Volume 26 Number 4
A descriptive exploratory survey of incontinence-associated dermatitis in the intensive care setting: the ADDrESS study
Yi-Chen Lee, Jill Campbell, Anna Doubrovsky, Adele McGarry and Fiona Coyer
Keywords Incontinence-associated dermatitis, Critically ill patients, intensive care, survey.
Abstract
Background: Incontinence-associated dermatitis (IAD) develops from skin exposure to urine, faeces or both. Previous studies have tested different IAD management practices in critically ill patients in the intensive care unit (ICU); however, actual clinical practices reported for IAD prevention and management remain unknown. This study aimed to understand Australian registered nurses’ current clinical practices to prevent and manage IAD in the ICU.
Method: This study used a cross-sectional exploratory survey design. Members of the Australian College of Critical Care Nurses (ACCCN) Ltd were invited to complete an electronic survey.
Results: Response rates were 5.6% (111/1967) of the total email invitations sent or 10.6% (111/1051) of those who read the invitation. Most participants were female (88.3%, n=98), with 69.4% (n=67) working in a metropolitan hospital, and 91% working in a public hospital. Participant recall of IAD frequency was 10.4%. Various methods were identified to prevent and manage IAD, including skin protection at the patients’ general bed-bath and at episodes of incontinence clean-up. The lack of IAD management policy in ICUs was common across the participant group.
Conclusion: Clinical practices to prevent and manage IAD were found to be the same. Formal IAD management protocols in ICUs were limited in number.
What is known on this topic?
- IAD is a common condition seen in critically ill patients in the intensive care environment.
- There is limited evidence addressing IAD in the ICU context.
- What this paper adds
- Clinical practices to prevent and manage IAD appear to be the same and include the application of a variety of products for skin protection at the time of the patient’s general bed-bath and episodes of incontinence clean-up.
- Formal IAD prevention and management protocols for the ICU are rarely used.
- IAD care should be guided by evidence-based best practice recommendations.
Introduction
Incontinence-associated dermatitis (IAD) is a type of moisture-associated skin damage characterised by skin surface inflammation, redness, oedema and, in some cases, bullae containing clear exudate1,2. Damage to the epidermis may be present in varying depths and in some instances the epidermis may be completely eroded, exposing a moist, weeping dermis3. It is found in patients who are incontinent and is a condition that causes considerable pain and discomfort to patients. Furthermore, from a clinician’s perspective, IAD can be difficult, time-consuming, and even costly to manage4. This condition can be found in all types of care settings, with prevalence ranges from 20% to 95%4-6 while the incidence rate of IAD in intensive care unit (ICU) patients is 25–40% of all patients with incontinence6,7.
In the ICU, patients are incontinent due to their immobility and critical illness and often experience high rates of diarrhoea8. Most ICU patients are cared for with an indwelling urinary catheter in situ9,10, with faecal incontinence more commonly seen in these patients than urinary incontinence11. Patients with loose or liquid stool are more likely to develop IAD than patients who have formed stools3. Patients with continuous acute faecal incontinence with diarrhoea have a higher rate of skin excoriation or IAD development12. Diarrhoea in ICU patients is a multifactorial problem and the leading cause of IAD13. High rates of diarrhoea in ICU patients is common and often attributable to enteral tube feeding, the use of antibiotics, infection, other treatments such as vasoactive agents for hypotension, and the patient’s critical condition13-15. The factors that increase the risk of diarrhoea can, in turn, increase the risk of IAD due to the causal relationship between diarrhoea and IAD3.
Background
A search of databases for articles was conducted to identify literature focusing on IAD prevention and management in ICUs. The initial search terms were “IAD” or “incontinence-associated dermatitis” or “perineal dermatitis”, and these were narrowed by adding “intensive care” or “ICU” or “critical care”. The search was conducted during September and October in 2017, and updated in February 2018, using electronic databases including the Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost), Cohort library (including all cohort reviews, other reviews, trials, methods studies, technology assessments, economic evaluations, and Cochrane Groups), Embase, Joanna Briggs Institute (Ovid), Medline (via EBSCOhost), and PubMed.
The search included information on IAD in the critical care setting within journals, books, handbooks, and abstracts of published articles. Boolean mode operators including “or”, “and”, and “not” were used to combine the search terms. Literature searches were not date limited. Selected literature included articles in English or in Chinese where English abstracts were included. Articles were included in the review if they reported IAD in the critical care or intensive care setting, and IAD in other settings with critical care or intensive care data reported separately.
The search identified 12 publications that addressed IAD in the ICU. One publication, a summation of literature, did not provide new information or data on IAD and was, therefore, excluded. Of the remaining 11 publications, one randomised controlled trial (RCT) was identified16, and five studies employed a mixture of quasi-experimental methods ranging from before and after studies17, prospective two group observation methods10, one group prospective study7, prospective observation methods9, and one comparison cohort18. Two studies were publications of conference abstracts with limited data presented19,20, and two papers reported quality improvement projects21,22. The studies are presented in Table 1.
Table 1: Overview of published studies
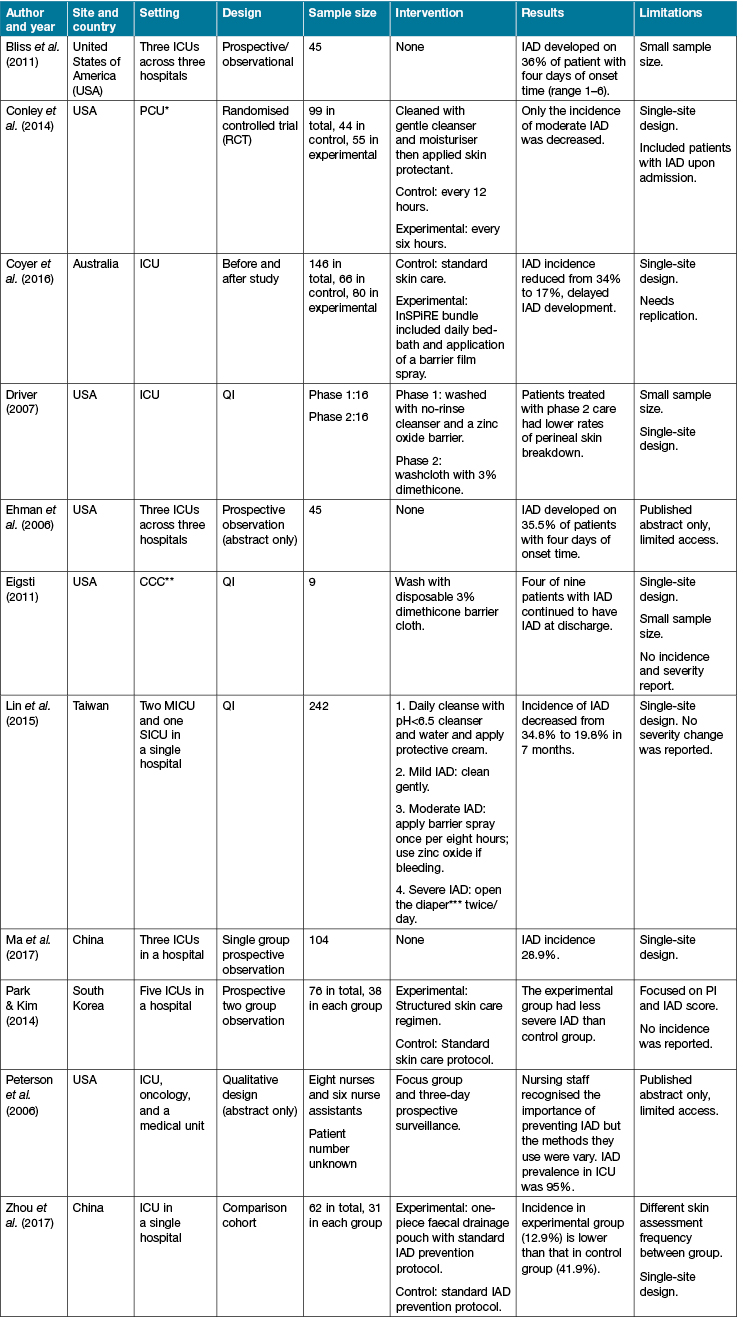
Notes: *PCU = Progressive care unit; **CCC = Critical care centre; ***Adult diaper use is a common clinical practice in Taiwan
There is a paucity of literature addressing IAD in the ICU context. Global consensus IAD prevention and treatment guidelines published in 20153 do not present ICU-specific recommendations. Reported care interventions to prevent and manage IAD are widely varied. Most of the studies identified in our review address the prevention of IAD. Some studies identified that the application of a product at the end of the daily bed-bath to protect the skin for those patients at risk of IAD was beneficial6,10,15. One study found the application of cleanser with moisturiser and the application of skin protectant could prevent the development of IAD9. Some studies found that a single intervention such as a no-rinse washcloth with 3% dimethicone21,22 alone could provide better protection to the skin than the use of multiple products. However, most of studies suggested that the combined use of hygiene strategies and products in a care bundle can prevent IAD more effectively6,10,17,18. A care bundle is defined as a group of three to five evidence-based interventions which, when delivered together, demonstrate better health outcomes than if they were delivered individually23. For example, low-pH cleanser (pH<5.5) with long-term protection cream6 or disposable washing cloth and other skin protectant18. A care bundle could also improve the assessment and documentation of skin integrity17 or include other methods like a pouch to remove incontinence for the patient’s skin18 and reduce the incidence of IAD.
In the majority of published studies (Table 1) the management of IAD was rarely discussed. One study found that more frequent general bed-bathing with reapplication of skin protectants was effective to reduce the severity of IAD16; however, this study did not include or account for the frequency of incontinence clean-up, and the types of products used during incontinence clean-up, thus confounding the findings. The use of different products may depend on the perceived severity of IAD6. Further, referral to, and consultation by, a skin care expert could improve the outcome of IAD management6,10. Considerable variation still exists in the reported practice for the management of IAD.
Aims and questions
The aim of this study was to gain an understanding of Australian ICU registered nurses’ (RNs) clinical practice in the prevention and management of IAD. The research questions underpinning this study were:
- What is RNs’ recall and self-reported frequency of IAD in the ICU?
- What practices do ICU RNs use to prevent IAD?
- What practices do ICU RNs use to manage IAD?
Design
This study used a cross-sectional cohort design. An investigator-developed questionnaire was delivered to all participants using an online platform, Key Survey (WorldApp Key survey, Version 8.17, 2017).
Setting and Participants
The study used a convenience sample of RN members of the Australian College of Critical Care Nurses (ACCCN) Ltd. This is the national professional body of critical care and intensive care registered nurses in Australia, with approximately 2500 members. Only ACCCN members who worked in an adult ICU were invited to participate in the survey. Members who worked in coronary care or high-dependency settings or paediatric or neonatal ICUs were excluded. This reduced the potential sample to approximately 60% of the total membership, or 1500 potential participants.
Instruments
The survey was developed by the authors based on clinical experience and the available literature. The survey addressed participants’ characteristics, the ICU context, and clinical care practices in the prevention and management of IAD. The participant characteristics included gender, age, academic level, current working state, working experience in ICU, and the experience in caring IAD patients or not. IAD-related items addressed type of patient incontinence, recall of the frequency of caring for patients with IAD. The ICU context included the type of hospital, the location of hospital, type of ICU and the number of operational beds. Care practices comprised the bed-bath (frequency, products choice), clean-up of incontinence (time to clean up, product choice), IAD documentation (record chart for IAD, use of formal severity tools, use of IAD guideline, bowel management protocol), the application of clean, sheet or pad.
The survey was developed using the online platform Key Survey (WorldApp Key survey, Version 8.17, 2017). The survey was pilot-tested with a small sample of three ICU RNs (non-ACCCN members) and one skin integrity nursing clinical expert. Modifications were made to the survey based on the pilot sample feedback. Feedback from the pilot sample suggested that the questionnaire was easy to interpret and that the time allocated to complete the survey was appropriate. Minor modifications were made to four questions.
Procedure
Following approval from the University Human Research Ethics Committee and ACCCN approval, all members of the ACCCN who had agreed to be contacted for research purposes were invited to participate in the survey during January and February 2018. An email inviting participation was distributed by an ACCCN administrative assistant through their member database. Embedded in the email was an online survey link in the form of a master uniform resource locator. An information sheet outlining the requirements for participation was attached to the email. Participants completed and submitted the survey online. Submission of the completed survey signified participants' consent to participate. This survey was anonymous, and no incentives or gifts were given to participants to encourage completion of the survey. The survey took approximately 10 minutes to complete. As per ACCCN’s policy, no reminder email was sent to the membership. The survey was available online for four weeks.
Data Analysis
Data was extracted from Key Survey and transported to the Statistical Package for the Social Sciences (SPSS, version 24, 2016) for analysis. Confidentiality of data was maintained through the use of electronic password-protected files. Data was cross-checked for missing responses. Descriptive data was analysed using means, standard deviations, modes, and interquartile ranges, as appropriate.
This study contained categorical data, as most of the questions provided forced choice responses for participants to choose from. Variables that could be formed into a small table (2X2) were tested by Pearson’s Chi-square. Fisher’s exact test continuity correction was used where the data did not fulfil the assumption of Pearson’s Chi-square. Variables that formed larger cell or tables were tested by linear-by-linear association24.
Ethics
This study was approved by the University Human Research Ethics Committee; approval number 1800000031. Approval was also received from ACCCN Ltd to conduct the study.
Results
A total of 1967 survey invitations were sent to ACCCN members by email, 1051 of these were opened, with 149 people clicking on the master link to the survey in the invitation mail and opening the survey. Of these 149 activated surveys, there were 111 valid responses comprising responses rates of 5.6% (111/1967) of the total email invitations sent or 10.6% (111/1051) of those who had read the invitation mail.
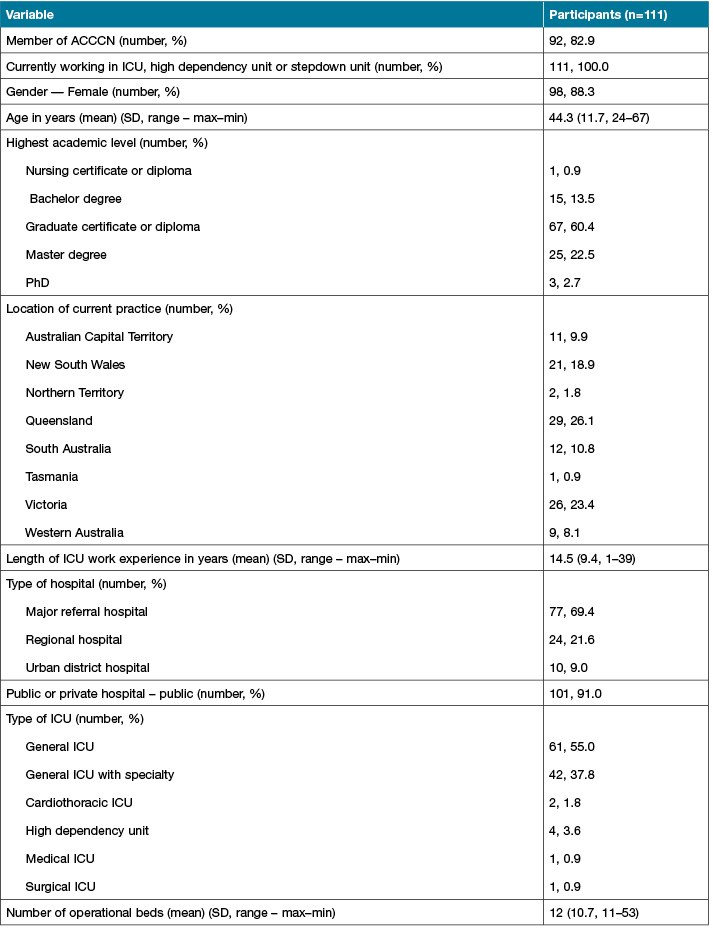
Of the 111 participants, 98 (88.3%) were female, mean age was 44 years (SD 11.7, range 24–67 years), and the mean length of ICU work experience was 14.5 years (SD 9.4, range 1–39). The majority of participants (n=67, 60.4%) had completed a graduate certificate or diploma, and most participants worked in Queensland (n=29, 26.1%) and in a major referral hospital (n=77, 69.4%) in the public health care sector (n=101, 91%). Participant and workplace demographics are presented in Table 2.
Table 2: Participant and workplace demographics
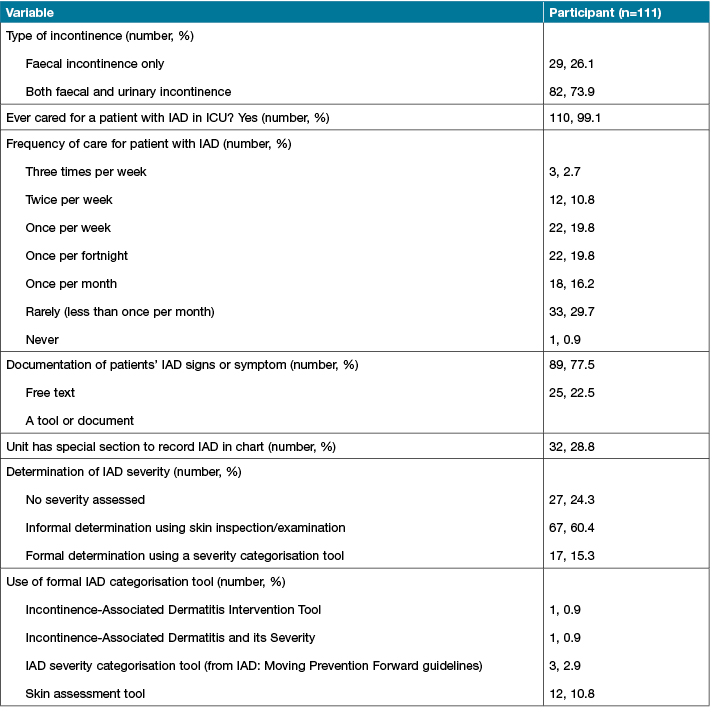
Combined faecal and urinary incontinence was the most commonly noted patient presentations (n=82, 73%). Almost all participants (99%, 110) had experienced caring for a patient with IAD in the ICU, and half of the participants reported caring for at least one patient with IAD every two weeks. However, 34 (30.6%) RNs reported that they rarely or never saw an IAD case.
Almost all (n=110, 99%) participants inspected patients' skin at least once per shift. The majority (n=89, 77.5%) of participants reported recording the clinical assessments of IAD in free text form in the patient’s notes. Thirty-two (28.8%) participants reported that their unit had a special section in each patient’s notes to record IAD. This special charting was identified as the ICU clinical information system, for example, Metavision, or other tools such as Riskman, Woundman, pressure injury (PI) tools (such as the Braden scale score), skin assessment tools, wound assessment tools, or the regular chart. More than half of the participants (n=67, 60.4%) determined the severity of IAD using a visual skin inspection and their own clinical judgement, while a formal categorisation tool was rarely used in the ICU (n=17, 10.8%). The results of RNs’ experience in caring for patients with incontinence are shown in Table 3.
Table 3: RNs’ experience of taking care of patients with incontinence
RNs reported that patients were provided with a general bed-bath at least once a day, and most participants (64%, 71/111) applied moisturiser regularly after a general bed-bath. Most RNs reported using a combination of products when providing a general bed-bath to their patients. The state or territory where the RNs worked was found to influence their choice of products (p<0.05) where participants working in Victoria were significantly associated with using soap.
Table 4: Participant demographic associations with processes of care for IAD within the ICU
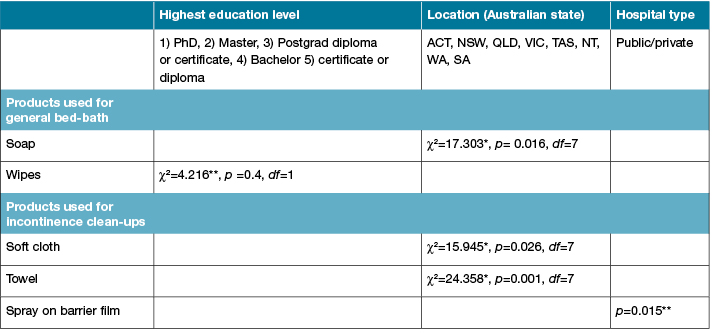
Participants with higher academic qualifications (master or doctorate degrees) were associated with not using no-rinse cleanser to clean up patients’ incontinence (X2=4.216, p<0.05). Products to prevent IAD were routinely applied by 83 participants and most (n=77, 70%) used these products after cleaning up an episode of incontinence. Participants who worked in private hospitals used spray-on barrier film more (80%) than those who worked in public hospitals (37.6%). Almost half (n=55, 49.5%) of the participants regularly applied a pad, under pad, or Kylie absorbent bed pad between their patients and sheets. The products used for the general bed-bath, clean-up after an episode of incontinence, and prevention of IAD are presented in Figures 1 to 3.
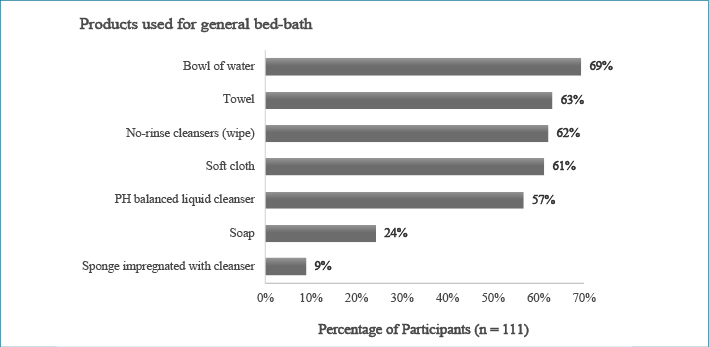
Figure 1: Percentage of participant use of products used for general bed-bath
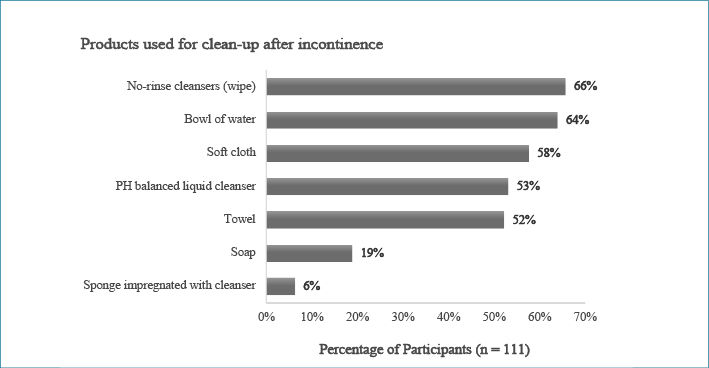
Figure 2: Percentage of participant use of products used for clean-up after an episode of incontinence
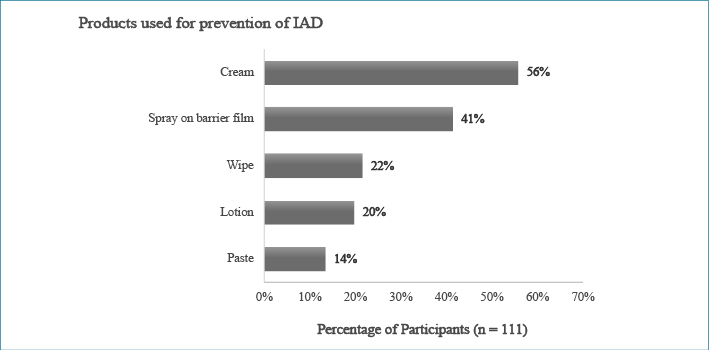
Figure 3: Percentage of participant use of products routinely applied to prevent IAD
Most of the ICUs had a bowel management protocol in place (n=98, 88%), and the majority of the protocols included the administration of aperients and prokinetics. IAD policy or guidelines were addressed in a general skin management document in some participants’ units (n=18). However, most of the ICUs (n=87, 78.3%) did not have a policy or guideline for IAD prevention and management.
Discussion
This small but unique study contributes to the understanding of Australian ICU RN clinical practice in the prevention and management of IAD. We identified that nearly all RNs had cared for a patient with IAD in the ICU and that clinical judgement rather than a formal IAD categorisation tool was used to assess the severity of IAD in the patient record, with 70% of RNs using products to prevent IAD. These findings highlight that while IAD is a common condition in the ICU, there remains variability in clinical practice in the ICU.
In this study, the RN recall of the frequency of IAD in ICU settings was 10.4%. This finding falls outside the IAD prevalence range of 32–95% in ICU patients reported by studies using direct observation data6,17,19,20. A possible explanation for the difference may be recall bias, which can be found in self-reported questionnaires when participants provide responses depending on their ability to recall past events. Recall bias can often underestimate the occurrence of the phenomenon under investigation25.
IAD documentation
This study found that most RNs reported that clinical documentation of IAD severity was recorded in free text form. The use of a special section in other assessment tools or charts suggested that participants in some ICUs (28.8%, n=32) were already aware that IAD is a skin problem that differs from other types of skin injury.
The lower perception regarding the magnitude of the IAD problem found in this study may be attributable to documentation. Historically, a number of tools exist for the categorisation and scoring of IAD severity, for example, the Identifying Incontinence-Associated Dermatitis and its Severity tool26, and Incontinence-Associated Dermatitis Intervention tool27. These tools have mostly been applied in research studies with no evidence found to suggest that they have been applied in the general clinical setting. Recently, an international consensus document provided a simple IAD Severity Categorisation Tool and management flow chart3. The lack of clear documentation pathways may contribute to RNs’ miscalculation or misperception of the number of patients with IAD.
In this study, RNs reported that the most common frequency for patients' general bed-baths was at least once a day. This reported frequency is consistent with recent global studies6,17,28. A well-delivered bed-bath maintains body hygiene and skin moisture balance29. Dry skin could increase the risk of PI development through an increase in friction against the skin30; while overhydration from excessive moisture, such as incontinence, wound exudate, or perspiration, could lead to all types of moisture-associated skin damage29. The application of a skin protectant or moisture barrier after the general bed-bath can further reduce the risk of IAD by avoiding or reducing skin exposure to moisture and irritants3.
Participants in this study reported the use of various products to deliver bed-baths including pre-packaged cloths and a basin, water, cleanser and soft cloth. This result is similar to Peterson et al.20 who reported water, wipes or cloths and a pH-balanced cleanser were the most commonly used products (all above 55%). Variations in product combinations were also found in most of the reviewed studies20.
Cleansing removes the irritants found in urine and faeces from the skin3. This study found participants reported the choice of product for incontinence cleansing episodes was similar to the products used in the general bed-bath. Products for incontinence clean-up used in other studies in the ICU were water and pH-balanced cleansers6,16,17, wash cloths with 3% dimethicone21,22, or wipes10,18. Soap and water were also used by some participants (n=21, 18.9%) in this study, and were also found to be the most common products to clean up after patients had an episode of incontinence in long-term care and acute care settings31,32.
The application of protection products after the bed-bath and incontinence clean-up can avoid or minimise skin exposure to incontinence substances3. In this study, most participants routinely applied a skin protectant (70%, n=77) and moisturiser (64%, n=71) after cleansing the patients' skin. Bliss et al.9 found that applied moisturiser or use of a cleanser containing moisturiser could reduce the development of IAD. More than half (n=62, 55.8%) of the participants in the current study reported that they used cream as a skin protectant, while some (n=37, 33%) reported they also used paste and lotion to protect their patients’ skin. The active ingredients in these products can significantly vary the protection provided33.
Wipes or disposable washcloths have been reported as being used to prevent IAD in ICUs in many studies10,21,22. A washcloth impregnated with 3% dimethicone has been found to be an efficient way to reduce the prevalence of IAD21,22,34. RNs in the current study reported that wipes were more commonly used for general bed-bathing (n=68, 61.2%) and incontinence clean-ups (n=73, 65.7%) than for skin protection (n=23, 20.7%). A possible explanation is that a washcloth with 3% dimethicone is a more expensive product than plain wipes. However, the general term “wipe”, which could mean a common disposable wipe or a specialised product such as a 3 in 1 wipe or wipe impregnated with 3% dimethicone, was used in the survey. Therefore, it was not possible to determine the exact product types participants used in their ICU.
Many RNs in the current study (n=45, 40.5%) also noted that they used a preventative product in the form of a spray as a skin protectant. This product has been shown to be effective in preventing IAD in the long-term care setting5. Some ICU RNs have also recently begun to apply sprays in IAD care bundles6,17; most respondents in the current study worked in private hospitals. The significant difference in application of this product in public and private hospitals could be due to the cost. Brunner et al.35 suggested that wipes are more cost-effective than sprays in acute and critical settings. However, decision-making about product choice is also influenced by ease of use (for example, a one-step product compared to multiple applications), clinical effectiveness and environmental impact (spray products generate less waste)35.
In the current study, almost half of the participants (n=55, 49.5%) noted that they used a pad, under pad, or Kylie absorbent pad between the patient and the sheet. An incontinence pad can absorb urine and water in faecal matter and thus reduce the skin’s exposure to moisture3. These products are used to manage urinary incontinence in bed-bound patients36,37. However, some studies have identified adverse effects when pads are used constantly, for example, skin protectants applied to the patient’s skin may transfer from the skin to the pads38 and creases and folds generate pressure on the skin and could contribute to PI development35.
The Moving Prevention Forward IAD guideline3 suggests that clinicians treat the cause of IAD, which in the intensive care environment is diarrhoea. A bowel management protocol in the ICU can help clinicians to manage patients’ bowel movements and maintain the bowel function with evidence-based, consistent practice39,40, thus reducing diarrhoea, and, subsequently, IAD. Studies have suggested that the implementation of a bowel management protocol in an ICU could reduce the incidence and duration of constipation and diarrhoea39,41. However, there is a lack of an international guideline for bowel management. Consequently, a number of studies abound that report the implementation of local bowel management protocols or guidelines in single-site ICUs. All of these studies reported the effective reduction of diarrhoea incidence or prevalence with the use of a protocol or guideline39,40,42. The results of the current study support these findings, with 88% (n=98) of participants reporting that their ICU used a bowel management protocol. This rate is higher than a previously reported survey in Europe, where 50% of ICU identified use of a guideline or protocol for bowel management43.
Knowles et al.44 found that a targeted protocol could help clinical staff to change their attitude towards evidence-based practice. Furthermore, Kortteisto et al.45 indicated that health professionals usually intend to follow a guideline in patient care; therefore, a formal protocol or guideline can improve the application of specific evidence-based practices. Thus, an IAD management protocol in the ICU should improve evidence-based practice of IAD prevention and management. However, despite the high rate of local site-specific bowel management protocols in ICUs generally, as reported in this study, and an international guideline for IAD prevention and management3, most ICUs in this study did not have a policy or guideline for IAD prevention and management.
Limitations
The sample might not accurately represent the total population of critical care nurses in Australia, as it excluded critical care nurses who were not members of ACCCN, potentially limiting the generalisability of the findings. However, members of ACCCN are a unique group; for example, membership suggests they are more likely to be interested in professional issues and education than non-members. The low response rate is acknowledged as a limitation; however, this situation is typical for electronic surveys conducted in organisations with no follow-up and no incentives46. A meta-analysis by Manfreda et al.47 also indicated that electronic surveys have an 11% lower response rate than paper-based surveys. Finally, general terms, such as "soap", "wipe", and "skin protectant", were used in this survey. As these terms were not defined it is impossible to know the exact type of products used.
Conclusion and recommendation
This study found that the RN reported frequency of IAD in ICUs in Australia was 10.4%. Clinical practices to prevent and manage IAD were the same and included applying a variety of products for skin protection at the time of the patient’s general bed-bath and episodes of incontinence clean-up. Formal IAD management protocols for the ICU were rarely reported by participants. IAD care should be guided by evidence-based best practice recommendations, such as those set forth by Beeckman et al.3. Further research is warranted to understand clinical practice regarding IAD prevention and management in the ICU setting.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Yi-Chen Lee
RN, BN, Master of Nursing
School of Nursing, Queensland University of Technology, QLD, Australia
Jill Campbell
RN, Grad Cert Intensive Care Nursing,
BHealthSc (Nursing), Grad Dip (Wound Care), PhD
Clinical Nurse, Skin Integrity Services,
Royal Brisbane and Women’s Hospital
Visiting Fellow, School of Nursing,
Queensland University of Technology
QLD, Australia
Anna Doubrovsky
BSc(Hons), MPH
Research Assistant, School of Nursing,
Queensland University of Technology
QLD, Australia
Adele McGarry
Research Assistant, School of Nursing,
Faculty of Health, Queensland University of Technology, QLD, Australia
Fiona Coyer*
RN, MSc (Nursing), PGCEA, PhD
Professor of Nursing, joint appointment Intensive Care Services, Royal Brisbane and Women’s Hospital and School of Nursing, Queensland University of Technology, QLD, Australia
Visiting Professor, Institute of Skin Integrity and Infection Prevention, University of Huddersfield, UK
Email f.coyer@qut.edu.au
* Corresponding author
References
- Gray M, Bliss DZ, Doughty DB et al. Incontinence-associated dermatitis: A consensus. J Wound Ostomy Continence Nurs 2007;34:45–56.
- Bryant RA, Nix DP (eds). Acute and chronic wounds: Current management concepts. 5th edn. St Louis: Elsevier Mosby, 2016.
- Beeckman D. Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: Moving prevention forward. Wounds International. 2015. Downloaded 20/3/2018 www.woundsinternational.com
- Campbell JL, Coyer FM, Osborne SR. Incontinence-associated dermatitis: A cross-sectional prevalence study in the Australian acute care hospital setting: IAD — Acute care prevalence. Int Wound J 2016;13:403–411.
- Bliss DZ, Zehrer C, Savik K, Smith G, Hedblom E. An economic evaluation of four skin damage prevention regimens in nursing home residents with incontinence: Economics of skin damage prevention. J Wound Ostomy Continence Nurs 2007;34:143–152.
- Lin TR, Hwang MR, Nien HH, Liu CC, Shie PS, Chen SH. Reducing the incidence of incontinence-associated dermatitis in intensive care unit diarrhea patient. J Nurs 2015;60:81–89.
- Ma ZZ, Song JY, Wang M. Investigation and analysis on occurrence of incontinence-associated dermatitis of ICU patients with fecal incontinence. Int J Clin Exp Med 2014;10:7443–7449.
- Park K. The effect of a silicone border foam dressing for prevention of pressure ulcers and incontinence-associated dermatitis in intensive care unit patients. J Wound Ostomy Continence Nurs 2014;41;424–429.
- Bliss DZ, Savik K, Thorson MAL, Ehman SJ, Lebak K, Beilman G. Incontinence-associated dermatitis in critically ill adults: Time to development, severity, and risk factors. J Wound Ostomy Continence Nurs 2011;38:433–445.
- Park K, Kim K. Effect of a structured skin care regimen on patients with fecal incontinence A comparison cohort study. J Wound Ostomy Continence Nurs 2014;41:161–167.
- Kottner J, Blume-Peytavi U, Lohrmann C, Halfens R. Associations between individual characteristics and incontinence-associated dermatitis: A secondary data analysis of a multi-centre prevalence study. Int J Nurs Stud 2014;51:1373–1380.
- Binks R, De Luca E, Dierkes C, Franci A, Herrero E, Niederalt G. Prevalence, clinical consequences and management of acute faecal incontinence with diarrhoea in the ICU: The FIRST™ observational study. J Intensive Care Soc 2015;16:294–301.
- Bliss D, Mathiason M, Gurvich O. Incidence and predictors of incontinence-associated skin damage in nursing home residents with new-onset incontinence. J Wound Ostomy Continence Nurs 2017;44:165–171.
- Hill L, Kidson S, Michell W. Corticotropin-releasing factor: A possible key to gut dysfunction in the critically ill. Nutrition 2013;29(7–8):948–952.
- McCulloch K, Miranda NF, Lugowski M et al. Sepsis and pneumonia: Treatment and outcomes: Does pancreatic insufficiency in ICU patients recovering from septic shock manifest as diarrhea and malabsorption? Am J Respir Crit Care Med 2014;189:1.
- Conley P, McKinsey D, Ross O, Ramsey A, Feeback J. Does skin care frequency affect the severity of incontinence-associated dermatitis in critically ill patients? Nursing 2014;44:27–32.
- Coyer F, Gardner A, Doubrovsky A. An interventional skin care protocol (InSPiRE) to reduce incontinence-associated dermatitis in critically ill patients in the intensive care unit: A before and after study. Int Crit Care Nurs 2016;40:1–10.
- Zhou X, He Z, Chen Y, Zuo L. Effect of a 1-piece drainable pouch on incontinence-associated dermatitis in intensive care unit patients with fecal incontinence: A comparison cohort study. J Wound Ostomy Continence Nurs 2017;44;568–571.
- Ehman S, Thorson M, Lebak K, Bliss D, Savik K, Beilman G. Development of perineal dermatitis in critically ill adults with fecal incontinence. Am J Crit Care 2006;15:333.
- Peterson K, Bliss D, Nelson C, Savik K. Practices of nurses and nursing assistants in preventing incontinence dermatitis in acutely/critically ill patients. Am J Crit Care 2006;15:325.
- Driver DS. Perineal dermatitis in critical care patients. Crit Care Nurs 2007;27:42–46.
- Eigsti JE. Innovative solutions: Beds, baths, and bottoms: A quality improvement initiative to standardize use of beds, bathing techniques, and skin care in a general critical-care unit. Dim Crit Care Nurs 2011;301:69–176.
- Horner D, Bellamy M. Care bundles in intensive care. Cont Ed Anaes Crit Care Pain 2012;12(4):199–202.
- McClave JT, Sincich T. Statistics. 13th edn. New York: Pearson, 2018.
- Althubaiti A. Information bias in health research: Definition, pitfalls, and adjustment methods. J Multidiscip Healthcare 2016;9:211–217.
- Borchert Z, Bliss M, Savik M, Radosevich M. The incontinence-associated dermatitis and its severity instrument: development and validation. J Wound Ostomy Continence Nurs 2010;37:527–535.
- Junkin J. An incontinence assessment and intervention bedside tool (IADIT) assists in standardising the identification and management of incontinence-associated dermatitis. Poster presented at Wounds UK, Harrowgate, 2014.
- Avşar P, Karadağ A. Efficacy and cost-effectiveness analysis of evidence-based nursing interventions to maintain tissue integrity to prevent pressure ulcers and incontinence-associated dermatitis. Worldviews Evid Based Nurs 2017.
- Gray M, Black JM, Baharestani MM. Moisture-associated skin damage: Overview and pathophysiology. J Wound Ostomy Continence Nurs 2011;38:233–241.
- Lechner A, Lahmann N, Neumann K, Blume-Peytavi U, Kottner J. Dry skin and pressure ulcer risk: A multi-center cross-sectional prevalence study in German hospitals and nursing homes. Int J Nurs Stud 2017;73:63.
- Campbell J, Gosley S, Coleman K, Coyer F. Combining pressure injury and incontinence-associated dermatitis prevalence surveys: An effective protocol? Wound Prac Res 2016;24:170–177.
- Rönner R, Berland R, Runeman R, Kaijser R. The hygienic effectiveness of 2 different skin cleansing procedures. J Wound Ostomy Continence Nurs 2012;37:260–264.
- Mrdjenovich D, Fleck C. Consider skin hygiene and care beyond the wound. J Am Col Certif Wound Spec 2011;3:45–47.
- Beeckman D, Verhaeghe S, Defloor T, Schoonhoven L, Vanderwee K. A 3-in-1 perineal care washcloth impregnated with dimethicone 3% versus water and pH neutral soap to prevent and treat incontinence-associated dermatitis: a randomized, controlled clinical trial. J Wound Ostomy Continence Nurs 2011;38:627–634.
- Brunner M, Droegemueller C, Rivers S, Deuser W. Prevention of incontinence-related skin breakdown for acute and critical care patients: comparison of two products. Urol Nurs 2012;32:214.
- Fader M, Bain D, Cottenden A. Effects of absorbent incontinence pads on pressure management mattresses. J Adv Nurs 2004;48:569–574.
- Omli R, Skotnes L, Romild U, Bakke A, Mykletun A, Kuhry E. Pad per day usage, urinary incontinence and urinary tract infections in nursing home residents. Age Ageing 2010;39:549–554.
- Hart J. Assessment of the incontinence pad blocking potential of 3M™ Cavilon™ durable barrier cream compared with Sudocrem™ and zinc and castor oil. Nursing Scotland. 2002; 15. Accessed 7/5/2018 http://tinyurl.com/zufqje3
- Knowles S, McInnes E, Elliott D, Hardy J, Middleton S. Evaluation of the implementation of a bowel management protocol in intensive care: Effect on clinician practices and patient outcomes. J Clin Nurs 2014;23:716–730.
- McKenna S, Wallis M, Brannelly A, Cawood J. The nursing management of diarrhoea and constipation before and after the implementation of a bowel management protocol. Aust Crit Care 2001;14:10–16.
- McPeake J, Gilmour H, Macintosh G. The implementation of a bowel management protocol in an adult intensive care unit. Nurs Crit Care 2011;16:235–242.
- Ring M. Implementation of a bowel care protocol within ICU. Aust Crit Care 2012;24:73–74.
- Bayon Garcia C, Binks R, DeLuca E et al. Prevalence, management and clinical challenges associated with acute faecal incontinence in the ICU and critical care settings: The FIRST™ cross-sectional descriptive survey. Int Crit Care Nurs 2012;28:242–250.
- Knowles S, Lam L, McInnes E, Elliott D, Hardy J, Middleton S. Knowledge, attitudes, beliefs and behaviour intentions for three bowel management practices in intensive care: effects of a targeted protocol implementation for nursing and medical staff. BMC Nurs 2015;14:6.
- Kortteisto T, Kaila M, Komulainen J, Mantyranta T, Rissanen P. Healthcare professionals’ intentions to use clinical guidelines: a survey using the theory of planned behaviour. Implement Sci 2010;5:51.
- Magro M, Prybutok V, Ryan S. How survey administration can affect response in electronic surveys. Quality Quantity 2015;49:2145–2154.
- Manfreda K, Bosnjak M, Berzelak J, Haas I, Vehovar V. Web surveys versus other survey modes: A meta-analysis comparing response rates. Int J Market Res 2008;50:79.



