Volume 27 Number 2
Ankle Brachial Pressure Index and compression application: review summary
Victoria Team, Georgina Gethin, John D Ivory, Kimberley Crawford, Ayoub Bouguettaya and Carolina D Weller
Keywords Ankle Brachial Pressure Index (ABPI), clinical practice guidelines (CPG), compression, content analysis, venous leg ulcers (VLUs).
For referencing Team V et al. Ankle Brachial Pressure Index and compression application: Review summary. WP&R Journal 2019; 27(2):74-77.
DOI https://doi.org/10.33235/wpr.27.2.74-77
Abstract
Venous leg ulcers (VLUs) are a significant complication amongst persons with chronic venous insufficiency (CVI) that frequently follow a cycle of healing and recurrence. Current clinical practice guidelines (CPGs) recommend applying below knee compression to improve VLU healing. Compression could be applied if the Ankle Brachial Pressure Index (ABPI) rules out significant arterial disease, as sufficient peripheral arterial circulation is necessary to ensure safe compression use. We conducted a content analysis of 13 global CPGs on the accuracy of recommendations related to ABPI and compression application. Eight CPGs indicated that compression is recommended when the ABPI is between 0.8 and 1.2 mmHg. However, this review found there is disagreement between 13 global VLU CPGs, with a lack of clarity on whether or not compression is indicated for patients with ABPIs between 0.6 and 0.8 mmHg. Some CPGs recommend reduced compression for treatment of VLUs, while others do not recommend any type of compression at all. This has implications for when it is safe to apply compression, and the inconsistency in evidence indicates that specialist advice may be required at levels beyond the ABPI “safe” range listed above.
Question
What is the best available evidence to guide compression application in venous leg ulcers (VLUs) after a vascular assessment including ABPI?
Review summary
Venous leg ulcers (VLUs) are a significant complication amongst persons with chronic venous insufficiency (CVI) that frequently follow a cycle of healing and recurrence1. Current clinical practice guidelines (CPGs) recommend applying below knee compression to improve VLU healing2. Compression could be applied if the Ankle Brachial Pressure Index (ABPI) rules out significant arterial disease2, as sufficient peripheral arterial circulation is necessary to ensure safe compression use2. A content analysis of 13 global CPGs was conducted on the accuracy of recommendations related to ABPI measures and compression application, in order to aid clinicians’ understanding of the best available evidence in this area. Eight CPGs indicated that compression is recommended when the ABPI is between 0.8 and 1.2 mmHg. However, this review concluded that there is disagreement between 13 global VLU CPGs, with a lack of clarity on whether or not compression is indicated for patients with the ABPI between 0.6 and 0.8 mmHg. Some CPGs recommend reduced compression for treatment of VLUs, while others do not recommend any type of compression altogether. This has implications for when it is safe to apply compression, and the inconsistency in evidence indicates that specialist advice may be required at levels beyond the ABPI “safe” range listed above.
Methodology
We conducted a qualitative and quantitative content analysis of 13 global CPGs on the accuracy of recommendations related to ABPI and compression application.
Search strategy
Global CPGs (or best practice recommendations) were located using a search of Scopus and PubMed (years 2000 to present). In addition, references in the CPG review article3 were manually searched to find more CPGs.
Inclusion criteria
CPGs were only included if they were described as such, were original, discussed VLUs or CVIs, and were published between 2000 and 2018. Additional items for assessment and incorporation included methodology used, population, personnel used for assessment, accuracy, specialist referral recommendations, indications, contradictions, and precautions for compression therapy. This search resulted in 2648 records, and after removing duplicates, 2502 titles and abstracts remained; 34 were retained for full text screening after assessing for eligibility, and in addition, 22 full text articles were added from a paper by Andriessen et al.3. Of these 56 articles, 43 did not meet all inclusion criteria, leaving 13 for analysis and synthesis. A PRISMA diagram was provided in the original review4.
Data analysis and synthesis
Three independent reviewers (under the guidance of senior co-authors) completed data analysis and synthesis. The quantitative analysis focused on ABPIs as a score, while the qualitative analysis analysed the description of methodology, evidence rating, ABPI measurement, compression indication and contraindication, precaution, and referrals.
ABPI and compression application
ABPI measurement recommendations
As arterial disease can complicate treatment of VLUs4, measurement of a patient's ABPI to assess peripheral arterial insufficiency is necessary. The most commonly recommended method is Doppler ultrasound.
Across 13 CPGs, 102,6-14 explicitly recommended that ABPI should be assessed to rule out any form of peripheral arterial disease. Nine of these recommended that Doppler be used, and one did not specify a method7. Only three15-17 did not explicitly state that ABPI should be measured to rule out peripheral arterial disease.
In patients with VLUs, appropriately trained or suitably trained medical staff should assess ABPI, including GPs and physicians8,9,12,13. The quality of the initial assessment is vital to ensure that patients receive appropriate management of their VLU. However, the CPGs do not suggest when to refer patients for specialist assessment.
ABPI interpretations
Six CPGs report that ABPI scores should be interpreted with caution, pending assessment for arterial calcification or diseases that lead to arterial calcification2,7,9,10-12. Of these, four CPGs suggested or explicitly stated that ABPI should be interpreted in the context of the patient’s general health, including combined clinical features, heart or vascular risk factors, or other assessments of peripheral arterial disease2,6,9,10. One CPG2 suggested that untrained personnel may incorrectly interpret ABPI scores, further suggesting that ABPI assessment and interpretation should be done by appropriately trained personnel.
ABPI and compression indication
Eight of the 13 CPGs provided recommendations for indication for compression (using ABPIs). Five CPGs2,6,7,10,12 proposed that compression could be safely initiated when the ABPI is above 0.8, although the suggested level of compression varied. Of these CPGs, one14 suggested 0.6 or above was safe, with two suggesting that an ABPI at 0.86,12 was safe as well. Two CPGs8,15 suggested that low level or reduced compression was safe to begin below 0.8, with one8 suggesting the minimum threshold for low compression was 0.5, and the other 0.615. One CPG suggested that compression can be safely used below 1.314, and another suggested safe compression below 1.210.
ABPI and compression contraindications
There was little agreement between the six CPGs on the ABPI at which compression was contradicted. These contraindications ranged from < 0.58, ≤ 0.56,14, < 0.615, <0.77 and <0.8 > 1.22.
Compression with close monitoring was generally recommended at certain ABPIs, although the exact index was varied. Two CPGs6,8 recommended compression with close monitoring when the ABPI is greater than 0.5. Three CPGs2,8,12 also suggested close monitoring with compression when the ABPI is below 0.8. One CPG suggested compression with close monitoring is necessary when the ABPI is above 1.2.
ABPI and referrals to specialists
CPG recommendations for when to refer to specialists differed between the reviewed guidelines. When ABPI is below 0.8, specialist referral was recommended by two CPGs2,12. One CPG6 suggested that referral is needed when the ABPI is lower than 0.9. When the ABPI is above 1.2, two CPGs recommended referral2,12. When the ABPI is above 0.85, one CPG10 suggested superficial venous surgery with compression. Another CPG suggests investigation when the ABPI is less than 0.8, specifically via duplex scan9.
ABPI and VLU prevention
Eight of the 13 reviewed CPGs2,6-8,10-12,15 discussed the use of compression for VLU prevention. Of the eight that discussed the use of compression for prevention, five provided the level of compression2,8,10,11,15. This was varied; one recommended that compression should start at 20 mmHg at the minimum, and increase to the highest tolerable pressure to an ideal of 30–40 mmHg10. Another suggested between 18–30 mmHg pressure2, while yet another suggested between 40–50 mmHg8.
Conclusions and future directions:
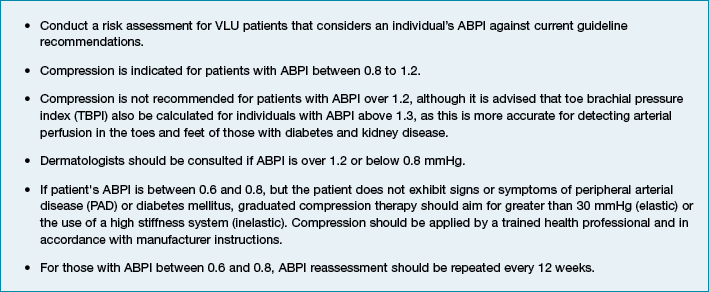
From this research, it is strongly recommended that clinicians treating VLUs follow the clinical practice recommendations as outlined in Box 1. As a considerable amount of variation exists in CPGs about how VLUs should be treated, clinicians should also consider speaking to wound specialists, and/or seek further training when unsure. Future research should address the safety of compression when the ABPI is below 0.8, as a degree of uncertainty exists. Specifically, future research should employ a longitudinal prospective design to address the efficacy and safety of compression in those with the ABPI between 0.6 and 0.8, as there is insufficient high-quality data in this area.
Box 1: Clinical practice recommendations
Acknowledgements
The original review was published in the International Wound Journal:
Weller CD, Team V, Ivory JD, Crawford K, Gethin G. ABPI reporting and compression recommendations in global clinical practice guidelines on venous leg ulcer management: A scoping review. Int Wound J 16(2):406-419.
Funding
Prof. Carolina Weller is funded by the National Health and Medical Research Council Translating Research into Clinical Practice (TRIP) Fellowship APP1132444.
Conflict of interest
The authors have no conflict of interest to declare.
Author(s)
Victoria Team
DrPH
Monash Nursing and Midwifery
Melbourne, VIC, Australia
Monash Partners
Monash Centre for Health Research and Implementation (MCHRI) Building
Clayton, VIC, Australia
Georgina Gethin
PhD
School of Nursing & Midwifery
National University of Ireland
Galway, Republic of Ireland
John D Ivory
MSc
School of Nursing & Midwifery
National University of Ireland
Galway, Republic of Ireland
Kimberley Crawford
PhD
Monash Nursing and Midwifery
Melbourne, VIC, Australia
Ayoub Bouguettaya
PhD
Monash Nursing and Midwifery
Melbourne, VIC, Australia
Carolina D Weller*
PhD
Monash Nursing and Midwifery
Melbourne, VIC, Australia
Email carolina.weller@monash.edu
* Corresponding author
References
- Franks PJ et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care 2016;25(Suppl 6):S1–s67.
- Australian Wound Management Association and New Zealand Wound Care Society. Australian and New Zealand Clinical Practice Guidelines for Prevention and Management of Venous Leg Ulcers. 2011; Available from: www.awma.com.au/publications/2011_awma_vlug.pdf.
- Andriessen A et al. Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications — a review of present guidelines. J Eur Acad Dermatol Venereol 2017;31(9):1562–1568.
- Weller CD, Team V, Ivory JD, Crawford K, Gethin G. ABPI reporting and compression recommendations in global clinical practice guidelines on venous leg ulcer management: A scoping review. Int Wound J 2019;16(2):406-419.
- Mosti G. Compression and venous surgery for venous leg ulcers. Clin Plast Surg 2012;39(3):269–280.
- O’Donnell TF, Jr, Passman MA. Clinical practice guidelines of the Society for Vascular Surgery (SVS) and the American Venous Forum (AVF) — Management of venous leg ulcers. Introduction. J Vasc Surg 2014;60(2 Suppl):1s–2s.
- Marston W et al. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen 2016;24(1):136–44.
- Kelechi TJ, Johnson JJ. Guideline for the management of wounds in patients with lower-extremity venous disease: an executive summary. J Wound Ostomy Continence Nurs 2012; 39(6):598–606.
- Sinha S, Sreedharan S. Management of venous leg ulcers in general practice — a practical guideline. Aust Fam Physician 2014;43(9):594–8.
- 1Haute Autorité de Santé [Management of leg ulcers of predominantly venous origin (dressing excluded)]. J Mal Vasc 2007;32(2):100–11.
- Neumann M C-TA, Jünger M, Mosti G, Munte K, Partsch H, Rabe E, Ramelet AA, Streit, M. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatol Venereol 2016;30(11):1843–1875.
- Scottish Intercollegiate Network (SIGN). Management of chronic venous leg ulcers: A national clinical guideline 2010. Available from: http://www.sign.ac.uk/assets/sign120.pdf.
- Schweitzer BPM et al. NHG Practice Guideline: Venous leg ulcer, 2014.
- Ebner H et al. Linee guida flebo-linfologiche SIF-SICVE 2016 della Società Italiana di Flebologia e della Società Italiana di Chirurgia Vascolare ed Endovascolare. Minerva Cardioangiol 2016;64(4):1–80.
- Agus GB et al. Guidelines for the diagnosis and therapy of the vein and lymphatic disorders. Int Angiol 2005;24(2):107–68.
- Bartkowski R et al. Lokal therapie chronischer wunden bei patienten mit den risiken periphere arterielle verschlusskrankheit. Diabetes mellitusm chronische cenose insuffizienz. 2012 April 2018. Available from: https://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Leitlinien/Evidenzbasierte_Leitlinien/091-001l_S3_Lokaltherapie_chronischer_Wunden_2012-06.pdf.
- Junger M et al. Leitlinien Deutsche Gesellschaft fur Phlebologie: Phlebolischer Kompressionsverband (PKV). Phlebologie 2009;38:168–171.




