Volume 27 Number 2
Methods for chronic wound research - a scoping systematic review of the recommendations, guidelines and standards
CN Parker, A Francis and KJ Finlayson
Keywords chronic wound, Wound management research methods, guidelines, recommendations.
For referencing Parker CN et al. Methods for chronic wound research — A scoping systematic review of the recommendations, guidelines and standards. WP&R Journal 2019; 27(2):62-73.
DOI https://doi.org/10.33235/wpr.27.2.0001
Abstract
Background This scoping systematic review aimed to investigate the existing literature for recommendations, guidelines and standards for research on chronic wound diagnosis, assessment, management and prevention; to identify gaps in this literature; and produce recommendations to support future wound management research.
Methods A scoping systematic literature review was undertaken in 2017–2018, which aligned with PRISMA guidelines and searched academic databases and grey literature published between 2007 and 2017.
Results Eighty-nine documents included recommendations or outcomes on research methods for studies on chronic wound diagnosis, assessment, management and/or prevention; covering the areas of research design, sampling, randomisation and blinding, independent and outcome measures and interventions for research in chronic wounds. Common themes regarding research gaps and flaws were identified.
Conclusion This review identified existing evidence, guidelines, recommendations and standards regarding the conduct of chronic wound research internationally. Recommendations include the need for standardised vocabulary, standardised checklists for wound research, development of core outcome datasets and an agreed and standardised set of economic parameters and methodology for cost-effectiveness. Establishment of a centralised national methodology service for wound research to assist with methodology design would be beneficial.
Introduction
Chronic wounds do not proceed through an orderly or timely reparative process, instead having a delayed, arrested or repetitive cycle of the phases of wound healing, with progress ceasing or slow1-3. These wounds are costly, with up to 4% of the total health care expenditure consumed on chronic wound care in Western countries4,5. In Australia, this equates to up to A$5 billion annually6. These wounds can be debilitating and often result in a decreased quality of life7,8.
The health care challenges associated with chronic wounds has seen increasing numbers of health professionals completing studies in this area. However, rigorous, high-quality evidence outcomes from research can be difficult to achieve. While some organisations have published guidelines, consensus documents and protocols addressing research methods in the areas of wound assessment, management and prevention, studies or recommendations on research methods are rarely reported in the literature, usually occurring as an add-on to other research projects. Previous articles concluded that guidelines were needed for research methodologies for chronic wounds9,10, as inconsistencies in study protocols, designs and themes, along with inadequate reporting often limit recommendations in relation to research outcomes.
This study aimed to review the literature on current evaluations, recommendations, guidelines and standards for research on chronic wound diagnosis, assessment, management and prevention; and identify gaps in this literature to guide recommendations on development of standards for wound management research to assist clinicians, researchers, academics, industry and policy makers.
Materials and methods
Search strategy
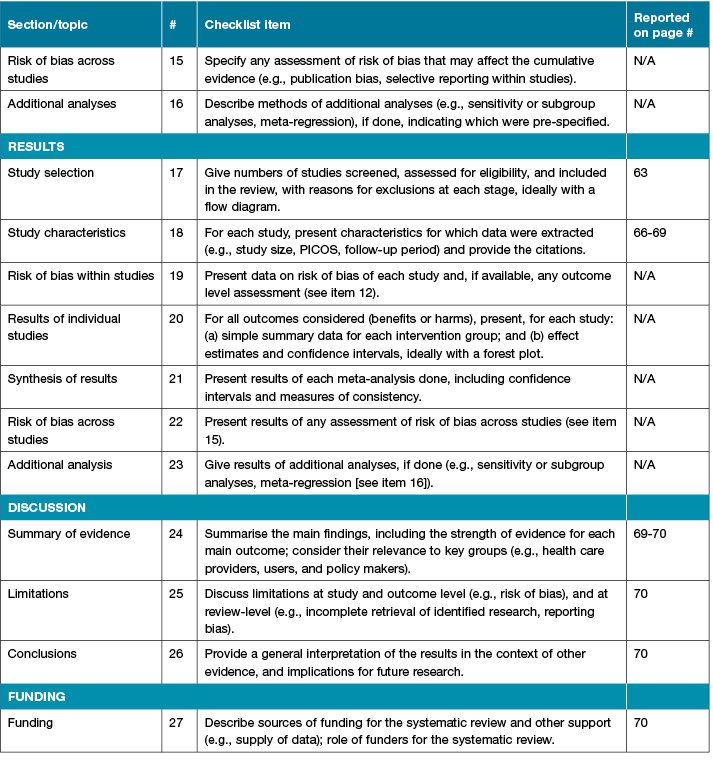
This descriptive scoping systematic review is reported in accordance with preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines11. For further information, see Table 1. Academic databases searched included: CINAHL, Medline, Embase, Joanna Briggs Institute Library (JBI), and the Cochrane Library. Grey literature sources searched included websites and publications of the Association for the Advancement of Wound Care (AAWC); Wounds Australia (formerly Australian Wound Management Association (AWMA)); European Pressure Ulcer Advisory Panel (EPUAP); European Wound Management Association (EWMA); International Compression Club (ICC); International Diabetes Federation (IDF); National Guideline Clearinghouse; ethics-related publications and guidelines; National Health and Medical Research Council (NHMRC) guidelines; National Institute for Health and Care Excellence (NICE); Registered Nurses Association of Ontario (RNAO); Scottish Intercollegiate Guidelines Network (SIGN); Wounds Canada; Wounds International; World Union of Wound Healing Societies (WUWHS), Wounds UK and the Welsh Wound Innovation Centre (WWIC).
Table 1: PRISMA checklist

Table 1 continued: PRISMA checklist
Selection criteria
Search terms were refined to: (guide* OR method* OR consensus* OR position OR best practice OR protocol* OR recommend*) AND (research OR stud* OR investigat*) AND (wound* OR ulcer* OR tear OR pressure) AND [(chronic) NOT (pulmonary) NOT (respiratory) NOT (renal) NOT (kidney) NOT (gastrointestinal)].
Inclusion criteria for documents included:
- published documents from 2007 to July 2017;
- published in English;
- document types included guides, guidelines, method studies or papers, consensus statements, recommendations, position statements, systematic reviews or grey literature;
- documents pertaining to or including recommendations on, or evaluations of, research methods for diagnosis, assessment, management or prevention of chronic wounds.
Exclusion criteria were individual studies not meeting the inclusion criteria, and those not providing data and/or evaluation on methods for chronic wound management research.
Data extraction
Results from searches of the databases were imported into Endnote libraries and stored on a secure access drive. Two reviewers independently extracted and assessed all documents for eligibility. A third reviewer arbitrated documents where there was disagreement. This process was followed for the screening phase, abstract eligibility assessment phase, and final full-text assessment for inclusion in the literature review.
Data analysis
Due to the lack of quantitative research studies evaluating research methods in chronic wound research, a narrative synthesis was undertaken of the reported outcomes and recommendations in the articles found from the search strategy.
Results
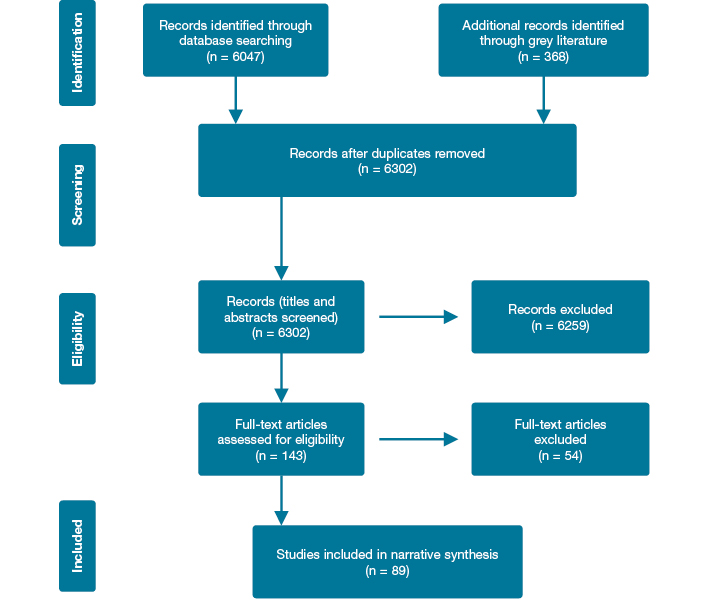
The initial database and grey literature searches resulted in 6415 articles. After excluding duplicates and articles not fitting the inclusion criteria, 89 eligible articles remained (Figure 1).
Figure 1: Flowchart of literature review
The majority of these articles were systematic/scoping reviews (n=58) and literature/narrative reviews (n=8) on various chronic wound topics, which included recommendations for research methodology. The remaining articles included recommendations based on expert opinion (n=12); clinical practice/evidence-based guidelines (n=8), and descriptive, prospective or survey studies (n=3). These studies are summarised in supplementary Table 2, which can be found in the electronic version of this article at https://doi.org/10.33235/wpr.27.2.62-73.
Only a few of the included articles specifically evaluated any methods for wound research. Two articles reviewed clinical data collection and analysis methods of wound trials12,13, and another aimed to provide specific recommendations to product developers and clinical researchers on the design of comparative effectiveness studies for the treatment of chronic wounds14. Liu et al. analysed the nature and specification of outcomes in Cochrane systematic reviews related to wound care15, while Jeffcoate et al. summarised the core details required in the planning and reporting of intervention studies for diabetic foot ulcers16. Consequently, the findings of this review comprise a summary of expert opinion based on systematic reviews and expert consensus, which were often secondary discussions from studies.
The results have been grouped and reported under the methodology sections of research design; sampling; randomisation and blinding; independent and outcome measures; interventions and analysis for research in chronic wounds.
Results
Findings on research design
Forty-three articles included recommendations on research design, covering study development and overall design. These articles included 35 systematic/literature/narrative reviews, seven expert opinion/consensus documents and one prospective study. The literature identified a gap in high-quality evidence from well-designed and rigorous, prospective studies17,18 or randomised controlled trials (RCTs)14,19-28, with poorly designed studies leading to the inability to pool study results and conduct meta-analysis29. The use of a CONSORT statement has been recommended in multiple articles to improve reporting of controlled trials22,24,26,30-42, to show valid and reliable results30,43,44 and to allow trials to be accurately assessed by readers and reviewers21,36.
A study design should commence with a clearly defined research question/s and hypotheses based on a thorough literature search using patient problem, intervention, comparison and outcome (PICO) and basing studies on a conceptual framework that is clearly defined45. Two systematic reviews concluded that it was essential that research questions be developed that are of a high priority to patients and other decision makers, and collaboration of stakeholders is needed to be able to answer the questions most relevant to patient care46,47.
Any RCTs should include a suite of uniform methodologies18,48, treatment comparisons and outcomes49 and process evaluations50,51 in order to establish the efficacy of commonly used therapies/interventions on chronic wounds49. It is essential that research be conducted by independent researchers and reporting be free from suggestion that the analysis or conclusions were substantially influenced by people with commercial or other personal interests in the findings16,26.
Management in the control group should be what is expected in routine clinical practice16, with the standard-of-care arm receiving widely accepted care that follows high-quality, evidence-based clinical guidelines14,39. Data should be collected in a uniform manner to capture the scale of the problem52 using specific tools, if appropriate53. Basic care, conventional care or standard care must be defined and standardised12,45,54 and specific details reported as this is likely to vary — particularly in dressing type, debridement type, frequency and intensity, and follow-up54,55; and all interventions should use the same models/versions of devices for all patients14,45 to ensure replicability16.
Six systematic reviews and one expert opinion document13,14,32,38,39,42,56 recommend a need for multicentre studies across a range of settings, as single-centre studies may be challenged to recruit sufficient participants for studies researching specific areas35.
Findings on sample considerations
Thirty-eight studies included some recommendations on sample considerations such as sample sizes and inclusion/exclusion criteria. These included 33 systematic/scoping/literature reviews, four expert opinion/consensus documents and one clinical practice guideline. The recommendations include the need for research studies to include analysis of power size and calculation of sample size22,25,41,45,57. Trials should estimate and have adequate, appropriate and large enough sample sizes32,43,44,58-61. They should be appropriately and adequately powered for clinically important primary outcome measures in order to detect significant treatment effects that are able to detect differences in wound healing rates, where sample size estimates10,19-21,31,33,34,36,39,40,62-68 should be based on a priori sample size calculation16,35,38,62. An RCT sample size should be large enough to be able to detect both a statistically significant effect and to allow for subgroup analyses23. Trials need clear inclusion and exclusion criteria for participants43,44,62, particularly with reference to baseline infection and the definitions of criteria and procedures for subject withdrawal or discontinuation45,68.
There must be consideration of the extent to which a recruited population is likely to represent patients seen in clinical practice, for example with respect to mobility, ulcer size and presence of ulcer infection14,24,31,40,55. It is recommended to clearly report baseline participant characteristics including defining the setting and location of data collection, patient characteristics, number of patients, time lines, allocation ratio of patients to the groups being compared, the procedures for diagnosing different wound types and the stage of the wound(s)16,30,33,34,45. There should be comparable groups at baseline (that is, stratification for ulcer size and duration)21,63, where the comparators used in the clinical study are clearly characterised36,41,68,69.
Findings on randomisation and blinding
Thirty-two studies included recommendations for randomisation and blinding, which comprised 25 systematic/literature reviews, four expert opinion/consensus documents and three clinical practice guidelines. The majority of these documents recommend that RCTs should employ robust valid methods of randomisation and include random sequence generation, treatment allocation and concealment of allocation of procedures (treatment) to minimise the risk of bias10,16,21,24,31,35,40,43,44,57,58,63,68,70,71. Studies should match ulcer characteristics, participant characteristics and the various interventions for appropriate randomisation, adjustment and stratification23. This could include computer-generated randomisation programmes with allocation concealment, for example, by using a remote, telephone randomisation service38,62. Clear reporting of the methods of randomisation is also essential to help ensure comparability of treatment groups at baseline45,62.
Trials should apply concealed allocation strategies, with effective blinding of participants (to study groups and outcome measures), personnel (where assessors should be blinded to treatment allocation), the data analyser and outcome assessment or measures; and these methods of blinding should be clearly reported10,14,16,21,22,24,25,30,32,35-37,40,43,45,46,57,60,62,63,72,73. While blinding in some studies is difficult and would clearly be unethical, one of the main weaknesses reported for many RCTs was a lack of blinding74. With adequate resources, blinding is often possible and is recommended39.
Findings on independent and dependent variable measures
Fifty-three studies included recommendations regarding independent and dependent variable measures. These included 44 systematic, narrative/literature or scoping reviews, six expert opinion/consensus documents and three clinical practice guidelines.
Importantly, there should be agreement and definition of which outcome measures should be used in studies at the outset of a study75, including a rationale for why the outcome measures were selected72. A 2017 systematic review of outcomes in wound care studies recommended development of core outcome data sets (as in other health care fields), as this would enable examination and comparison of the effectiveness of different clinical interventions based on a core set of outcomes15. Outcome measures should be objective, consistent, clinically relevant and standardised in terms of what is included and how these are measured12,16,32,46,68,73,76-78, using pre-defined, widely accepted criteria or definitions for measurement12,79 that matter to patients, carers and health professionals47,67. Development should involve a consensus process to define outcome measures, be data-driven, iterative, and prepared by expert working groups, including patients, wound specialists, health professionals, trialists, methodologists, scientists from industry, health economists and regulators15. This will facilitate comparison of results57,72.
It is important to define both primary and secondary outcome measure(s)45. Such variables are likely to include: incidence, time to complete wound healing, ulcer-free survival following treatment, healing rates and ulcer area, time to ulcer closure, percentage and absolute change in wound size (surface area or volume), quality of life measures, mortality, health resource utilisation and cost of treatment, pain, acceptability, cosmetic outcomes, patient comfort, accessibility of interventions, satisfaction, ulcer recurrence rates and adverse events, such as infection26,30,35,36,39,46,58,59,64,77,80,81.
Different core outcome sets will be needed for different wound types15. For example, identification of the time of surgical wound healing is difficult, hence it may be more relevant to count surgical wound healing problems (such as infection or dehiscence)15. Different wounds should be considered different entities as the aetiology and cause of the injury are different37, 74 and for each patient, a single reference ulcer should be selected for a study38. Trials that combine different types of conditions (acute, sub-acute and chronic) should present results of each condition group separately27.
Outcomes should be tested for effectiveness and impact82 — including parameters such as individual self-management strategies83. Clinically relevant endpoints (such as ulcer healing or amputations) may mean more in practice but may be only partially dependent on any effect of the chosen intervention84. Conversely, surrogate endpoints (such as change in wound bed appearance or ulcer area) may more closely relate to the effect of the product being tested but have little relevance to clinical outcomes84. It is important that studies are designed to include meaningful endpoints, even in participants where wound healing is never achieved73, and that alternative endpoints to healing are evaluated as being equally suitable for the evaluation of various wound interventions12,85.
Trial protocols need to address outcome measure heterogeneity — an issue in the absence of agreement about key outcomes65. Reporting outcomes is often very heterogeneous, with some trials reporting mean or median time for complete wound closure; however, few used relative risk or odds ratio, survival analysis, or reported hazard ratios31,73.
There is a need for clinical outcomes and health-related quality of life (HRQoL) that should be considered in clinical trials as routine alongside clinical and economic criteria86 to be reported using validated assessment tools59,70. Instruments that measure the generic factors of HRQoL, as well as the disease-specific domains should be used62,86. HRQoL assessment should be undertaken using a standardised, valid and reliable assessment instrument with findings reported in full24,31,37,40,46 — and should include patient satisfaction of any intervention28.
Outcome measures to investigate the subset of disease severity/classification most likely to benefit from the therapy/intervention and the expected duration of benefits should be included and reported25. Trials should analyse particular participant subgroups to ascertain potential differing responses and, if efficacy is demonstrated, to establish the point in a treatment regimen at which it should be applied36. Assessment of subgroup effects is important because it is likely that treatment effects will vary across the spectrum of patients13.
Trials must include well-defined, long-term adequate follow-up for all participants10,42,44,56,64,66,81, be of sufficient duration to capture a meaningful proportion or effect of events and/or interventions21,38, be able to ascertain the completeness and durability of ulcer healing58 and should include detailed clinical assessment to detect the true rate of outcomes such as recurrence42. While there is little agreement in the literature regarding follow-up periods and they are likely to be different for different wounds, a recent review found that most studies used only a short follow-up period (that is, 14 days), and therefore concluded that longer follow-up periods were needed59. Long-term follow-up is needed to provide evidence on ulcer recurrence and the occurrence of lower limb amputations73 and this includes long-term follow-up examinations after completion of the studies and reporting of this data60. A range of trial durations have been suggested including: 20 weeks to allow for comparisons to be made across trials, and provide a more robust evaluation of the benefits and harms of interventions35, at least six months ‘was essential’ for any wound healing effect to be detectable in chronic wounds46, and at least 30 days postoperatively was required to ensure all complications of surgical wounds were reported57.
Findings on interventions
Eight studies included some recommendations on intervention considerations in wound research. These included six systematic reviews and two expert opinion/consensus documents.
The intervention must be described and accurately and appropriately reported in sufficient detail to allow replication of studies, and include: the rationale behind the intervention, details of the intervention (for example, compression), prognostic factors, administration, treatment regimen, other components of treatment, rationale for control or comparator, description of control or comparator, setting and context of the intervention, and the background/qualifications and training of the responsible clinicians46,69. Practical clinical information, such as the location of wounds, frequency of treatment application, combination or sequencing and duration of treatment modalities, must be documented; and compliance must be reported36,39,55,58,87.
Findings on analysis
Databases and analysis
Twenty-four studies included some recommendations on analysis considerations in relation to databases. These included 22 systematic or literature reviews, one expert opinion/consensus document and one clinical practice guideline. Appropriate statistical analysis16,57,58 and reporting all patients’ flow through studies, analysis of losses to follow-up and missing data58 is recommended. Where participants have been lost to follow-up, appropriate and valid methods of imputation should be used and reported with the patient the unit of randomisation and analysis, rather than individual wounds31,37. For consistency of multicentre trials, standards are required to standardise analysis of results between sites58.
Standards are required for statistical methods to account for confounding, effect modification and clustering of patients58,88. Control for confounding may occur by restricting the study population, using pre-stratification (for example, aetiology), and statistical adjustment13. Multiple ulcers on a patient should not be randomised individually and considered independent unless the trial has been specifically designed to accommodate this, and appropriate statistical analysis, that accounts for clustering, should be specified38.
Intention-to-treat and time-to-event principle analysis should be adopted in order to minimise bias10,16,21,24,31,35-38,40,43,58,62,63,65,66 and have a pre-determined method for dealing with missing data to minimise the potential for attrition bias65. Where trials are measuring a time-to-event outcome such as time to healing, they should employ survival analysis37,38,40-42 or approaches which account for censoring37, with adjustment for prognostic covariates such as ulcer area and duration24,31,41.
A National Wounds Registry may aid research by identifying the scope of the wound burden, benchmarking healing, and aiding cost-effectiveness analyses10,49,89. There is a need to register all trials with a register that meets World Health Organization (WHO) criteria and principal investigators should keep their contact details up to date on the register31,37. Outcomes of a trial should be prospectively declared in a clinical trial database, including the nature and timing of the primary outcome65.
Analysis of costs
Thirty studies included some recommendations on analysis considerations in relation to costs. These included 18 systematic or literature reviews, four clinical practice guidelines, five expert opinion/consensus documents and three prospective/survey/descriptive studies. It is agreed across a breadth of literature that health economic studies are essential for future research56,75,82, including a need for objective evidence on the costs and benefits of evidence-based wound management86,89 and for an economic case to be developed for wound care and wound care services90 taking a holistic approach61,91. Wound care as a clinical area suffers from a paucity of robust economic data and, therefore, a true understanding of the costs52. Trials should include and report full clear and meaningful economic evaluations and cost–effectiveness analysis so that health care providers can make informed decisions about which technique is more efficient and cost-effective24,25,27,31,40-44,57,68,80,85,92-95. Studies should strive to calculate a disease-specific cost or net cost using a matched non-disease cohort96 including measurement of the costs of alternative treatments and assessment and reporting of the cost-effectiveness37 of interventions25,70,71. Standardising methods for cost-of-illness studies in general will allow researchers and policy makers to establish and understand the importance of chronic ulcers in comparison with other diseases and may encourage research and policy initiatives for the prevention and treatment of chronic wounds96.
Discussion
Common themes regarding research gaps and flaws have been identified in the areas of research design, sampling, randomisation and blinding, independent and outcome measures and interventions. In general, there is a need to improve the quality of evidence from wound care research, formulate guidelines within differing areas and investigate different types of evidence that might be required by different authorities12. Due to variation of definitions in terms related to wound care and wound healing across the literature there is also a need for the language to be standardised in a way that allows for comparison of studies. Chronic, delayed healing, non-healing, standard care, basic care and conventional care are all examples of varying terminology used interchangeably across the range of studies; however, these are not always with identical meanings.
There is a need for greater inclusion of translation and implementation methods to ensure the issues related to chronic wound research are addressed. Outside dedicated trial settings, there is also a need to ascertain and clearly document how to translate best wound care practices into all clinical (that is, ‘typical’) settings where therapies are delivered by ‘typical’ clinicians10. Examining system factors will promote or support delivery of best practice recommendations and could include the use of facility-wide protocols that guide the delivery of chronic wound care interventions41.
A national approach to the issue of wound care research is essential for improving the quality of evidence in relation to assessment, management and prevention of wounds. An evidence-based wound assessment minimum data set may reduce unwarranted variation in chronic wound care90 and assist in the highest priorities for research, which have been noted to in the literature to include areas such as debridement, risk assessment, diabetic foot problems, nutrition and pressure redistribution97-99.
Inconsistencies in study protocols, language/definitions, designs and themes, along with inadequate reporting, often limit recommendations in relation to wound care research. This systematic literature review has informed a number of high-priority areas that are likely to improve the quality, usefulness and value of future wound care research.
Limitations
While 89 articles from the literature review met the broad inclusion criteria, there were only a few that were specifically related to methods of research in assessment, management and prevention of chronic wounds. The majority of information was scattered across the literature and often difficult to find. Examples include a single sentence on a recommendation for future research methods at the end of an RCT. Hence, there was no one document that contained a comprehensive list of all the requirements for quality research in this area.
The various websites with relevant grey literature proved difficult to reliably navigate and contained numerous cross-references to other relevant bodies and associations. Some documents were readily and freely available; others required registration but were free, while some sites would not permit access to documents that appeared relevant. Some of these documents were available via formal academic libraries and publications.
Conclusion and recommendations
Recommendations to address the issue of chronic wound research include the establishment of working groups and a consensus process for production and publication of consensus documents pertinent to research on chronic wound diagnosis, assessment, management and prevention. These should include:
- Standardised vocabulary and definitions for chronic wound research criteria.
- A standardised checklist for chronic wound research and the standardised reporting of trials.
- Development of core outcome data sets for all chronic wound research.
- Development of an agreed and standardised set of economic parameters and appropriate and applicable economic evaluations/tools/methods that can be incorporated into all chronic wound research studies.
- Establishment and development of a centralised methodology service for chronic wound research to provide guidance and assistance with methodology design and review, appropriate power analysis and a remote randomisaton, blinding, and blinded outcome assessment service.
The robustness, usefulness and value of wound care research will be greatly enhanced if results of study/trials are published according to standardised, tailored wound research methodology checklists using standardised definitions, outcome measures and economic analysis measures. The outcomes of future wound research will then have more complete, consistent and uniform elements/components. This will allow for more robust and meaningful pooling of data/studies and therefore higher quality evidence from systematic reviews and/or meta-analyses, which ultimately will deliver better clinical care and improved patient outcomes.
Ultimately, it is impossible to be confident that 100% of potentially relevant grey literature information has been found, despite extensive efforts to do so. Ultimately, a lot of useful information is already available relating to wound care research; however, it is difficult to reliably find and access and there is no single simple, central, well-organised repository for the information that already exists.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
This publication was supported by a grant from Wounds Australia.
Supplementary table
Supplementary Table 2 can be accessed in the electronic version of this article at https://doi.org/10.33235/wpr.27.2.62-73.
Author(s)
CN Parker*
PhD
Faculty of Health, Institute of Health and Biomedical Innovation
Queensland University of Technology
Kelvin Grove, QLD, Australia
Email christina.parker@qut.edu.au
A Francis
MBBS (Hons) FRCPA
Faculty of Health, Institute of Health and Biomedical Innovation
Queensland University of Technology
Kelvin Grove, QLD, Australia
Faculty of Medicine
The University of Queensland
Brisbane, QLD, Australia
KJ Finlayson
PhD
Faculty of Health, Institute of Health and Biomedical Innovation
Queensland University of Technology
Kelvin Grove, QLD, Australia
* Corresponding author
References
- Casey G. Chronic wound healing: Leg ulcers. Kai Tiaki Nursing New Zealand 2011;17(11):24–9.
- Harvey D. New, improved Kerraboot: a tool for leg ulcer healing. Br J Community Nurs 2006 Jun;11(6):S26,S8–30.
- Wyffels JT, Edsberg LE. Granulation tissue of chronic pressure ulcers as a predictive indicator of wound closure. Adv Skin Wound Care 2011;24(10):464–73.
- Bennett G, Dealy C, Posnett J. The cost of pressure ulcers in the UK. Age Ageing 2004;33:230–5.
- Posnett J, Franks P. The cost of skin breakdown and ulceration in the UK. The silent epidemic. Hull: Smith and Nephew Foundation; 2007.
- Australian Institute of Health and Welfare. Health expenditure Australia 2015–16. Australian Institute of Health and Welfare; 2012 [02/8/18]. Available from: http://www.aihw.gov.au/publication-detail/?id=10737423009&tab=3.
- Chase SK, Whittemore R, Crosby N, Freney D, Howes P, Phillips TJ. Living with chronic venous leg ulcers: a descriptive study of knowledge and functional health status. J Community Health Nurs 2000 Spring;17(1):1–13.
- Persoon A, Heinen MM, van der Vleuten CJM, de Rooij MJ, van de Kerkhof PCM, van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004;13(3):341–54.
- Sonnad SS, Goldsack JC, Mohr P, Tunis S. Methodolocial recommendations for comparative research on the treatment of chronic wounds. J Wound Care 2013;22(9):470–80.
- Jones KR. Wound healing in older adults. Aging Health 2009;5(6):851–66.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4(1).
- Gottrup F, Apelqvist J, Price P. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care 2010;19(6):237–68.
- Vollenweider D, Ebneter I, Mayer D, Hafner J, Steurer J, Puhan MA. Dealing with heterogeneous populations in randomized wound trials: Challenges and potential solutions. Wound Repair Regen 2012;20(4):466–72.
- Sonnad SS, Goldsack JC, Mohr P, Tunis S. Methodological recommendations for comparative research on the treatment of chronic wounds. J Wound Care 2013;22(9):470–80.
- Liu Z, Saldanha IJ, Margolis D, Dumville JC, Cullum NA. Outcomes in Cochrane systematic reviews related to wound care: An investigation into prespecification. Wound Repair Regen 2017;25(2):292–308.
- Jeffcoate WJ, Bus SA, Game FL, Hinchliffe RJ, Price PE, Schaper NC. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality. Lancet Diabetes Endocrinol 2016;4(9):781–8.
- Gélis A, Dupeyron A, Legros P, Benaïm C, Pelissier J, Fattal C. Pressure ulcer risk factors in persons with SCI: part I: acute and rehabilitation stages. Spinal Cord 2008;47(2):99.
- Grocott P, Campling N. A methodology for evaluating wound care products in complex chronic wounds. Wounds UK 2009;5(4):28–34.
- Wu B, Lu J, Yang M, Xu T. Sulodexide for treating venous leg ulcers. Cochrane Wounds Group 2013;8.
- Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;5:CD006899.
- McGaughey H, Dhamija S, Oliver L, Porter-Armstrong A, McDonough S. Pulsed electromagnetic energy in management of chronic wounds: a systematic review. Phys Ther Rev 2009;14(2):132–46.
- Queirós P, Santos E, Apóstolo J, Cardoso D, Cunha M, Rodrigues M. The effectiveness of cleansing solutions for wound treatment: a systematic review. JBI Database System Rev Implement Rep 2014;12(10):121–51.
- Fernando ME, Seneviratne RM, Tan YM, Lazzarini PA, Sangla KS, Cunningham M et al. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev 2016;1:CD010764.
- O’Meara S, Martyn-St James M, Adderley UJ. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev 2015;8:CD010182.
- Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2015;6(6):CD004123.
- Marti-Carvajal AJ, Knight-Madden JM, Martinez-Zapata MJ. Interventions for treating leg ulcers in people with sickle cell disease. Cochrane Database Syst Rev 2014;8(12):CD008394.
- Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev 2014;10(10):CD009261.
- Bellmunt-Montoya S, Escribano JM, Dilme J, Martinez-Zapata MJ. CHIVA method for the treatment of chronic venous insufficiency. Cochrane Database Syst Rev 2015;(6):CD009648.
- Allahabadi S, Haroun KB, Musher DM, Lipsky BA, Barshes NR. Consensus on surgical aspects of managing osteomyelitis in the diabetic foot. Diabet Foot Ankle 2016;7(1).
- Aziz Z, Cullum N. Electromagnetic therapy for treating venous leg ulcers. Cochrane Database Syst Rev 2015;7:CD002933.
- Cullum N, Liu Z. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev 2017;5(5):CD001180.
- Araujo DN, Ribeiro CT, Maciel AC, Bruno SS, Fregonezi GA, Dias FA. Physical exercise for the treatment of non-ulcerated chronic venous insufficiency. Cochrane Database Syst Rev 2016;12(12):CD010637.
- Weller CD, Buchbinder R, Johnston RV. Interventions for helping people adhere to compression treatments for venous leg ulceration. Cochrane Database Syst Rev 2016;3(3):CD008378.
- de Oliveira Carvalho PE, Magolbo NG, De Aquino RF, Weller CD. Oral aspirin for treating venous leg ulcers. Cochrane Database Syst Rev 2016;2(2):CD009432.
- Gethin G, Cowman S, Kolbach DN. Debridement for venous leg ulcers. Cochrane Database Syst Rev 2015;(9):CD008599.
- Aziz Z, Bell-Syer SEM. Electromagnetic therapy for treating pressure ulcers. Cochrane Database Syst Rev 2015.
- Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2015;3:CD005083.
- Wilkinson EAJ. Oral zinc for arterial and venous leg ulcers. Cochrane Database Syst Rev 2014;9:CD001273.
- Samuel N, Carradice D, Wallace T, Smith GE, Chetter IC. Endovenous thermal ablation for healing venous ulcers and preventing recurrence. Cochrane Wounds Group 2013;10.
- O’Meara S, Martyn St-James M. Foam dressings for venous leg ulcers. Cochrane Wounds Group 2013;5.
- Jones JE, Nelson EA, Al-Hity A. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev 2013;1:CD001737.
- Al-Khamis A, McCallum I, King PM, Bruce J. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev 2010;20(1):CD006213.
- Dat AD, Poon F, Pham KB, Doust J. Aloe vera for treating acute and chronic wounds. Cochrane Wounds Group 2012;2.
- Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev 2012;2:CD003861.
- Price P, Gottrup F, Abel M. EWMA Study recommendations: for clinical investigations in leg ulcers and wound care. J Wound Care 2014;23(Suppl 5c):S1.
- Vermeulen H, van Hattem JM, Storm-Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007;1:CD005486.
- Dumville JC, Stubbs N, Keogh SJ, Walker RM, Liu Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database Syst Rev 2015;2(2):CD011226.
- Horkan L, Stansfield G, Miller M. An analysis of systematic reviews undertaken on standard advanced wound dressings in the last 10 years. J Wound Care 2009;18(7):298–304.
- Jones KR. Identifying best practices for pressure ulcer management. JCOM 2009;16(8):375–81.
- Pagan M, Trip H, Burrell B, Gillon D. Wound programmes in residential aged care: A systematic review. Wound Practice & Research 2015;23(2):52–60.
- Harlin SL, Willard LA, Rush KJ, Ghisletta LC, Meyers WC. Chronic wounds of the lower extremity: a preliminary performance measurement set. Plast Reconstr Surg 2008;121(1):142.
- Harding K, Posnett J, Vowden K. A new methodology for costing wound care. Int Wound J 2013;10(6):623–9.
- Augustin M, Schmitt J, Herberger K, Goepel L, Heyer K, Dissemond J et al. The German national consensus on wound documentation and outcomes: Rationale, working program and current status. Wound Medicine 2014:8–13.
- Elraiyah T, Domecq JP, Prutsky G, Tsapas A, Nabhan M, Frykberg RG et al. A systematic review and meta-analysis of debridement methods for chronic diabetic foot ulcers. J Vasc Surg 2016;63(2):37S–45S.e2.
- Tatsioni A, Balk E, O’Donnell T, Lau J. Usual care in the management of chronic wounds: a review of the recent literature. J Am Coll Surg 2007;205(4):617–24.e57.
- Wounds International. International consensus. Acellular matrices for the treatment of wounds. An expert working group review. London: Wounds International; 2010.
- Sandy-Hodgetts K, Watts R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post-surgical wound complications: a systematic review and meta-analysis. JBI Database System Rev Implement Rep 2015;13(1):253–303.
- Lazarus G, Valle MF, Malas M, Qazi U, Maruthur NM, Doggett D et al. Chronic venous leg ulcer treatment: Future research needs. Wound Repair Regen 2014;22(1):34–42.
- Micheli C, Palese A, Canzan F, Ambrosi E. No sting barrier film to protect skin in adult patients: findings from a scoping review with implications for evidence-based practice. Worldviews Evid Based Nurs 2017;14(5):403–11.
- Moore C, Young J. Effectiveness of silver in wound care treatment. Phys Ther Rev 2011;16(3):201–9.
- Munro G. Causes and consideration with chronic wounds: A narrative review of the evidence. Wound Practice & Research 2017;25(2):88–97.
- Adderley UJ, Holt IG. Topical agents and dressings for fungating wounds. Cochrane Database Syst Rev 2014;5:CD003948.
- European and US National Pressure Ulcer Advisory Panels (EPUAP and NPUAP) and Pan Pacific Pressure Injury Alliance. International Pressure Ulcer Guidelines. Osborne Park, Australia: Cambridge Media; 2014.
- Goel RR, Abidia A, Hardy SC. Surgery for deep venous incompetence. Cochrane Database Syst Rev 2015;2(2):CD001097.
- Ingram JR, Woo P-N, Chua SL, Ormerod AD, Desai N, Kai AC et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev 2015;10:CD010081.
- Norman G, Dumville JC, Mohapatra DP, Owens GL, Crosbie EJ. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst Rev 2016;3(3):CD011712.
- Norman G, Westby MJ, Stubbs N, Dumville JC, Cullum N. A ‘test and treat’ strategy for elevated wound protease activity for healing in venous leg ulcers. Cochrane Database Syst Rev 2016;1(1):CD011753.
- O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2013;12(12):CD003557.
- Rabe E, Partsch H, Jünger M, Abel M, Achhammer I, Becker F et al. Guidelines for Clinical Studies with Compression Devices in Patients with Venous Disorders of the Lower Limb. Eur J Vasc Endovasc Surg 2008;35(4):494–500.
- Australian Wound Management Association. Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury. Osborne Park, WA: Cambridge Media; 2012.
- The Australian Wound Management Association Inc. and the New Zealand Wound Care Society Inc., editor. Australian and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers: Cambridge Publishing; 2011.
- Gottrup F, Apelqvist J, Bjarnsholt T, Bjansholt T, Cooper R, Moore Z et al. EWMA Document: Antimicrobials and Non-healing Wounds: Evidence, controversies and suggestions. J Wound Care 2013;22(Supp5):S1–S89.
- Santema TBK, Poyck PPC, Ubbink DT. Systematic review and meta-analysis of skin substitutes in the treatment of diabetic foot ulcers: Highlights of a Cochrane systematic review. Wound Repair Regen 2016;24(4):737–44.
- Bardy J, Slevin NJ, Mais KL, Molassiotis A. A systematic review of honey uses and its potential value within oncology care. J Clin Nurs 2008;17(19):2604–23.
- Wounds International. International consensus. Making the case for cost-effective wound management. London, UK: Wounds International Enterprise House; 2013.
- Walker M, Kralik D, Porritt K. Fasciotomy wounds associated with acute compartment syndrome: a systematic review of effective treatment. JBI Database System Rev Implement Rep 2014;12(1):101–75.
- Gurusamy KS, Koti R, Toon CD, Wilson P, Davidson BR. Antibiotic therapy for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) in non surgical wounds. Cochrane Wounds Group 2013;(11).
- Arsenault KA, McDonald J, Devereaux PJ, Thorlund K, Tittley JG, Whitlock RP. The use of transcutaneous oximetry to predict complications of chronic wound healing: A systematic review and meta-analysis. Wound Repair Regen 2011:n/a–n/a.
- Jørgensen LB, Sørensen JA, Jemec GB, Yderstræde KB. Methods to assess area and volume of wounds — a systematic review. Int Wound J 2016;13(4):540–53.
- Wounds International. Appropriate use of silver dressings in wounds. London: Wounds International; 2012.
- Heuch L, Streak Gomersall J. Effectiveness of off-loading methods in preventing primary diabetic foot ulcers in adults with diabetes: a systematic review. JBI Database System Rev Implement Rep 2016;14(7):236–65.
- Registered Nurses’ Association of Ontario (RNAO). Assessment and Management of Foot Ulcers for People with Diabetes. 2nd Edn. Toronto, Ontario: Registered Nurses’ Association of Ontario; 2013.
- Registered Nurses’ Association of Ontario (RNAO). Strategies to Support Self-Management in Chronic Conditions: Collaboration with Clients. Toronto, Ontario: Registered Nurses’ Association of Ontario; 2010.
- Game FL, Hinchliffe RJ, Apelqvist J, Armstrong DG, Bakker K, Hartemann A et al. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Chichester, UK: John Wiley & Sons, Ltd; 2012, pp. 119–41.
- Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes: IWGDF Guidance on Interventions to Enhance Chronic Ulcers Healing. Diabetes Metab Res Rev 2016;32:75–83.
- Augustin M, Langenbruch AK, Herberger K, Baade K, Goepel L, Blome C. Quality of life measurement in chronic wounds and inflammatory skin diseases: definitions, standards and instruments. Wound Medicine 2014:29–38.
- Mordiffi SZ, Kent B, Phillips N, Chi Tho P. Use of mobility subscale for risk assessment of pressure ulcer incidence and preventive interventions: A systematic review. JBI Libr Syst Rev 2011;9(56):2417.
- Glass GE, Nanchahal J. The methodology of negative pressure wound therapy: Separating fact from fiction. J Plast Reconstr Aesthet Surg 2012;65(8):989–1001.
- Norman RE, Gibb M, Dyer A, Prentice J, Yelland S, Cheng Q et al. Improved wound management at lower cost: a sensible goal for Australia. Oxford, UK: Blackwell Publishing Ltd; 2016. pp. 303–16.
- Adderley U, Evans K, Coleman S. Reducing unwarranted variation in chronic wound care. Wounds UK 2017;13(4):22–7.
- Santema TB, Stoekenbroek RM, van Steekelenburg KC, van Hulst RA, Koelemay MJ, Ubbink DT. Economic outcomes in clinical studies assessing hyperbaric oxygen in the treatment of acute and chronic wounds. Diving Hyperb Med 2015;45(4):228–34.
- World Union of Wound Healing Societies (WUWHS). Consensus Document. Closed surgical incision management: understanding the role of NPWT. London: Wounds International; 2016.
- Cowman S, Gethin G, Clarke E, Moore Z, Craig G, Jordan-O’Brien J et al. An international eDelphi study identifying the research and education priorities in wound management and tissue repair. J Clin Nurs 2012;21(3–4):344–53.
- Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic venous leg ulcers — A national clinical guideline. Edinburgh: SIGN; 2010.
- Carter M. Economic Evaluations of Guideline-Based or Strategic Interventions for the Prevention or Treatment of Chronic Wounds. Appl Health Econ Health Policy 2014;12(4):373–89.
- Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care 2017;26(Supp4):S4.
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. UK: NICE; 2014.
- National Institute for Health and Care Excellence (NICE). Diabetic foot problems: prevention and management. UK: NICE; 2015.
- Stopher L, Jansen S. Systematic review of the impact and treatment of malnutrition in patients with chronic vascular wounds. Wound Practice & Research 2017;25(2):71–80.



