Volume 26 Number 3
The use of topical calcineurin inhibitors in chronic wound management
Geoff Sussman
Keywords Calcineurin inhibitors, tacrolimus, pimecrolimus, pyoderma gangrenosum.
Abstract
The management of wounds has evolved over many years and with a greater understanding of the mechanisms of skin repair, a recent approach to treatment is to address the changes in tissue. Complex chronic wounds place a significant challenge on health professionals working with these patients, in accurately identifying the underlying cause of the wound and the tissue factors impacting on wound healing. Many different types of dressings are used with limited success; however, dressings do not address the imbalances in tissue. Once a clear diagnosis has been established, the approach is to try to address this underlying imbalance in the tissues. Calcineurin inhibitors were approved for topical use in 2000–2001 for the treatment of atopic dermatitis. Since that time, their use has expanded to treat a number of chronic wounds, including pyoderma gangrenosum, cutaneous lupus erythematosus and other uncommon chronic wounds, with some success. This approach, in contrast to dressings that only impact on the wound environment, addresses the need to restore tissue balance and thus encourage wound healing.
Background
The use of topical calcineurin inhibitors in chronic wounds has developed since early 2000, with their extensive use in dermatology, in atopic dermatitis and for mild to moderate eczema. Over the past 10 years, their use has expanded to include use in complex chronic wounds1. There were concerns expressed by the FDA2 over risks, including skin cancer and lymphoma; however, these concerns have not been demonstrated in the evidence3,4 The report of the American Academy of Dermatology Association Task Force conference found no causal proof that topical calcineurin inhibitors cause lymphoma or non-melanoma skin cancer5.
Calcineurin inhibitors were originally prescribed to prevent allograft rejection post transplant. They have been demonstrated to have anti-inflammatory activity and immunosuppression6-8. They have also been shown to have little risk of skin atrophy when compared with topical corticosteroids7. They also have been shown to induce initial release of substance P that helps to explain their efficacy in pruritis and sensory side effects9.
Calcineurin inhibitors form complexes with cytoplasmic immunophilins, which block the action of calcineurin in activated T-cells. This prevents production of interleukin-2 and other cytokines that normally stimulate T-cell proliferation and differentiation10. Calcineurin inhibitors are used for the prevention of solid organ transplant rejection11 and for the prevention and treatment of graft-versus-host disease in stem cell transplants. These drugs are normally administered by IV injection or orally12.
Clinical Use
Over the past 10 years calcineurin inhibitors such as tacrolimus have been employed in the treatment of a wide range of dermatological diseases and chronic wounds. They promote melanocyte and melanoblast growth and create a favourable milieu for cell migration via keratinocytes: possible mechanisms of how tacrolimus ointment induces re-pigmentation in patients with vitiligo13-16.
Topical tacrolimus and pimecrolimus are now used more extensively in the treatment of pyoderma gangrenosum with good results; a case study of this use is included17-22 ,24-35.
Some of the more recent uses of calcineurin inhibitors are in the treatment of necrobiotic xanthogranuloma, where tacrolimus is applied topically in a 0.1–0.02% ointment. Topical tacrolimus does not negatively impact acute skin wound healing. Altieri et al. reported on the success of topical tacrolimus for parastomal pyoderma gangrenosum in two patients21. Rice et al.23 used topical tacrolimus 0.1% ointment for the treatment of cutaneous Crohn’s disease in an open-label, observational study of 20 patients with heterogeneous forms of cutaneous Crohn’s disease, using topical tacrolimus 0.1% ointment once-daily to the affected areas for 12 weeks. Of the 17 patients completing the 12-week study, 15 improved using a specifically designed physicians’ global severity scale. One patient cleared, four showed a pronounced improvement (51–75%) and 10 demonstrated a mild (1–25%) or moderate improvement (25–50%) in 12 weeks. They concluded that “0.1% tacrolimus ointment was safe and effective in treating cutaneous manifestations of Crohn’s disease, particularly perineal disease and pyoderma gangrenosum, yet it seldom cleared the condition”.
Topical calcineurin inhibitors have been used in the management of a number of other conditions, including lichen planus and lichen sclerosus26-29, cutaneous lupus erythematosus and dermatomyositis30,31, necrobiosis lipoidica, pemphigus vulgaris, pityriasis versicolor, nail psoriasis and the treatment of secondary lymphoedema32-37.
Elidel® is the only commercial form of Pimecrolimus cream available in Australia. In the case of topical tacrolimus, the ointment form is required to be compounded by a pharmacy (see Table 1).
Table 1

Published Clinical Studies
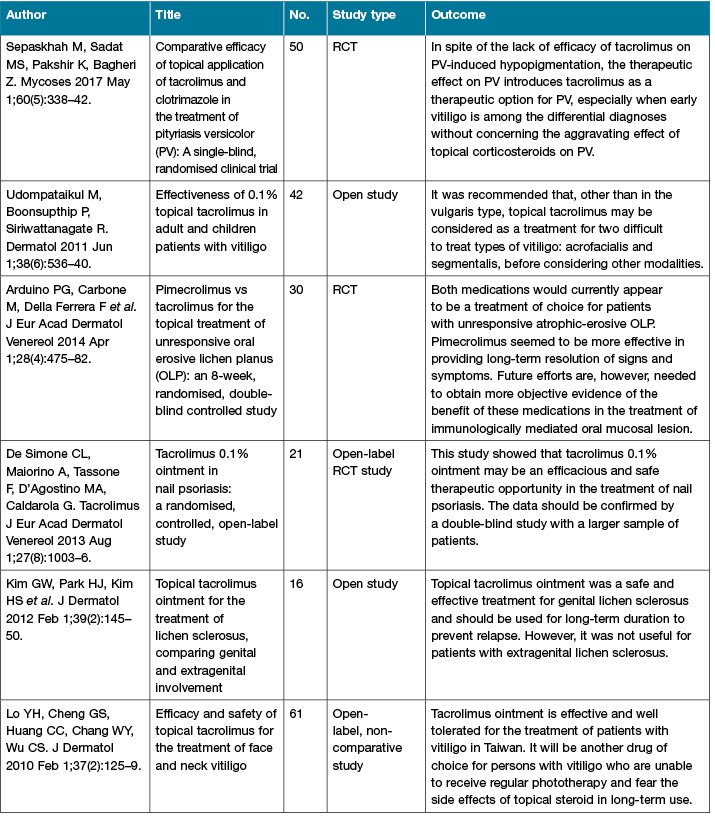
There are an increasing number of published studies of the clinical use of calcineurin inhibitors (Table 2). The more recent conduct of RCTs helps to strengthen the level of evidence for their use.
Table 2: Calcineurin inhibitors clinical studies
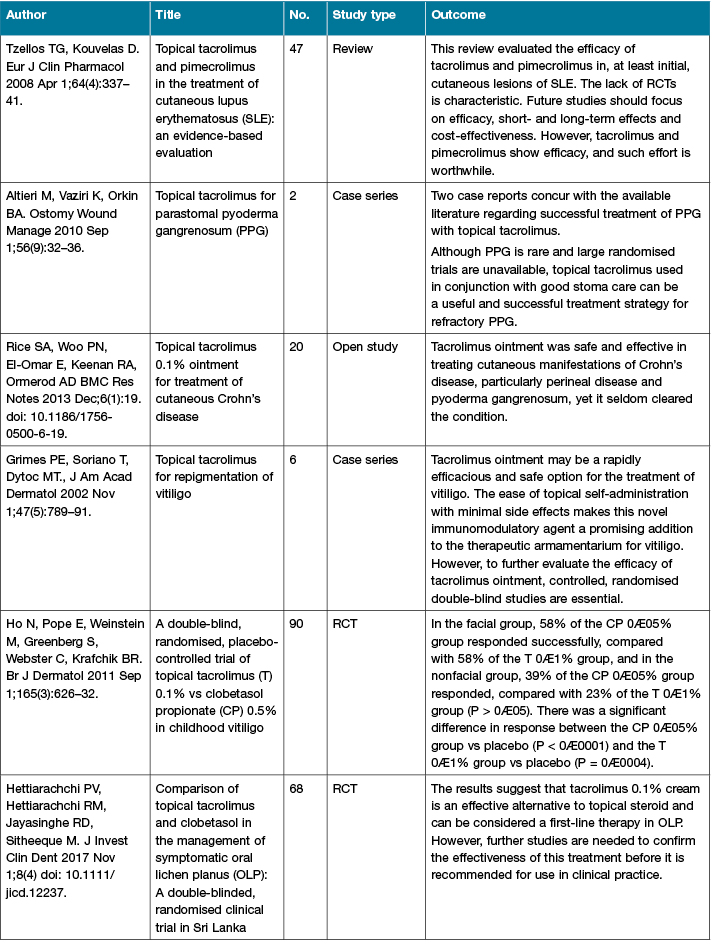
Table 2 (continued): Calcineurin inhibitors clinical studies
Precautions
On 15 February 2005, the Pediatric Advisory Committee of the Food and Drug Administration (FDA) recommended ‘black box’ warnings for pimecrolimus cream (Elidel®) and tacrolimus ointment (Protopic®) because of concerns of potential safety risks (including skin cancer and lymphoma). On 10 March 2005, the FDA issued a Public Health Advisory2 informing health-care providers of the Agency’s safety concerns associated with use of these drugs. However, there is no evidence that topical use of pimecrolimus and tacrolimus causes malignancies3. A consensus statement published in Dermatology4 stated that the recommendations of the Pediatric Advisory Committee and the FDA Health Alert are not justified based on the scientific evidence and should be reconsidered. The report of the American Academy of Dermatology Association Task Force5 conference found no causal proof that topical calcineurin inhibitors cause lymphoma or non-melanoma skin cancer.
Clinical Case Studies of the use of Calcineurin inhibitors
Case 1: Use in necrobiotic xanthogranuloma (NXG)
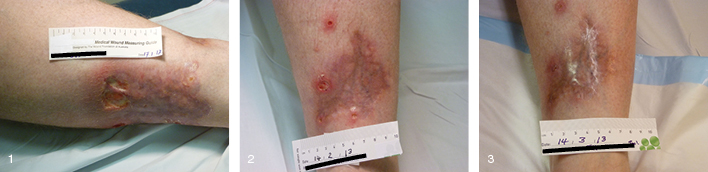
NXG is a rare, chronic granulomatous disorder characterised by indurated plaques and nodules of the skin10. NXG initially presents with yellowish papules and nodules that coalesce into indurated plaques, usually 0.5–2.0 cm. Lesions often show superficial telangiectasias and can scar and ulcerate in 40–50% of patients. Skin lesions can recur rapidly and lesion size typically increases with recurrence. Despite these potential complications, incisional biopsy is recommended to confirm the diagnosis when NXG is suspected clinically. Most NXG skin lesions (60–70%) first appear on the trunk or extremities. Patient JH was a 55-year-old male with a history of diagnosed NXG, who had a number of different treatments with limited success. Treatment with topical tacrolimus was commenced on 17 January 2013 (Figure 1), reviewed in four weeks (Figure 2) and continued treatment until healing was achieved by 14 March 2013 (Figure 3).
Case 2: Use in pyoderma gangrenosum (PG)
PG is a rare, inflammatory, non-infective skin disease resulting in destructive, painful, rapidly enlarging ulcers that are irregular and raised, with reddish borders, a necrotic base and undermined bluish or purplish-red edges. It is associated with inflammatory bowel diseases and immune system abnormalities. PG is difficult to diagnose and is mostly diagnosed by exclusion of the typical causes of leg ulcers. A wound biopsy will often help to exclude other causes. It is often the case if a biopsy is taken that the wound will enlarge; this is described as pathergy. PG is difficult to treat as this involves pain management, maintaining a moist environment, and the systemic use of medication including steroids, cyclosporin or dapsone.
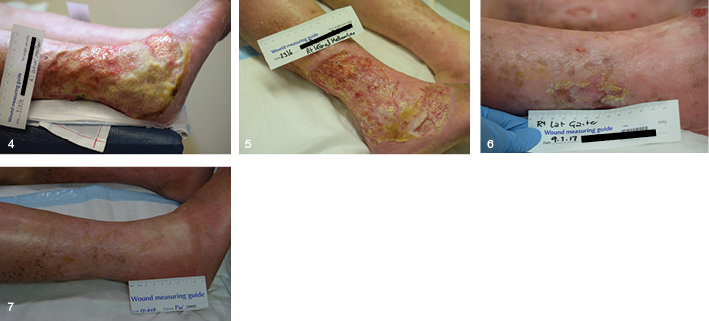
Patient PW was an 84-year-old female with a long history of non-healing leg ulcers. They had been treated as venous leg ulcers with dressings and compression. One ulcer on the right medial malleolus deteriorated and a new diagnosis of PG was made. Topical tacrolimus was commenced on 9 June 2016 (Figure 4) and within five weeks of use there was a significant improvement in wound size and condition (Figures 5 and 6). The treatment was continued over the next few months and the wounds rapidly improved and, on review in March 2017, were healed (Figure 7).
Discussion
Immunosuppression administered systemically is often part of the management of wounds related to PG, vasculitis and other complex autoimmune diseases. The difficulty faced with this method of administration is that given that the dosage needs to be at a level for a therapeutic outcome, the risk of side effects is much greater. The use of topical immunosuppression is one option that may overcome the potential side effect profile of many of the medications used. Topical calcineurin inhibitors, such as tacrolimus, have been shown to suppress T-cell activation and act as an immune suppressor without the risks of significant side effects. They do not cause skin atrophy, they work locally and, because of the very low strength, there is little risk of systemic absorption of the drug, which does not have a negative impact on wound healing.
Conclusion
Topical calcineurin inhibitors have been used dermatologically for many years in the management of eczema and atopic dermatitis. The extension of their use in the management of complex, chronic, non-healing wounds is increasing with the publication of their successful use in a wider range of wounds, including some RCTs. At present, there is no commercial form of topical tacrolimus; it needs to be manufactured by a compounding pharmacy or hospital pharmacy department.
There is a need for more clinical studies of the use of topical calcineurin inhibitors and publication of results to build a sound level of evidence for the role of these compounds in wound practice to become more widespread.
Conflict of Interest
The author declares no conflicts of interest.
Funding
The author received no funding for this study.
Author(s)
Geoff Sussman
OAM, FPS, FACP, FAIPM, FAWMA, FRVAHJ
Associate Professor of Wound Care
Monash Institute for Health and Clinical Education
Faculty of Medicine, Nursing and Health Science
Monash University, VIC, Australia
Email geoff.sussman@monash.edu
References
- Wollina U, Hansel G, Koch A, Abdel-Naser MB. Topical pimecrolimus for skin disease other than atopic dermatitis. Expert Opin Pharmacother 2006 Oct 1;7(14):1967–75.
- Novartis and Fujisawa FDA Briefing Statements. Pediatric Advisory Committee Meeting of the US Food Drug Administration. Washington, DC, 2005. Available at: http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4089b2.htm.
- Berger TG, Duvic M, Van Voorhees AS, Frieden IJ. The use of topical calcineurin inhibitors in dermatology: safety concerns: report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol 2006 May 1;54(5):818–23.
- Bieber T, Cork M, Ellis C et al. Consensus statement on the safety profile of to pical calcineurin inhibitors. Dermatology 2005;211(2):77–8.
- Fonacier L, Spergel J, Charlesworth EN et al. Report of the topical calcineurin inhibitor task force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2005 Jun 1;115(6):1249–53.
- Stuetz A, Baumann K, Grassberger M, Wolff K, Meingassner JG. Discovery of topical calcineurin inhibitors and pharmacological profile of pimecrolimus. Int Arch Allergy Immunol 2006;141(3):199–212.
- Grassberger M, Steinhoff M, Schneider D, Luger TA. Pimecrolimus — an anti-inflammatory drug targeting the skin. Exp Dermatol 2004 Dec 1;13(12):721–30.
- Lazarous MC, Kerdel FA. Topical tacrolimus Protopic. Drugs Today 2002;38:7–15.
- Pereira U, Boulais N, Lebonvallet N, Pennec JP, Dorange G, Misery L. Mechanisms of the sensory effects of tacrolimus on the skin. Br J Dermatol 2010 Jul 1;163(1):70–77.
- Sussman G. Biologicals in wound care In: Harikrishna K R Nair. Diabetic Foot. Selangor Darul Ehsan Malaysia Uriquelink 2017;241–257.
- Lee CF, Lo YC, Cheng CH et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep 2015 Oct 27;13(4):760–70.
- Elgarten CW, Arnold DE, Bunin NJ, Seif AE. Single-agent calcineurin-inhibitor (CNI) for the prevention of graft versus host disease (GVHD) in pediatric patients undergoing HLA-identical sibling bone marrow transplant (BMT). Biol Blood Marrow Transplant 2017 Mar 1;23(3):S366.
- Ho N, Pope E, Weinstein M, Greenberg S, Webster C, Krafchik BR. A double-blind, randomized, placebo-controlled trial of topical tacrolimus 0·1% vs clobetasol propionate 0· 05% in childhood vitiligo. Br J Dermatol 2011 Sep 1;165(3):626–32.
- Udompataikul M, Boonsupthip P, Siriwattanagate R. Effectiveness of 0.1% topical tacrolimus in adult and children patients with vitiligo. J Dermatol 2011 Jun 1;38(6):536–40.
- Lo YH, Cheng GS, Huang CC, Chang WY, Wu CS. Efficacy and safety of topical tacrolimus for the treatment of face and neck vitiligo. J Dermatol 2010 Feb 1;37(2):125–9.
- Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol 2002 Nov 1;47(5):789–91.
- Feldman SR, Lacy FA, Huang WW. The safety of treatments used in pyoderma gangrenosum. Expert Opin Drug Saf 2018 Jan 2;17(1):55–61.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: Classification and management. J Am Acad Dermatol 1996;34:395–409.
- Kontos AP, Kerr HA, Fivenson DP, Remishofsky C, Jacobsen G. An open-label study of topical tacrolimus ointment 0.1% under occlusion for the treatment of pyoderma gangrenosum. International J Dermatol 2006;45:138–5.
- Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273–83.
- Altieri M, Vaziri K, Orkin BA. Topical tacrolimus for parastomal pyoderma gangrenosum: a report of two cases. Ostomy Wound Manage 2010 Sep 1;56(9):32–36.
- Reich K, Vente C, Neumann C, Topical tacrolimus for pyoderma gangrenosum Br J Dermatol 1998;139:755–757.
- Rice SA, Woo PN, El-Omar E, Keenan RA, Ormerod AD. Topical tacrolimus 0.1% ointment for treatment of cutaneous Crohn’s Disease. BMC Res Notes 2013 Dec;6(1):19. doi: 10.1186/1756-0500-6-19
- Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB Jr, White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore) 2000;79:37–46.
- Reichrath J, Bens G, Anette Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005 Aug 1;53(2):273–83.
- Vente C, Reich K, Rupprecht R, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol 1999 Feb 1;140(2):338–42.
- Hettiarachchi PV, Hettiarachchi RM, Jayasinghe RD, Sitheeque M. Comparison of topical tacrolimus and clobetasol in the management of symptomatic oral lichen planus: A double-blinded, randomized clinical trial in Sri Lanka. J Investig Clin Dent 2017 Nov 1;8(4). J Invest Clin Dent 2017 Nov 1;8(4) doi: 10.1111/jicd.12237.
- Arduino PG, Carbone M, Della Ferrera F et al. Pimecrolimus vs. tacrolimus for the topical treatment of unresponsive oral erosive lichen planus: a 8 week randomized double-blind controlled study. J Eur Acad Dermatol Venereol 2014 Apr 1;28(4):475–82.
- Kim GW, Park HJ, Kim HS et al. Topical tacrolimus ointment for the treatment of lichen sclerosus, comparing genital and extragenital involvement. J Dermatol 2012 Feb 1;39(2):145–50.
- Tzellos TG, Kouvelas D. Topical tacrolimus and pimecrolimus in the treatment of cutaneous lupus erythematosus: an evidence-based evaluation. Eur J Clin Pharmacol 2008 Apr 1;64(4):337–41.
- Avgerinou G, Papafragkaki DK, Nasiopoulou A, Arapaki A, Katsambas A, Stavropoulos PG. Effectiveness of topical calcineurin inhibitors as monotherapy or in combination with hydroxychloroquine in cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol 2012 Jun 1;26(6):762–7.
- Peckruhn M, Tittelbach J, Elsner P. Update: Treatment of necrobiosis lipoidica. JDDG: J Dtsch Dermatol Ges 2017 Feb 18;2(15):151–7.
- Binamer Y, Sowerby L, El-Helou T. Treatment of ulcerative necrobiosis lipoidica with topical calcineurin inhibitor: case report and literature review. J Cutan Med Surg 2012 Nov;16(6):458–61.
- Harman KE, Brown D, Exton LS et al. British Association of Dermatologists’ guidelines for the management of pemphigus vulgaris 2017. Br J Dermatol 2017 Nov 1;177(5):1170–201.
- Sepaskhah M, Sadat MS, Pakshir K, Bagheri Z. Comparative efficacy of topical application of tacrolimus and clotrimazole in the treatment of pityriasis versicolor: A single blind, randomised clinical trial. Mycoses 2017 May 1;60(5):338–42.
- De Simone CL, Maiorino A, Tassone F, D’Agostino MA, Caldarola G. Tacrolimus 0.1% ointment in nail psoriasis: a randomized controlled open-label study. J Eur Acad Dermatol Venereol 2013 Aug 1;27(8):1003–6.
- Gardenier JC, Kataru RP, Hespe GE et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat Commun 2017 Feb 10;8:14345.



