
Background
PI is defined as an injury on or underneath the skin that can occur in less than one hour under certain constant pressures5-7. Hospital-acquired PI (HAPI) refers to the development of a PI during hospitalisation. Prevalence rates of HAPI among all PI cases in acute health settings vary widely in different regions and countries, as shown in Table 1. HAPI risk in surgical specialties was reported as being high. HAPI incidences vary based on the type of surgery. For instance, 14.3% to 18% of cardiovascular surgery patients8,9, while 31.7% of orthopaedic surgery patients10, and 10% to 37% of head and neck surgery patients11 were at risk of developing a HAPI.
Table 1: International prevalence rates of HAPI (2001–2016)
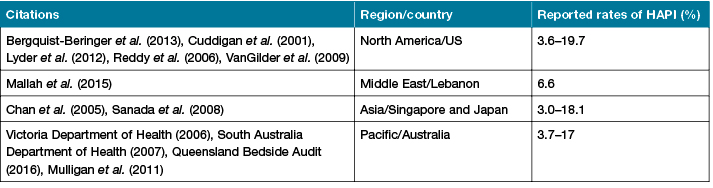
HAPI may have devastating effects on patients and their families, and impose a heavy financial burden on health care services. Adverse outcomes to patients include reduced quality of life, longer hospitalisation, and increased morbidity; these complications can contribute to death12-16. For health care organisations, the estimated opportunity costs of PI on the Australian health care system were A$285 million nationally in 200512, and increased to A$820 million per annum in 2012 to 201317. Treatment costs of PI in hospitals cost A$983 million annually17, the costs associated with PI in both Australian public and private hospitals in 2010–2011 were approximately A$2.11 billion18. The financial burden associated with preventing and treating HAPI for health care organisations has also been recognised globally19-24. For example, in the US, treating HAPI costs up to US$3.6 billion a year (approximately A$4.63 billion)25-27. Yet the extent to which the perioperative setting contributes to the overall incidence of HAPI is still relatively unknown and there is a paucity of evidence about current perioperative practice compliance to the international guidelines in relation to PIP. Therefore, understanding compliance of current perioperative health care practitioners’ practice with the international PIP guidelines prior to reducing the incidence of HAPI is imperative. As such, this study focuses on HAPI to best describe perioperative practice in relation to PIP in a tertiary hospital.
Aim
The overarching aim of this study was to describe the extent to which perioperative health professionals at a large tertiary hospital in Southeast Queensland implement PIP strategies in compliance with international PIP guidelines. Subsumed in this aim, are the following research questions:
- To what extent are PIP strategies implemented by intraoperative health professionals in relation to: skin inspection; support surfaces to positioning aids; thermal regulation; medical devices and/or equipment; and interprofessional communication?
- Is there a relationship between the number of co-morbidities and number of PIP strategies implemented intraoperatively?
- Is there a relationship between adult patients’ temperature on admission to the post-anaesthetic recovery unit (PACU) and the number of thermal regulation PIP strategies implemented intraoperatively?
- Is there a relationship between the length of surgery and total number of PIP strategies implemented intraoperatively?
- Is there a relationship between the supine surgical position and total number of PIP strategies implemented intraoperatively?
- Is there a relationship between the number of medical devices and/or equipment implemented intraoperatively and the total number of PIP strategies implemented intraoperatively?
Methods
In this prospective observational study, a structured observation tool was developed, tested and used to collect patients’ demographic and clinical data, health care professionals’ implementation of PIP strategies, as well as data from a systematic head-to-toe skin inspection on postoperative day 2. Thus, the time period of observation included the preoperative (in induction room), intraoperative (in operating room) and postoperative (the first half hour when the patient is admitted to the post-anaesthesia care unit [PACU]) periods.
Setting and sample
This study was conducted in 2016 at a 750-bed tertiary facility with 22 commissioned operating rooms (ORs). Approximately 16,000 surgeries are performed each year. In this study, surgical procedures were purposively selected across seven specialties. Inclusion and exclusion criteria were applied: Adult patients were included if their length of surgery exceeded 60 minutes, and their anticipated hospital length of stay as an inpatient was a minimum of 48 hours after surgery. Patients were excluded from participation if they were unable to provide informed consent, or speak, read or understand English in the absence of an interpreter. All perioperative practitioners who worked in the OR department were invited to participate.
Observational tool development and testing
A structured data collection tool was developed based on the international PIP guidelines and other literature2,4,8,10,12,22,27-58, and a data dictionary containing conceptual and operational definitions was used in conjunction with the observational tool. The tool was piloted by two trained observers who independently observed four surgical cases, and the interrater agreement of the tool was tested. Using this structured data collection tool, observations started when the patient entered the induction room and ended half an hour after the patient’s admission at the PACU. During each surgical procedure, observers documented explanatory field notes to give further description of case-specific nuances that may have had a bearing on the PIP of the patient; for example, neurology and orthopaedic surgeries. These field notes gave further context to support the structured observations.
Ethics clearance
This study was conducted in accordance with the National Health and Medical Research Council’s National Statement on Ethical Conduct in Human Research (2007); ethics approval for this study was granted by the Gold Coast Hospital and Health Service District (HREC/16/QGC/43), and Griffith University (HREC#2016/583) Human Research Ethics Committees.
Measures and data collection
Observations were conducted from August to December over five continuous months during 2016, taking approximately 300 hours. Perioperative teams were observed from the time the patient arrived in the induction room until half an hour after their admission to the PACU. During each surgical procedure, the research student kept explanatory field notes to support the structured observations of key variables. The categorical variables include surgical position and medical devices and/or equipment, which were dummy-coded as ‘0’ or ‘1’. Continuous variables included the following: the patient’s BMI, the American Society of Anesthesiologists (ASA) scores, the number of patient co-morbidities, Waterlow risk assessment scores, length of surgery, patient’s temperature on admission to the PACU, and the number of PIP strategies used.
On the day of surgery, surgical patients were screened according to the inclusion and exclusion criteria, and recruited using convenience sampling. The patients’ surgical procedure and its approximate length were verified by a registered nurse (RN) in the surgical admission unit (SAU). Consenting patients were then allocated a study number and their demographic data were collected. The consented patients were followed and perioperative PIP practice was observed in an unobtrusive manner. On postoperative day 2, a systematic, head-to-toe skin inspection on the ward was undertaken after reconfirming the patients’ consent verbally, as well as access to their electronic medical charts for data collection. The results of the skin inspection were cross-referenced and reconciled with patients’ earlier data, and categorised according to the six stages of PI, defined by the international guidelines22. The assessment was then verified by the ward RN on the day.
Data analysis
The interrater reliability testing of the data collection tool using Cohen’s kappa coefficient was performed. Data were entered and cleaned prior to analysis using the statistical program, SPSS 24.0 for Windows software (SPSS Inc., Chicago, Illinois). Descriptive statistics were used to describe the characteristics of the patients, surgical specialties, and frequency and type of PIP strategies implemented. Analyses were undertaken according to the level of the data and their distribution, and the results were reported as absolute (n) and relative (%), medians, and interquartile ranges (IQRs) in tables, as appropriate. Spearman’s rho test was used in the inferential analyses (hypothesis testing) to describe the relationships between clinical and/or case factors, and the total number of PIP strategies implemented intraoperatively.
Results
Descriptive results
In total, this study recruited and observed 278 staff, including 149 staff from the nursing team (comprising anaesthetic nurses, instrument nurses, scout nurses and PACU nurses), 109 staff from the medical team (comprising surgeon consultants/registrars and anaesthetist consultants/registrars) and 20 operational staff. The Kappa interrater agreement between two independent observers for the data collection tool was 98% (p < .0005), indicating almost perfect agreement.
Figure 1 illustrates the flow of surgical patients who participated in the study. During the study, the research student approached 75 patients who met the inclusion criteria on the day of surgery for recruitment in the surgical admission unit (SAU) or induction room were approached. Of the 75 patients screened and approached, 74 consented to participate in the study. Of these 74 patients, one patient was excluded from the study because of cancellation of their surgery. The remaining consenting 73 patients continued their participation in the study. Observational data based on the 73 patients were collected in the induction room, OR and PACU. Data relating to skin inspection were collected on day 2 following surgery on wards to capture postoperative PI incidence. Eight post-surgical patients did not have day 2 skin inspection, as they were discharged prior to day 2. However, their demographic and clinical data collected following enrolment in the study were included in the descriptive and correlational analyses. Therefore, the demographic and relationships between variables were analysed based on 73 observed procedures, and the outcome data in relation to PI development were analysed based on 65 observed procedures.
Figure 1: Flow diagram of data collection for PIP study.
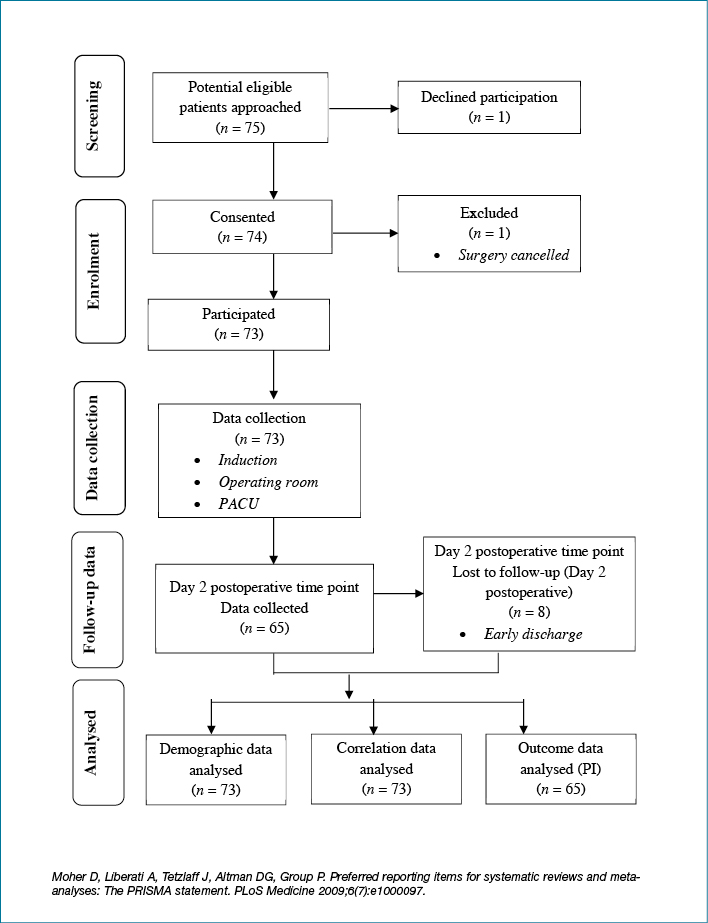
Table 2 presents patients’ demographic and clinical factors. Of the 73 patients included in the final analysis, the median age across the sample was 67 years (IQR: 20.5, range: 23–93 years), comprising 34/73 (46.6%) females, representing just under half of the total sample. The majority of patients (n=68/73, 93.1%) had up to eight co-morbidities. In relation to BMI, 32/73 (43.8%) and 20/73 (27.4%) were classified as being obese or overweight, respectively. Only 36/73 (49.3%) of patients had a Waterlow risk assessment score over 10, indicating they were at risk of developing a PI. Twenty-seven patients (37.0%) had an ASA score of 3, indicating severe systemic disease, while 32 (43.8%) were prescribed three to six medications before induction.
Table 2: Demographic and clinical characteristics of the patient sample (n = 73)
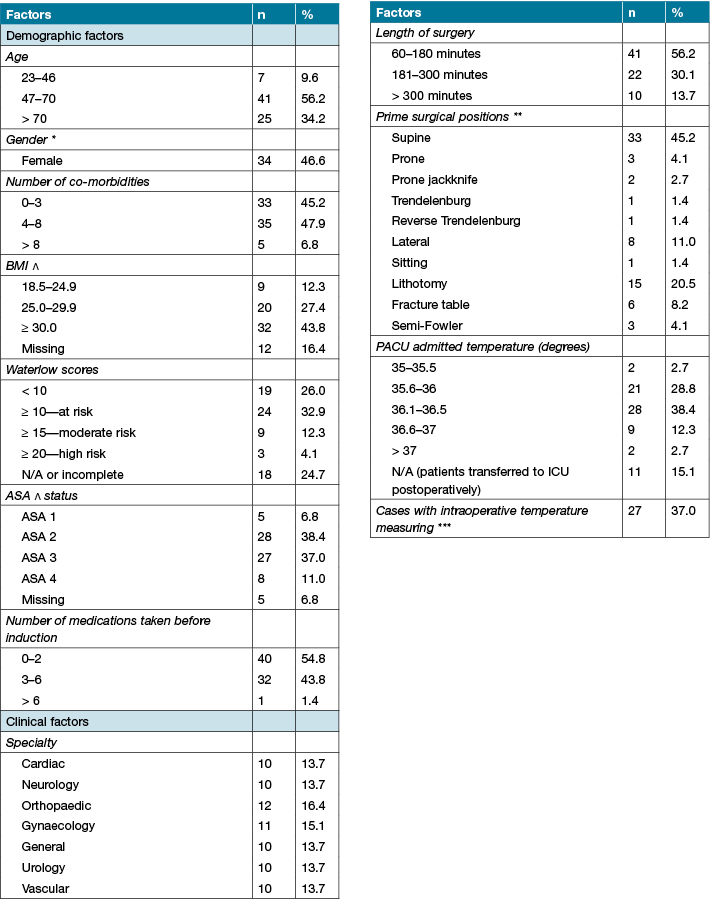
Clinical factors such as type of surgery, length of surgery, surgical position and patients’ temperature on PACU admission were examined in relation to the total number of PIP strategies implemented. Just over half of the surgeries (41/73 surgeries, 56.2%) lasted from one to three hours, while 10 observed procedures (13.7%) lasted longer than five hours. Across the 73 observed procedures, the median length of surgery was 173 minutes (IQR=137.5). The predominant surgical position was supine (n=33, 45.2%), while lithotomy (n=15, 20.5%) was the second most common position used. One-third of consenting patients admitted to the PACU had lower recorded temperatures ranging from 35 to 36 degrees (n=23, 31.5%). Following surgery, 11 patients bypassed the PACU and were transferred straight to the intensive care unit (ICU). Of the 73 observed procedures, patients’ temperature was measured intraoperatively in 27/73 (37%) cases.
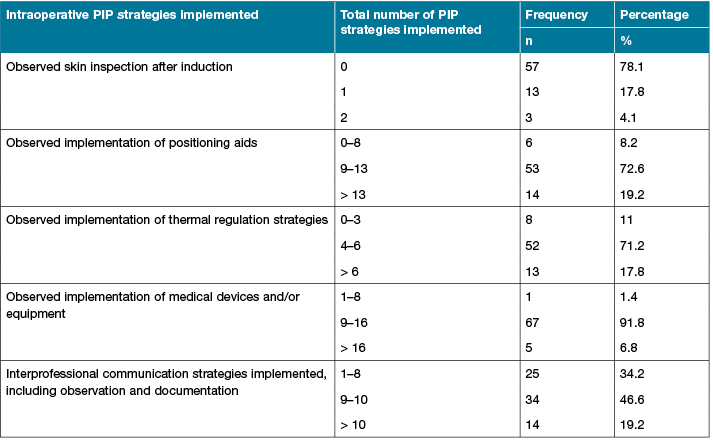
Table 3 reports the number of the five key PIP strategies observed intraoperatively across the 73 observed procedures. Skin inspection after induction was observed as only being performed in 16/73 cases (21.9%). Implementation of nine or more positioning aids intraoperatively to prevent PI was observed in 67/73 (91.8%) cases. Implementation of thermal regulation, medical devices or/and equipment, and interprofessional communication was frequently observed intraoperatively in 65/73 (89%) cases, 72/73 (98.6%) cases and 48/73 (65.8%) cases, respectively.
Table 3: Total number of PIP strategies patients received intraoperatively (n=73)
Relationships analyses
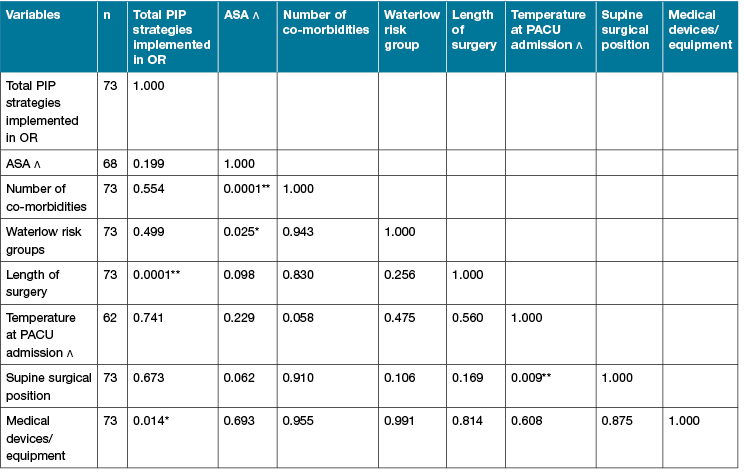
As shown in Table 4, Spearman’s rho test showed a moderate positive correlation (ϒ=0.603, p<0.01) between the length of surgery and total number of PIP strategies implemented intraoperatively across the sample (n=73). Thus, the longer the surgery, the more PIP strategies were implemented in the OR. There was also a positive correlation (ϒ=0.287, p<0.05) between the number of medical devices or/and equipment used intraoperatively and the total number of PIP strategies instigated during surgery. This finding suggests that the more medical devices and/or equipment used intraoperatively, the higher number of intraoperative PIP strategies are implemented. However, there were no significant correlations between the total number of PIP strategies implemented intraoperatively and the number of patients’ co-morbidities, patients’ temperature on admission to the PACU, or supine position during the surgical procedure.
Table 4: Correlation matrix (n=73)
Discussion
In this study, two important results are highlighted. Firstly, the total number of PIP strategies implemented intraoperatively across the sample ranged from 27 to 49. This study is the first to report the frequency of key PIP strategies used intraoperatively per surgery, while the broader literature has not provided such information. Based on the number of approaches used intraoperatively, the perioperative setting is necessarily an area where the use of PIP strategies is high. The sheer number of implemented PIP strategies observed during the intraoperative period particularly reinforces that patients in this setting are most vulnerable to developing a HAPI while they are anaesthetised.
Secondly, there were significant relationships between the total number of PIP strategies implemented intraoperatively, the length of surgery and number of medical devices and/or equipment used during surgery. This finding signifies the unique risk factors of HAPI development in perioperative settings in that both the length of surgery and number of medical devices and/or equipment used can be difficult to modify directly during surgery. This finding is consistent with the findings in the broader literature4,36 that informed the rationale for identifying the key PIP strategies for this study.
Currently, there is very little research examining the effect of medical devices and/or equipment on HAPI development. The European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Ulcer Advisory Panel (NPUAP) and Pan Pacific Pressure Injury Alliance (PPPIA) (2014) strongly recommends at least two skin inspections of the area around medical devices or equipment. The guidelines also recommend not applying medical devices or equipment directly on patients where possible, based on limited empirical evidence (that is to say, studies with healthy humans, studies with humans with other types of chronic wounds, or animal models). However, there is a lack of clarity around implementation of this guideline. While the need for these various types of medical devices and equipment demonstrates the difficulties in providing effective PIP, and highlights the existing challenges in developing guidelines for their use. In the current study, we observed the frequent use of medical devices and/or equipment on patients during surgery.
Strengths and limitations
This study was theoretically grounded and informed by the international clinical practice guidelines and other best available evidence around PIP in the literature. The data collection tool was developed based on the modifiable and non-modifiable risk factors identified in the literature, and pilot tested by two independently trained raters. This is the first prospective observational study in relation to PIP practices used in the perioperative settings, and the first to use a structured observational data collection tool. Other published research in perioperative settings has mainly relied on randomised controlled trials of support surfaces and retrospective chart audits of incidence and risk factors of PI4,46,59. Data collection in this study encompassed the entire patient surgical journey, commencing at the preoperative stage and continuing to day 2 postoperative, which enabled more comprehensive observation and collection of data in relation to practitioners’ PIP practice.
However, this study was conducted at a single hospital site, which may be atypical in relation to other hospitals in the region. The use of convenient sampling methods may give rise to selection bias. There is the possibility of the Hawthorn effect as health professionals may have changed their PIP practice due to awareness of being observed. However, with the prolonged presence of the observer, it is likely that the Hawthorn effect was somewhat mitigated, as perioperative staff become accustomed to the presence of the observer60. Further, observation of some aspects of preoperative PIP care could have been missed because of workflow issues and limited space in the induction room, but these instances were documented as field notes to give more detailed information about these types of situations. The period of 30 minutes' observation during the postoperative period was relatively brief, as the priority of care in the PACU is the patients’ airway, breathing and circulation, and limited PIP practice was observed. Finally, outcome data related to postoperative PI may have been missed, as PI can develop after day 2 postoperatively40; this study had limited time and budgetary resources for additional postoperative skin assessments. A larger multisite study may be able to address some of these limitations.
Conclusion
Based on the identified five key PIP strategies from literature, this first observational study of health care practitioners’ practice in relation to perioperative PIP confirms that: 1) skin inspection and interprofessional communication as important PIP strategies is compromised in practice; 2) there is a lack of research on guiding how to use medical devices and/or equipment in relation to perioperative PIP; and 3) further research on how these five key PIP strategies interact with each other in clinical practice may improve perioperative PIP practice in the future.
Author(s)
Isabel Wang*
BN(Hons), RN
School of Nursing & Midwifery, Griffith University, Gold Coast, Qld, Australia
Email i.wang@griffith.edu.au
Rachel Walker
PhD, RN, BN, BA, MA (Research), Research Fellow
School of Nursing & Midwifery, Griffith University, Gold Coast, Qld, Australia
Princess Alexandra Hospital, Qld, Australia
Brigid M Gillespie
PhD, RN, FACORN
School of Nursing & Midwifery, Griffith University, Gold Coast, Qld, Australia
Gold Coast Hospital and Health Service, Qld, Australia
* Corresponding author
References
- Engels D, Austin M, McNichol L, Fencl J, Gupta S, Kazi H. Pressure ulcers: Factors contributing to their development in the OR. AORN J 2016;103(3):271–81.
- Rao AD, Preston AM, Strauss R, Stamm R, Zalman DC. Risk factors associated with pressure ulcer formation in critically ill cardiac surgery patients: A systematic review. J Wound Ostomy Continence Nurs 2016;43(3):242–7.
- Rego A. Pressure ulcers or moisture lesions: The theatre perspective. J Perioper Pract 2016;26(4):84.
- Yoshimura M, Iizaka S, Kohno M et al. Risk factors associated with intraoperatively acquired pressure ulcers in the park-bench position: a retrospective study. Int Wound J 2016;13(6):1206–13.
- Loorham-Battersby CM, McGuiness W. Heel damage and epidural analgesia: Is there a connection? J Wound Care 2011;20(1):28, 30, 2–4.
- Kosiak M. Etiology of decubitus ulcers. Arch Phys Med Rehabil 1961;40(2):62–9.
- Grous C, Reilly NJ, Gift AG. Skin integrity in patients undergoing prolonged operations. J Wound Ostomy Continence Nurs 1997;24(2):86–91.
- Pokorny ME, Koldjeski D, Swanson M. Skin care intervention for patients having cardiac surgery. Am J Crit Care 2003;12(6):535–44.
- Ginés DG, Palma MR, Pavón FG, Molle RA, Bou JETI. Pressure ulcers in the operating room: Intraoperative incidence in patients undergoing cardiac surgery. Gerokomos 2009;20(4):176–80.
- Shaw LF, Chang PC, Lee JF, Kung HY, Tung TH. Incidence and predicted risk factors of pressure ulcers in surgical patients: experience at a medical center in Taipei, Taiwan. Biomed Res Int 2014;2014:416896.
- Wright KM, Van Netten Y, Dorrington CA, Hoffman GR. Pressure injury can occur in patients undergoing prolonged head and neck surgery. J Oral Maxillofac Surg 2014;72(10):2060–5.
- Australian Wound Management Association [AWMA]. Pan Pacific clinical practice guidelines for prevention and management of pressure injury, 2012.
- Lyder CH, Wang Y, Metersky M et al. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. J Am Geriatr Soc 2012;60(9):1603–8.
- Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs 2007;57(5):494–504.
- Collier M, Potts C, Shaw E. Use of a coverlet system for the management of skin microclimate. Br J Nurs 2014;23(15):S28, S30–5.
- Gorecki C, Brown JM, Nelson EA et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 2009;57(7):1175–83.
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Aust Health Rev 2015;39(3):329–36.
- Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Practice & Research 2014;22(1):20–33.
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age Ageing 2004;33(3):230–5.
- Berlowitz D, Van Deusen L, Parker V et al. Preventing pressure ulcers in hospitals: A toolkit for improving quality of care, 2011.
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care 2012;21(6):261–6.
- National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Ulcer Advisory Panel (EPUAP), Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers: Quick reference guide, 2014.
- Schuurman JP, Schoonhoven L, Defloor T, van Engelshoven I, van Ramshorst B, Buskens E. Economic evaluation of pressure ulcer care: A cost minimization analysis of preventive strategies. Nursing Econ 2009;27(6):390–400.
- Severens JL, Habraken JM, Duivenvoorden S, Frederiks CMA. The Cost of Illness of Pressure Ulcers in the Netherlands. Adv Skin Wound Care 2002;15(2):72–7.
- Centers for Medicare & Medicaid Services Medicare Program. Proposed changes to the hospital inpatient prospective payment systems and fiscal year 2009 rates. Proposed additions to hospital acquired conditions for fiscal year 2009, 2009.
- Primiano M, Friend M, McClure C et al. Pressure ulcer prevalence and risk factors during prolonged surgical procedures. AORN J 2011;94(6):555–66.
- Zaratkiewicz S, Whitney JD, Lowe JR, Taylor S, O’Donnell F, Minton-Foltz P. Development and implementation of a hospital-acquired pressure ulcer incidence tracking system and algorithm. J Healthc Qual 2010;32(6):44–51.
- Yusuf S, Okuwa M, Shigeta Y et al. Microclimate and development of pressure ulcers and superficial skin changes. Int Wound J 2015;12(1):40–6.
- Yoo C, Ayello EA, Robins B et al. Perioperative use of bispectral (BIS) monitor for a pressure ulcer patient with locked-in syndrome (LIS). Int Wound J 2014;11(5):540–5.
- Wu T, Wang ST, Lin PC, Liu CL, Chao YF. Effects of using a high-density foam pad versus a viscoelastic polymer pad on the incidence of pressure ulcer development during spinal surgery. Biol Res Nurs 2011;13(4):419–24.
- Wright KM, Van Netten Y, Dorrington CA, Hoffman GR. Pressure injury can occur in patients undergoing prolonged head and neck surgery. J Oral Maxillofac Surg 2014;72(10):2060–5.
- Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol 2005;63(1):5–18; discussion
- Webster J, Lister C, Corry J, Holland M, Coleman K, Marquart L. Incidence and risk factors for surgically acquired pressure ulcers: a prospective cohort study investigators. J Wound Ostomy Continence Nurs 2015;42(2):138–44.
- Tschannen D, Bates O, Talsma A, Guo Y. Patient-specific and surgical characteristics in the development of pressure ulcers. Am J Crit Care 2012;21(2):116–25.
- Torossian A, Brauer A, Hocker J, Bein B, Wulf H, Horn EP. Preventing inadvertent perioperative hypothermia. Dtsch Arztebl Int 2015;112(10):166–72.
- The European Commission. Medical devices: Guidance document, 2010.
- Sutherland-Fraser S, McInnes E, Maher E, Middleton S. Peri-operative nurses’ knowledge and reported practice of pressure injury risk assessment and prevention: A before-after intervention study. BMC Nurs 2012;11:25.
- Stevens J, Nichelson E, Linehan WM et al. Risk factors for skin breakdown after renal and adrenal surgery. Urology 2004;64(2):246–9.
- Shafipour V, Ramezanpour E, Gorji MA, Moosazadeh M. Prevalence of postoperative pressure ulcer: A systematic review and meta-analysis. Electron Physician 2016;8(11):3170–6.
- Rondinelli JL. Establishing risk for patients with medical device related hospital acquired pressure ulcers in intensive care: A multi-site study (Order No. 3619292). 2014.
- Rich SE, Shardell M, Hawkes WG et al. Pressure-redistributing support surface use and pressure ulcer incidence in elderly hip fracture patients. J Am Geriatr Soc 2011;59(6):1052–9.
- Polit DF, Beck CT. Nursing research: Generating and assessing evidence for nursing practice. 9 ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2012.
- Pittman J, Beeson T, Kitterman J, Lancaster S, Shelly A. Medical device-related hospital-acquired pressure ulcers: Development of an evidence-based position statement. J Wound Ostomy Continence Nurs 2015;42(2):151–4; quiz E1–2.
- Pham B, Teague L, Mahoney J et al. Support surfaces for intraoperative prevention of pressure ulcers in patients undergoing surgery: a cost-effectiveness analysis. Surgery 2011;150(1):122–32.
- Minnesota Hospital Association. Pressure ulcer prevention in the OR — Recommendations and guidance 2013 [Available from: https://www.mnhospitals.org/Portals/0/Documents/ptsafety/skin/OR-pressure-ulcer-recommendations.pdf.
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2015(9):CD001735.
- McInnes E, Jammali-Blasi A, Bell-Syer S, Dumville J, Cullum N. Preventing pressure ulcers — Are pressure-redistributing support surfaces effective? A Cochrane systematic review and meta-analysis. Int J Nurs Stud 2012;49(3):345–59.
- MacDonald JJ, Washington SJ. Positioning the surgical patient. Anaesth Intensive Care Med 2012;13(11):528–32.
- Lumbley JL, Ali SA, Tchokouani LS. Retrospective review of predisposing factors for intraoperative pressure ulcer development. J Clin Anesth 2014;26(5):368–74.
- Kallman U, Bergstrand S, Ek AC, Engstrom M, Lindberg LG, Lindgren M. Different lying positions and their effects on tissue blood flow and skin temperature in older adult patients. J Adv Nurs 2013;69(1):133–44.
- Hooper VD, Chard R, Clifford T et al. ASPAN’s evidence-based clinical practice guideline for the promotion of perioperative normothermia: second edition. J Perianesth Nurs 2010;25(6):346–65.
- Glasgow D, Millen IS, Nzewi OC, Varadarajaran B. Device-related atypical pressure ulcer after cardiac surgery. J Wound Care 2014;23(8):383–4, 6–7.
- Gillespie BM, Chaboyer W, Kang E, Hewitt J, Nieuwenhoven P, Morley N. Postsurgery wound assessment and management practices: a chart audit. J Clin Nurs 2014;23(21–22):3250–61.
- Gillespie BM, Chaboyer W, Fairweather N. Interruptions and miscommunications in surgery: an observational study. AORN J 2012;95(5):576–90.
- European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Ulcer Advisory Panel (NPUAP). Prevention and treatment of pressure ulcers: Quick reference guide. Washington DC: National Pressure Ulcer Advisory Panel, 2009.
- Beeckman D, Defloor T, Schoonhoven L, Vanderwee K. Knowledge and attitudes of nurses on pressure ulcer prevention: A cross-sectional multicenter study in Belgian hospitals. Worldviews Evidence Based Nursing 2011;8(3):166–76.
- Baumgarten M, Margolis D, Orwig D et al. Use of pressure-redistributing support surfaces among elderly hip fracture patients across the continuum of care: adherence to pressure ulcer prevention guidelines. Gerontologist 2010;50(2):253–62.
- Aronovitch SA. Intraoperatively acquired pressure ulcers: Are there common risk factors? Ostomy Wound Manage 2007;53(2):57–69.
- Sewchuk D, Padula C, Osborne E. Prevention and early detection of pressure ulcers in patients undergoing cardiac surgery. AORN J 2006;84(1):75–96.
- Gillespie BM, Harbeck E, Kang E, Steel C, Fairweather N, Chaboyer W. Correlates of non-technical skills in surgery: a prospective study. BMJ Open 2017;7(1):e014480.



