Volume 26 Number 2
Evaluating the effectiveness of silicone multilayer foam dressing in preventing heel pressure injury among critically ill patients in Singapore
Teo Kai Yunn, Ang SY, Bian Luping, Cheah Engfeng Sharon, Somera Melanie Aquino, Ahmad Nurul Huda, Lim Shu Hui, Goh Hui Qi Ivy and Aloweni Fazila Abu Bakar
Keywords Prophylactic dressing, heel, pressure injury prevention, quasi-experimental.
Abstract
Background: The heel has become the second most common site for the development of pressure ulcers in recent years. Hospitals worldwide have been implementing preventive measures to tackle pressure ulcers in patients, such as the use of offloading devices and dressing.
Aim: To evaluate the effectiveness of a soft silicone multilayer heel dressing (Mepilex® Heel) in reducing the incidence of heel pressure injuries (HPI) among critical ill patients in the intensive care unit (ICU).
Method: This was a quasi-experimental, pre-, and post-intervention study design conducted in three adult ICUs in an academic teaching hospital. A convenience sampling was used to recruit 326 patients (195 patients in pre-intervention, 131 patients in intervention group).
Result: Statistical analysis was made using Fisher exact test to compare between the two groups. The results showed a reduction of 86% in the incidence of HPI between the two groups (pre-intervention: 10.8% versus post-intervention: 1.5%). Patients in the intervention group were less likely to develop HPIs (p=<0.007).
Conclusion: A prophylactic multilayer foam heel dressing has shown to be effective at reducing the incidence of HPIs among critically ill patients in ICU, even with the tropical climate in Singapore.
Introduction
Preventing hospital-acquired pressure injuries (PIs) remains a top priority of hospitals worldwide1. The incidence of PI is a quality-of-care indicator and a nursing-sensitive outcome2. Patients who develop PIs experience added morbidity, pain, infection, loss of function, extended hospitalisation stay, and increased health care expenditure cost3. The cost of treating PIs varies, ranging from US$20,000 to US$70,000 per wound4-6. Specifically, heel pressure injury (HPI) is a physically debilitating and painful condition that can lead to serious complications such as infection, cellulitis, osteomyelitis, septicaemia, limb amputation, and even death7.
Many factors contribute to the development of PIs, but the most common pathway is tissue ischaemia8. Studies have shown that tissues are only capable of sustaining pressure of around 30–32 mmHg for only a short duration of time, but when there is direct, sustained or moderate, repetitive pressure and shearing forces, it will lead to occlusion of the capillary vasculature and eventual tissue ischaemia9,10.
The heel is one of the common areas for PIs due to factors such as its calcaneus pointed shape and the bony prominence with limited fats11. Also, the muscle tissues entailing the heel are poorly equipped to absorb the compressive forces of pressure or shearing that are generated during limb movement or transfers11. Additionally, the skin around the heel is often dry due to the lack of sebaceous glands12,13.
A 10-year retrospective review (1990–2000) of PIs and a more recently published data on PIs have reported that HPI has risen from the sixth to the second most common site of PI14,15. The reported incidence of HPI is estimated to be as high as 60% in the acute care setting13. Specifically, critically ill patients, elderly persons and surgical patients are at greater risk of developing PIs due to the severity of their illness, the use of sedation, prolonged period of immobility, and over-reliance on medical devices16. Reported incidence of PIs among critically ill patients ranged from 3.3% to 34.4%17-24.
Prevention of HPI involves pressure relief and prevention of shearing or friction by using offloading devices8. The use of a multi-layer dressing in the prevention of PI is gaining popularity, especially after its efficacy was proven in the in-vitro computer modelling studies done by Levy and Gefen15,25. Their studies found that anisotropic (directionally dependent stiffness properties) dressing facilitated more soft tissue protection compared to isotropic (same stiffness in every direction) dressing and multilayer dressing seemed to be beneficial over single-layer dressing as it is able to dissipate tissue strains by promoting internal shear within the dressing, thus diverting the loads from the tissues and has a protective effect15,25.
A previous systematic review26 on the use of prophylactic dressing in the prevention of PI suggested that soft foam silicone dressings may help reduce PI incidence associated with medical devices, especially among immobile patients in intensive care units (ICUs). However, all of the 10 studies included in the analysis were from different care settings, and only three reported on heel injuries, while the rest of the studies reported on sacrum, trochanter and nose. Furthermore, all of the studies were conducted in a different climate compared to Asia and there was no specific recommendations on the type of dressing materials26.
Literature review
A search was conducted using the databases of PubMed and CINAHL from 2007 to 2017, with the following subject keywords: “pressure ulcer” OR “pressure injury” AND heels AND “prophylactic dressings” only found three relevant articles. The studies were varied in design, setting, and sample, as summarised in Table 1. In Santamaria’s paper, they only observed for any development of PI while their patients were in the emergency department, operating room and ICU but stop monitoring these patients once they were transferred to the general ward, hence it is possible that some of these patients may develop PI subsequently in the general ward. The findings from Sola et al.27 found that the group on hydrocellular heel pad had less incidence of HPI than the polyurethane heel dressing group (2.49% versus 3.37%). However, in their study, both heel pad and dressing were applied only at night; therefore, it is possible that patients may still be exposed to shear and friction during the day.
Table 1: Summary of literature 2007–2017 on the effectiveness of prophylactic dressing in preventing heel pressure injury
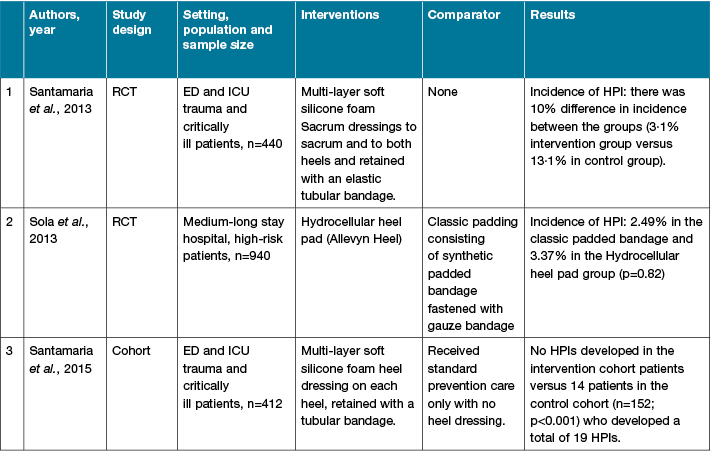
N: number of subjects; RCT: Randomised controlled trial; HPI: heel pressure injury; ED: emergency department; ICU: intensive care unit.
Although there seems to be some evidence on the effectiveness of prophylactic dressing in prevention of HPI, it is challenging to generalise the findings given the differences in skin structure between Caucasians and Asians, and differences in temperature and humidity between temperate and tropical countries. Studies have reported that Asian skin has the weakest barrier function upon mechanical challenge compared to Caucasian skin, although both types of skin possesses a similar basal transepidermal water loss (TEWL) and ceramide levels28,29. Hence, it is of interest to evaluate the effectiveness of prophylactic heel dressings among patients of Asian origin. Singapore is a tropical country with an average daily average daily temperature of 31º Celsius and mean humidity of 84%30, hence it is also of interest to evaluate if the dressings stayed on and served as an effective protective layer.
Therefore this study aimed to evaluate the effectiveness of a soft silicone multilayer heel dressing (Mepilex® Heel) in reducing the incidence of HPI among critical ill patients requiring ICU care in Singapore. To the best of the authors’ knowledge, this is the first study done in an Asian context that examines the effectiveness of a silicone multilayer heel dressing among critically ill patients.
Ethical considerations
This study was approved by the SingHealth Centralised Ethic Review Committee (ref no: 2016/2013) and has conformed to the ethical guidelines of the 1975 Declaration of Helsinki. A waiver of informed consent was granted by the review committee.
Methodology
Setting
This study was conducted in an academic 1751-bed, tertiary care institute in Singapore. The facility has three adult ICUs (surgical, medical, and neuroscience) with a total capacity of 26 beds. The nurse-to-patient ratio is 1:1 for all ICUs as per staffing practice internationally31.
Study design
A quasi-experimental, pre-, and post-intervention study design was adopted instead of a randomised controlled trial (RCT) because it was not possible to randomise patients or the ICU because of the high potential risk of contamination of the intervention in the busy critical care area and also the ethical concerns of only providing prophylactic care to some critically ill patients and not others.
Sample size calculation
To detect a decrease in incidence rate of HPI from 10% to 2%, a total of 108 patients per group was needed in order to achieve a power of 80% and alpha of 0.05.
Sampling method and study population
A convenience sampling approach was adopted; patients were eligible to participate in this study if they were over 21 years old and admitted to any of the ICUs during the study period.
Exclusion criteria:
- Patients with pre-existing HPI or trauma to the heels;
- Patients with suspected or actual spinal injury that prevent them from being turned;
- Patients who needed lower limb cast;
- Patients with a pre-existing skin condition over the heels that interfere with the application of the heel dressing; and
- Patients with moulted heel or ischaemic heels due to receiving high dose of inotropes.
Pre-intervention
The pre-intervention group’s study period ran from April 2016 to September 2016. All patients who met the inclusion criteria received the standard PI preventive measures such as daily PI risk assessment, regular repositioning, pressure-redistributing overlay or alternating air mattress and skin care such as barrier or emollient cream.
Intervention
The intervention group’s study period started from November 2016 to September 2017, which includes recruitment, data collection and training of nurses in all three ICUs on the application of the prophylactic heel dressings. All eligible patients received the standard PI preventive measures in addition to prophylactic foam heel dressings that were applied to both heels within four hours upon admission to the ICU. The heel dressing was changed every three days or whenever soiled.
The prophylactic heel dressing used in this study was Mepilex® Heel which is a soft, self-adherent foam dressing that has been theorised to substantially reduce the mechanical loading in the soft tissue and prevent shearing through its multilayer structure15,32. It also provides a protective barrier and cushioning between the surface of the patient’s skin and the bedding surface, thus reducing the impact of the pressure, friction and shear forces. Additionally, the dressing conforms to the body, manages excess moisture, and can easily be placed and removed to allow for visual inspection of the heels.
Measurements and outcome assessments
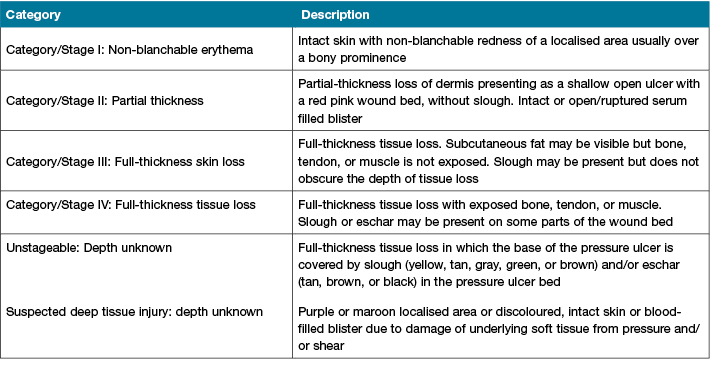
The patients' heels were assessed daily for development of PI to their heels and the conditions were documented by the registered nurses who cared for the patient as per standard hospital practice. Data were censored when patients were able to sit out of bed or discharged. The staging of PI was according to the National Pressure Ulcer Advisory Panel (NPUAP) and European Pressure Ulcer Advisory Panel (EPUAP)33 (Table 2).
Table 2: Pressure injury classification according to European Pressure Ulcer Advisory Panel (EPUAP)
Patients were also monitored if any sensitivity reactions developed such as redness or itchiness while on the heel dressing. If any sensitivity reactions occurred, the heel dressing will be removed immediately and patients will be taken out of the study.
To control for potential confounders, other data such as patients' demographic, medical and surgical information, severity of illness according to Acute Physiology and Chronic Health Evaluation II (APACHE) score, and total length of observation expressed in days were collected. All data were gathered via the hospital electronic patient management system.
APACHE II provides a general measure of severity of disease. An increasing score (range 0 to 71) is closely correlated with the subsequent risk of mortality34.
The primary endpoint was incidence rate of hospital-acquired HPI in both groups expressed as the number of patients with newly acquired HPI in each group and divide by the number of patients examined for HPI in each group during the study period (pre-intervention: 5 months; intervention: 10 months).
Statistical method
Data analysis was performed using the Statistical Package for Social Sciences, version 23.0, computer software (IBM, Armonk, New York). The continuous data are expressed as the mean and SD. Descriptive statistics were used to examine the distributions of the demographic, clinical, and hospitalisation data. Between group differences in demographics and APACHE II score, were evaluated using chi-square tests or student t-tests. HPI incidence was compared between the two groups using the Fisher exact test. Odds regression was used to control for differences in demographic or clinical factors among the two groups. The level of statistical significance was set at P <0.05.
Results
Data from 326 patients (195 patients in pre-intervention; 131 patients in intervention group) were analysed. Four patients dropped out in the intervention group; three were due to treatment-needs in ICU and one patient verbalised that the heel dressing was uncomfortable and requested to remove it.
The demographic, clinical and hospitalisation data revealed that both groups were comparable on major physiological and demographic characteristics on admission to the ICU except for gender and length of observation (Table 3). Patients in the pre-intervention group were observed for longer period than patients in the intervention group (6 versus 4 days; p=0.01).
Table 3: Demographic, clinical, and hospitalisation data between 2 groups
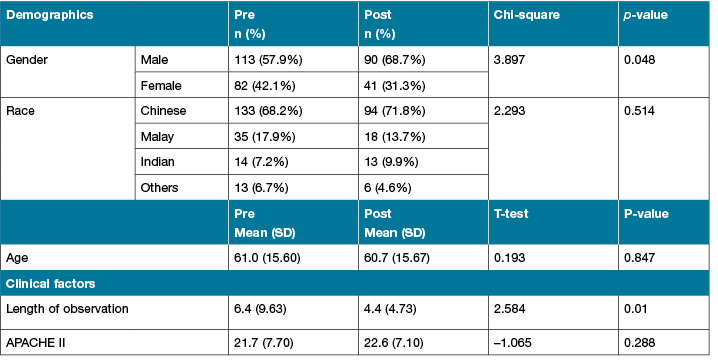
APACHE II: Acute Physiology and Chronic Health Evaluation II; NS: not significant; ICU: intensive care unit.
P value significant at < 0.05
There was an 86% reduction in the incidence rate of HPI between the two groups (pre-intervention: 10.8% versus 1.5%: post-intervention). Data on the incidence of HPI between the two groups and details of the HPIs are presented in Table 4.
Table 4: Details on heel pressure injuries
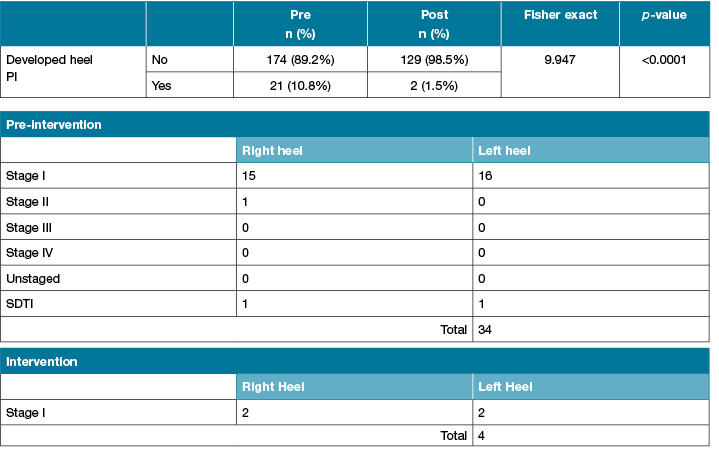
*Pressure injury stage according to EPUAP
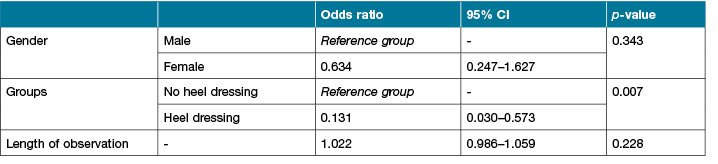
After controlling for gender, length of observation and groups (pre-intervention and intervention), the results of the regression indicated that patients in the intervention group were less likely to develop HPIs (odds ratio [OR], 0.13; 95% CI, 0.03 to 0.57; p= <0.007) (Table 5).
Table 5: Odds regression to control for differences between variables
Discussion
This is the first study done in the tropics and solely on Asian patients on the use of soft silicone multilayer foam heel dressing in preventing HPI. Our findings were similar to Santamaria et al.'s23 study, in which multilayer, soft silicone foam dressing was effective in reducing the incidence of HPI. Their study managed to achieve zero HPI in their intervention group, whereas we had two HPIs in our intervention group. The difference in findings may be due to factors such as the climate, skin type, ethnicity and differences in the starting point of the study. To illustrate, in Santamaria’s23 study, intervention started in the emergency department and continued to the operating room and the ICU, whereas we only began recruiting when the patients were admitted in ICU.
Our study’s endpoint was when patients were able to sit out of bed as it was cumbersome to walk with the heel dressing. However, studies have shown that mobile patients may also develop PI8,35; hence it may also be necessary to protect the heels when patients are sleeping at night. For example, in Sola et al.27, they found that a classic bandage heel pad was effective at preventing incidence of HPIs; therefore, future studies may consider using some form of heel protection at night for high-risk patients. Manufacturers may also consider designing a prophylactic heel dressing or pad that can be easily reapplied when patients are resting in bed.
Limitations and implications for future research
This was a single-site study and we were unable to conduct an RCT as we anticipate a high potential risk of contamination between the intervention and control group due to the busy setting of the ICU. Nonetheless the baseline characteristics of patients in all three ICUs were homogenous; hence, the data were comparable between the two groups.
Another limitation factor was that we were unable to blind data outcome assessors due to the nature of the study; however, we had employed different team members to perform cross-checking of the data collected in order to ensure its data accuracy. Therefore, our results can only be viewed in the context of the critically ill patients in an acute care setting and cannot be generalised to chronic care area.
However, although all three ICUs shared the same standard PI prevention measures, all three ICUs had different types of foam mattress from various manufacturers; thus we were unable to determine whether the use of different support surfaces could have influenced the results. Future studies may consider measuring the incremental effectiveness of support surfaces when used in conjunction with prophylactic silicone foam dressing in the prevention of PIs among critically ill patients.
Conclusion
A prophylactic multilayer foam heel dressing is effective at reducing the incidence of PI among critically ill patients in ICU. It seems that the Asian skin type and a more humid climate did not interfere with the effectiveness of the silicone foam dressing in prevention of heel injuries. The patients were able to tolerate the silicone dressing well and the heel dressing was able to stay in place when patients were not ambulating.
Disclosure of interest
The authors have no disclosure of interest to report.
Author(s)
Teo Kai Yunn*
BS (Bachelor of Nursing)
Nurse Clinician, Singapore General Hospital, Singapore
Email teo.kai.yunn@sgh.com.sg
Ang SY
MBA (Healthcare Management)
Singapore General Hospital, Singapore
Bian Luping
BS (Bachelor of Nursing)
Singapore General Hospital, Singapore
Cheah Engfeng Sharon
BS (Bachelor of Nursing)
Singapore General Hospital, Singapore
Somera Melanie Aquino
BSN (Bachelor of Science in Nursing)
Singapore General Hospital, Singapore
Ahmad Nurul Huda
BSc (Hons) Nursing Practice
Singapore General Hospital, Singapore
Lim Shu Hui
BS (Bachelor of Science)
Singapore General Hospital, Singapore
Goh Hui Qi Ivy
Diploma in Business Information Technology Singapore General Hospital, Singapore
Aloweni Fazila Abu Bakar
MSci (Health Research Methodology)
Singapore General Hospital, Singapore
* Corresponding author
References
1. Morehead D, Blain B. Driving hospital-acquired pressure ulcers to zero. Crit Care Nurs Clin North Am 2014;26(4):559–67.
2. Kavanagh KT, Cimiotti JP, Abusalem S, Coty M-B. Moving healthcare quality forward with nursing-sensitive value-based purchasing. J Nurs Scholarsh 2012;44(4):385–95.
3. Lyder C. Preventing heel pressure ulcers: Economic and legal implications. Nurs Manage 2011:4.
4. Nussbaum S, Carter MJ, Fife CE et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21(1):27–32.
5. Torra I Bou J, López JR, Camañes G et al. Preventing pressure ulcers on the heel: A Canadian cost study. Dermatol Nurs 2009;21(5).
6. Dreyfus J, Gayle J, Trueman P, Delhougne G, Siddiqui A. Assessment of risk factors associated with hospital-acquired pressure injuries and impact on health care utilization and cost outcomes in US hospitals. Am J Med Qual 2017:1062860617746741.
7. Fowler E, Scott-William S, McGuire JB. Practice recommendations for preventing heel pressure ulcers. Ostomy Wound Manage 2008;54(10):42–8, 50–2, 4–7.
8. Bhattacharya S, Mishra RK. Pressure ulcers: Current understanding and newer modalities of treatment. Indian J Plast Surg 2015;48(1):4–16.
9. Gefen A. The biomechanics of heel ulcers. J Tissue Viability 2010;19(4):124–31.
10. Gefen A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 1. Nurs Stand 2009;23(45):64, 6, 8 passim.
11. Wong V, Stotts NA. Physiology and prevention of heel ulcers: The state of science. J Wound Ostomy Continence Nurs 2003;30:8.
12. Lechner A, Lahmann N, Neumann K, Blume-Peytavi U, Kottner J. Dry skin and pressure ulcer risk: A multi-center cross-sectional prevalence study in German hospitals and nursing homes. Int J Nurs Stud 2017;73:63–9.
13. Langemo D, Thompson P, Hunter S, Hanson D, Anderson J. Heel pressure ulcers: Stand guard. Adv Skin Wound Care 2008;21:11.
14. Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care 2001;14(4):208–15.
15. Levy A, Frank MB, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability 2015.
16. Coyer F, Tayyib N. Risk factors for pressure injury development in critically ill patients in the intensive care unit: a systematic review protocol. Syst Rev 2017;6(1):58.
17. Fife C, Otto G, Capsuto EG et al. Incidence of pressure ulcers in a neurologic intensive care unit. Crit Care Med 2001;29(2):8.
18. Shahin E, Dassen T, Halfens RJ. Incidence, prevention and treatment of pressure ulcers in intensive care patients: a longitudinal study. Int J Nurs Stud 2009;46(4):413–21.
19. Gray-Siracusa K, Schrier L. Use of an intervention bundle to eliminate pressure ulcers in critical care. J Nurs Care Qual 2011;26(3):216–25.
20. Black J, Berke C, Urzendowski G. Pressure ulcer incidence and progression in critically ill subjects: influence of low air loss mattress versus a powered air pressure redistribution mattress. J Wound Ostomy Continence Nurs 2012;39(3):267–73.
21. Cremasco MF, Wenzel F, Zanei SS, Whitaker IY. Pressure ulcers in the intensive care unit: the relationship between nursing workload, illness severity and pressure ulcer risk. J Clin Nurs 2013;22(15–16):2183–91.
22. Santamaria N, Gerdtz M, Sage S et al. A randomised controlled trial of the effectiveness of soft silicone multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. International Wound Journal. 2013:7.
23. Santamaria N, Gerdzt M, Liu W et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: Border II Trial. J Wound Care 2015;24(8):6.
24. Kalowes P, Messina V, Li M. Five-layered soft silicone foam dressing to prevent pressure ulcers in the intensive care unit. Am J Crit Care 2016;25(6):e108–e19.
25. Levy A, Gefen A. Assessment of the biomechanical effects of prophylactic sacral dressings on tissue loads: A computational modeling analysis. Ostomy Wound Manage 2017;63(10):8.
26. Clark M, Black J, Alves P et al. Systematic review of the use of prophylactic dressings in the prevention of pressure ulcers. Int Wound J 2014:13.
27. Ferrer Solà, Espaulella Panicot J, Altimires Roset J, Ylla-Català Borè E, Moreno Susi M. Comparison of efficacy of heel ulcer prevention between classic padded bandage and polyurethane heel in a medium-stay hospital: randomized controlled trial. Rev Esp Geriatr Gerontol 2013;48(1):3–8.
28. Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci 2006;28(2):79–93.
29. Barrett-Hill F. Ethnic, Coloured & Caucasian: skin differences beyond colour alone. New Zealand: Beautymagonline.com; [Available from: http://beautymagonline.com/beauty-articles-2/907-coloured-skins-3.
30. The Weather and Climate of Singapore: National Environment Agency; [Available from: http://www.nea.gov.sg/weather-climate/climate.
31. Bray K, Wren I, Baldwin A et al. Standards for nurse staffing in critical care units determined by: The British Association of Critical Care Nurses, The Critical Care Networks National Nurse Leads, Royal College of Nursing Critical Care and In-flight Forum. Nurs Crit Care 2010;15(3):109–11.
32. Call E, Pedersen J, Bill B et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J 2015;12(4):408–13.
33. NPUAP Pressure Injury Stages: National Pressure Ulcer Advisory Panel (NPUAP) 2016 [Available from: http://www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-injury-stages/.
34. Knaus WA DE, Wagner DP, Zimmerman JE. APACHE II a severity of disease classification system. Crit Care Med 1985;13(10):12.
35. Wake WT. Pressure ulcers: What clinicians need to know. Perm J 2010;14(2):56–60.



