Volume 27 Number 2
The Surgical Patients’ Pressure Injury Incidence (SPPII) study: a cohort study of surgical patients and processes of care
Cintia M Martinez-Garduno, Jane Rodgers, Rosemary Phillips, Anoja W Gunaratne, Peta Drury and Elizabeth McInnes
Keywords pressure injuries, hospital-acquired, Prospective cohort, incidence, surgical patients.
For referencing Martinez-Garduno CM et al. The Surgical Patients’ Pressure Injury Incidence (SPPII) study: a cohort study of surgical patients and processes of care. WP&R Journal 2019; 27(2):86-94.
DOI https://doi.org/10.33235/wpr.27.2.86-94
Abstract
Background Surgical patients are at high risk of developing pressure injuries (Pls) due to anaesthesia-induced immobility as well as risk factors such as length of surgery and co-morbidities. Few Australian studies have investigated the incidence of PIs in surgical patients. This prospective cohort study assessed the incidence of post-surgical PIs and identified gaps in pressure injury prevention (PIP) for elective surgical patients.
Methods Consecutive elective surgery patients at an urban tertiary referral hospital were recruited who had an expected length of stay of >48 hours. Baseline PI risk (measured by the Waterlow scale) and PIP strategies implemented at five time points were collected from medical records. Two prospective outcome assessments were conducted at 24 and 48 hours post-operatively. Data were analysed descriptively.
Results One patient out of 150 (incidence rate 0.7) developed an intra-operative Stage 1 PI. Four patients developed skin tears. PIP strategies were applied inconsistently throughout the patient journey, regardless of risk status.
Conclusions While the incidence of surgically acquired PIs in this study was low, ongoing staff education is needed about the importance of consistent skin and risk assessments and of implementing strategies appropriate for level of PI risk.
What is already known:
- PIs are widely considered to be an adverse event of hospitalisation and are largely preventable.
- Surgical patients are at risk of developing a PI primarily due to immobilisation following anaesthesia, length of surgery and co-morbidities.
- There are few studies on PI incidence and prevention strategies used in the post-operative period.
What this manuscript contributes:
Although the incidence of post-surgical PIs among elective surgical patients was low, there are gaps in PIP for this group of patients, including for those deemed at high risk of PI. There is a need for clinicians to improve documentation of risk assessment and strategies implemented to reduce the risk of PIs, throughout the surgical patient journey.
Introduction
Each year, over 2 million surgeries are performed in Australia1, during which the patient is anaesthetised, immobilised and unable to perceive or voice pain and discomfort from unrelieved pressure to the surgical team2. These factors may lead to the development of a pressure injury (PI)2-4. Pressure injuries (PIs), also known as pressure sores, bed sores and decubitus ulcers are defined by the National Pressure Ulcer Advisory Panel as a “localized injury to the skin and/or underlying tissue usually over a bony prominence or related to a medical or other device”5. The financial impact of PIs for hospitals and health systems is significant, with the annual costs of medical treatment and extended hospitalisations estimated to be between £1.8 and £2.6 billion in the United Kingdom6 and US$11 billion in the United States (US)7. In Australia, the treatment costs of PIs have been estimated to be A$983 million per annum, representing approximately 1.9% of all public hospital expenditure8.
Surgical patients have been identified as at elevated risk for PI development9. PI development can occur between the first hour and 4–6 hours following sustained pressure10. Therefore, surgeries that are longer than four hours have been shown to increase the chance of PI development11-13. Development of surgery-related PI may result in reduced quality of life14,15, decreased mobility, increased pain, prolonged hospital stay, re-admission and negative psychological consequences16,17. Furthermore, hospital-acquired PIs (HAPIs), including surgery-related PIs, are regarded as a key performance indicator of the quality of care provided by health facilities, particularly of nursing care18,19. In Australia, the National Safety and Quality Health Service Standard 8 requires health service organisations to implement evidence-based systems and guidelines to prevent and manage PIs20. The classification of HAPIs as never events (US) or adverse events (Australia) and the introduction of non-payment or financial penalties for HAPIs have placed PIs as a priority for health services. In the US, Medicare introduced non-payment for hospital-acquired conditions including PIs in 2008, whilst financial penalties were introduced more latterly (2013) in Queensland, Australia17,21-23. The Australian Commission on Safety and Quality in Health Care (ACSQHC) has developed a national list of 16 hospital-acquired complications (HAC), which includes PIs, and developed a range of resources to support adoption of the HAC list24. Depending on the practice setting, the reported incidence and prevalence of HAPIs ranges from 0.0% to 72.5%7. For surgery-related PIs, the incidence varies, ranging from 1.3% to 66%, depending on the study population, the type of surgery and duration of the surgical procedure13,23,25-27. Evidence from a recent systematic review of 17 studies found the pooled incidence of surgery-related PIs was 0.15 (95% CI 0.14–0.16; range 0.003–0.574)28. Of note, none of the included studies were conducted in Australia, highlighting limited research in this area. Indeed, a prospective cohort study at a single-site investigating the incidence of HAPIs remains one of the few studies investigating surgery-related PI incidence in Australia23. Therefore, our knowledge of PI incidence is predominantly based on studies conducted in other countries and may not be indicative of the true incidence in Australian surgical patients.
Pressure injury prevention (PIP) is a global quality of care indicator and there are national and international evidence-based clinical guidelines to inform this area of nursing practice29. Conducting risk and skin assessments, coupled with attention to positioning, protecting and padding pressure-sensitive and vulnerable areas are primary PIP strategies for surgical patients30-32; as standard PIP processes of care they have the potential to reduce PI incidence33. However, there are only a few studies with information on PIP processes of care in relation to surgical patients31,34. Furthermore, these studies are limited as they do not evaluate PIP strategies for at-risk patients and there is a need to identify if evidence-based PIP processes of care for surgical patients occurs consistently throughout the entire surgical patient journey, including the pre-operative, peri-operative and post-operative phases. Therefore, this study aimed to determine the incidence of HAPIs among elective surgical patients and to describe the extent to which PIP processes of care were documented as adhered to throughout the surgical patient journey.
Methods
Design
A one-sample prospective cohort study design.
Setting
This study was conducted in a large public (402 beds), metropolitan, tertiary referral hospital in Sydney, New South Wales, Australia between July 2015 and March 2016.
Patients
Eligibility criteria
Patients were eligible for inclusion if they were greater than 18 years of age, scheduled for an elective surgical procedure and had an expected 48-hour minimum hospital stay following surgery. This inclusion criterion reflects findings from other studies, which suggest that PIs may take up to 48 hours to appear after relief from periods of pressure, friction or shearing13,35. Patients were excluded from the study if they were admitted for emergency surgery, admitted for elective surgery through the emergency department, or admitted into hospital a day or more prior to their elective surgery. These groups were excluded because of the uncertainty about how long they may have been immobile before their transfer to the operating suite.
Recruitment
Patients were recruited to the study if they met the inclusion criteria and attended the pre-admission clinic prior to surgery. Pre-admission patient lists provided by the admissions unit were used to identify those patients with an expected length of stay (LoS) of >48 hours. Using a non-probability sampling method, those patients who met the criteria were approached by a nurse research assistant (RA) in the pre-admission clinic. Patients were given verbal and printed information about the study, and if agreeable, signed their consent. If a patient declined to participate in the study, or was expected to have stay of < 48 hours, the next eligible patient was approached.
Data collection and outcome assessment
The following information was collected from patients’ medical records using a standardised data collection form: demographics, patient’s history of PIs in the previous 12 months, co-morbidities, length of time in surgery, total time in operating theatre and time in recovery, type of surgery, American Society of Anesthesiologists (ASA) score, patient transfer method to and from the operating table, patient position and positioning devices and PI prevention strategies implemented pre-, intra- and post-operatively.
Prior to the study commencement, RAs received training in the use of the data collection tool, the use of the Waterlow scale and the observation and classification, or staging, of PIs according to the European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel Classification System36. Inter-tester reliability was 92%, which is considered almost perfect agreement37.
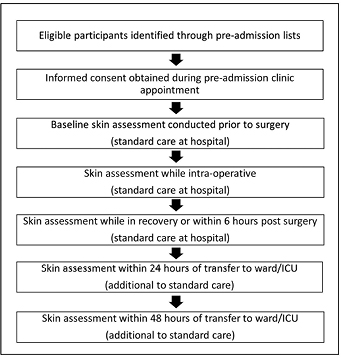
Skin assessments were recorded at five time points. The first three skin assessments were conducted before, during and after surgery and documented as part of the hospital’s standard of care in patients’ medical records. At 24 hours and 48 hours post-operatively, two additional skin assessments were undertaken by the trained RAs as part of the outcome assessment (PI presence) (Figure 1). The number and location of all PIs and any other changes to skin integrity signifying a developing area of PIs were recorded. The staging of any PI that occurred was verified by the wound management clinical nurse consultant (JR). Time to event (defined as from time in operating suite until development of PI) was also recorded.
Figure 1: Study processes
Sample size calculation
Sample size calculations were based on an assumption of PI incidence of 20%, as suggested by previous studies of high-risk surgical patients13,35. In consultation with a statistician, a sample size of 250 was estimated from tables for 95% confidence intervals with a 5% margin of error. However, the sample size was changed after recruitment of 150 patients and a low detection of PIs.
Data analysis
Data were entered and analysed using Statistical Package for Social Sciences, version 23 (SPSS, Chicago, Illinois). Baseline demographics and clinical characteristics, risk status and processes of care were reported as frequencies and percentages for categorical variables or means and standard deviations for continuous variables. Incidence was calculated using a binomial confidence interval (95%). Mean length of time in the operating suite was calculated from the time the patient entered the surgical unit, including time in the pre-operative bay and surgery length), to the time the patient was transferred from the operating suite to either the recovery or ICU. Time in recovery was calculated from the time the patient entered the recovery unit until transfer to ward.
Ethical approval
Ethics approval was given by the St Vincent’s Hospital Sydney Human Research Ethics Committee (HREC LNR/15/SVH/137). All patients provided written consent to participate. Patients who declined study participation or were unable to give informed consent were excluded.
Results
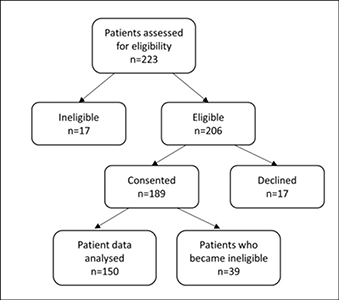
Two hundred and twenty-three elective surgery patients were assessed for eligibility (Figure 2). Of 206 patients assessed as eligible to participate, 189 consented to take part in the study. Thirty-nine patients became ineligible following recruitment because their post-operative LoS was <48 hours, surgery was cancelled or they were referred to palliative care; resulting in a final sample of 150.
Figure 2: Recruitment of study patients
Demographic and baseline characteristics of study participants (Table 1) showed the mean age was 60.6 (SD± 16.7, range 18.1-87.1), with an average body mass index (BMI) of 28.6 (SD ±6.3, range 18.3-56.4) and 63% (n=94) were males. All participants could reposition independently in bed (100%). The majority were continent (n=145; 97%), could ambulate (n=131; 87%) and lived independently (n=138; 92%). In terms of physical health status, as measured by the American Society of Anasthesiologists (ASA), score, most participants (45%) had mild systemic disease (ASA 2), and 34% moderate systematic disease (ASA3). Over 80% of the sample had one or more co-morbidities, such as hypertension (n=57; 38%), cardiovascular disease and heart failure (n= 49; 33%) or respiratory disease (n=30; 20%).
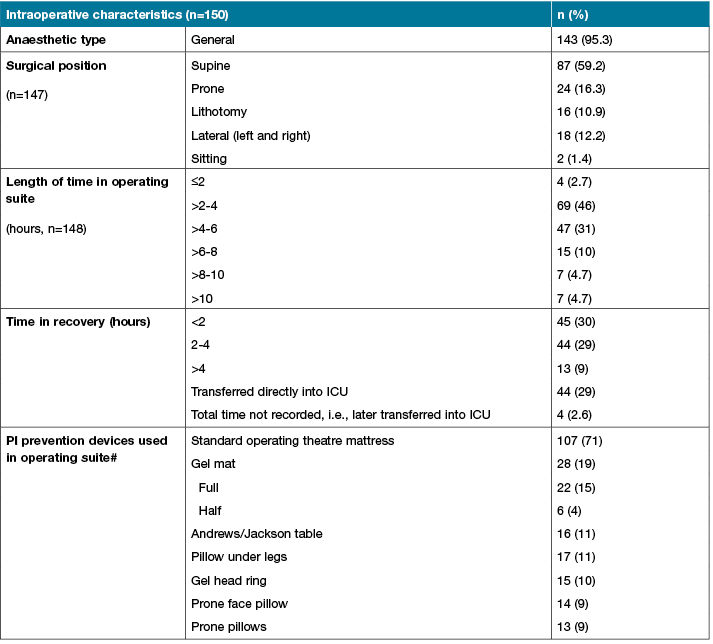
Intra-operative participant characteristics showed that the most common operations were neurology (n=38; 25%), orthopaedic (n=28; 19%) and cardiothoracic (n=21; 14%) (Table 1). The average length of time in the operating suite was 4.5 hours (SD±2.35); almost all participants received general anaesthetic (n=143; 94%) and over half were placed in a supine surgical position (n=87; 59%). A third were either transferred directly to ICU from the operating suite or stayed in recovery for less than 2 hours, or between 2 to 4 hours. The standard hospital operating theatre overlay was used in the majority of participants (71%).
Table 1: Baseline and intraoperative participant characteristics
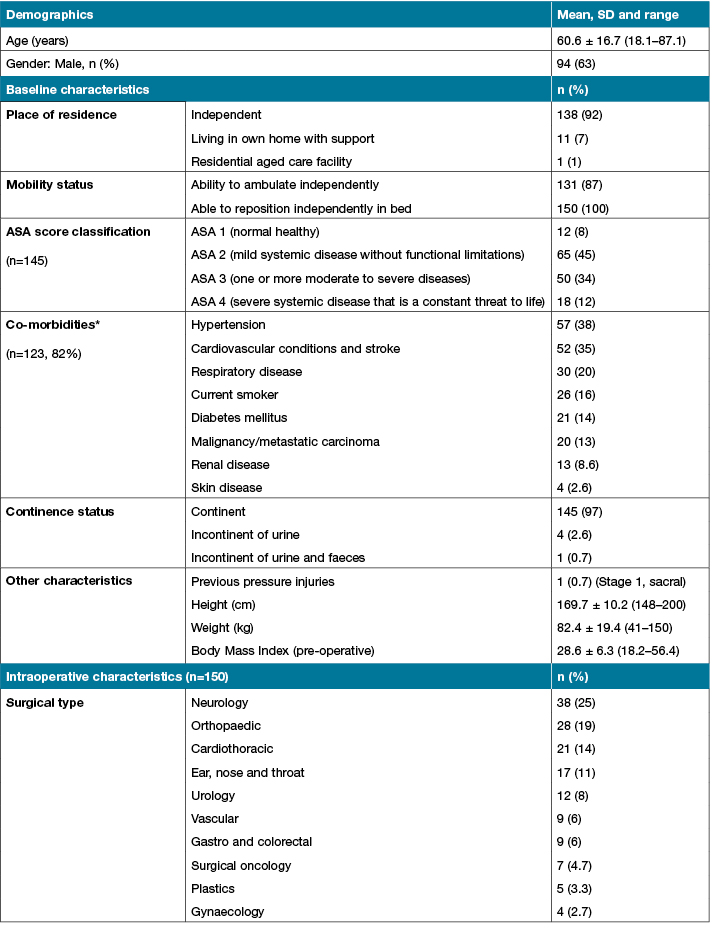
Table 1 (continued): Baseline and intraoperative participant characteristics
Pressure injury incidence
One participant was documented as having developed a PI (stage 1) in the left knee during the intra-operative period. The binomial confidence interval analysis showed the incidence of PIs was 0.7% (CI 0.0002, 0.037). Four patients (2.6%) had skin tears while in recovery. The PI and the skin tears resolved within 24 hours post-operatively and no other patient developed a PI during the study period.
Processes of care
PI risk assessment
The numbers of documented PI risk assessments decreased during the patient surgical journey. Prior to surgery, 80% (n=120) of participants were assessed using the Waterlow scale; this decreased to 41% (n=62) intra-operatively and 36% (n=54) post-operatively in recovery. All participants had a Waterlow assessment completed by the RAs at 24 and 48 hours following surgery.
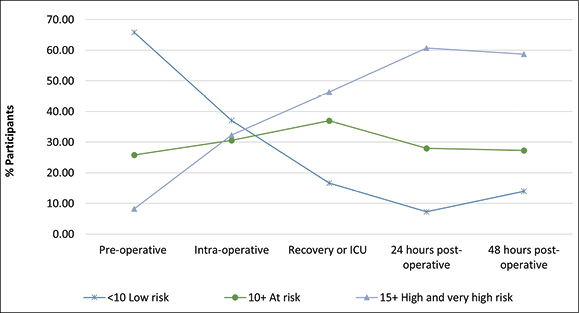
Figure 3 shows that a higher proportion of participants were classified as being at high to very high risk of PI as they progressed along the surgical journey. During the pre-operative period only 8% (n=10) of the sample were identified as being at high or very high risk of developing a PI; while at 48 hours post operatively, 59% (n=88) fell into the high to very high-risk category.
Figure 3: Waterlow assessment rates pre-operative to 48 hours post-operative (n=150)
Post-operative PI preventive strategies and devices
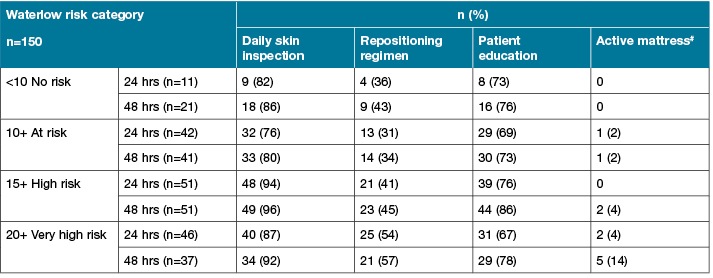
Documented PI preventive strategies (Table 2) for the post-operative period showed that less than a quarter of participants who were classified as at high or very high-risk of PI, received a specially designed support surface such as an alternating pressure mattress. Just over a half in this risk category had documentation of a repositioning regime. Over three-quarters of the sample received patient education and almost all had daily skin inspections.
Table 2: Post-operative processes of care by Waterlow risk category*
Discussion
The purpose of this study was to prospectively investigate the incidence of post-surgical PIs among elective surgical patients with a minimum hospital stay of 48 hours and to describe the processes of PI care received. Determining PI incidence, which counts the number of PIs developing after admission, rather than a snapshot of prevalence, provides the strongest evidence of quality of care38. The findings therefore add to the knowledge about PI quality of care for surgical patients, particularly those who have a hospital stay of 48 hours, because this group is generally regarded as being at high risk for developing PIs.
In our sample the incidence was low, with only one participant developing a PI (Stage 1) intraoperatively. This was identified and documented in the immediate post-operative period and resolved within 24 hours after surgery. Four patients developed intra-operative skin tears, which also resolved within 24 hours. Given that there is mandatory reporting of the occurrence of PIs in the facility in which the study took place, the likelihood of other PIs in this sample not being documented is low. While some studies have found higher post-operative PI incidence rates of up to 27%34,39-42, others such as a prospective study of 337 cardiac surgery reported a PI incidence rate of zero (that is, all patients had intact skin at the time they left the operating theatre)39. Our results were comparable to (albeit lower than) an Australian prospective cohort study comprising 534 patients that reported an immediate post-operative (defined as being within 1 hour of admission to the post anaesthetic care unit) PI incidence rate of 1.3%23.
Variation in reported incidence across studies may be attributable to the differences in the time frame between PI occurrence and data collection time during the post-operative period. Since our aim was to identify PIs attributable to surgery, follow-up to 48 hours post-operatively was selected on the basis that previous research has suggested that the 48-hour post-operative window is the time frame within which most PIs due to surgery develop13,35,43. Incidence of PIs outside this time frame is considered to be attributable to post-surgical care and not the surgery itself.
Our surgical patient cohort had risk factors for the development of PIs that had been previously identified in the literature. These are lengthy surgeries between 2 and 6 hours13,35,44-47; multiple co-morbidities including diabetes mellitus48; and either low or high BMI17. In addition, patients in our study underwent a broad range of surgical procedures including cardiac surgery, which has been identified in the literature as a risk factor for the development of PIs39,46,49. Several patients in our study, however, had pre-operative characteristics which may have had a protective effect against the development of PIs and therefore contributed to the low incidence of PIs observed. Several PI protective factors were reported in a study of surgical patients, including having healthy skin, being continent, being able to move independently and being admitted from home23. In our study, 87% of patients could ambulate independently pre-operatively, 97% were continent and 99% were admitted from their own home with only 1 patient admitted from an aged care facility. Therefore, the sample was relatively healthy. In addition, there were a wide range of pressure-relieving devices that were used intra-operatively in the majority of patients such as pillows, gel mats and head rings.
Our investigation of documentation of evidence-based PI care throughout the surgical patient journey indicated variability in processes of care. Gaps in documentation of PI were evident, with Waterlow completion rates for risk assessments in the intra-operative and immediate post-operative periods as low as 41% and 36%, respectively. Such low completion rates could be due to the fast turn-around of patients and the clinical imperative to quickly transfer patients to either the recovery or the intensive care unit; thereby making completion of risk assessment unfeasible. Moreover, this information was collected from the patients’ medical record, which may reflect a documentation issue rather than a lack of assessments performed. Lack of time by nursing staff has been previously reported to be a barrier to completing patient documentation, even though accurate, consistent and appropriate documentation is recognised as a fundamental part of patient care50 and essential for monitoring changes in PI risk status throughout the patient admission. Failure to achieve complete documentation at all time periods means that there is high potential for early identification of skin changes and a missed opportunity for instituting preventive strategies.
Only up to 14% of patients classified as being at high and very high risk of PI were allocated a pressure-relieving support surface and just over half were documented as having a repositioning regime. This suggests that improvement is urgently needed in the prescription of these interventions for high-risk patients51, especially given that HAPIs are regarded as a major patient safety issue and that in our sample the numbers classified as being at high risk increased exponentially from admission to 48 hours post-operatively. However, other processes of care documented were well performed, irrespective of risk category, such as patient education and daily skin inspections. At the study hospital, a multi-strategy approach and patient PIP education has been in place since 2011. Patient education has been proven to be an important component of PI prevention strategies because it provides patients and family with a degree of ownership for their care52,53. The reasons for strategies that are the cornerstones of evidence-based PIP guidelines, such as allocation of pressure-relieving devices and recording of a repositioning regime not being done requires investigation. Another study similarly found that even where formal risk assessment is well established, this is not necessarily followed up with appropriate PIP38.
Strengths and limitations
This study had a number of strengths. Our study design was a prospective cohort study, which is the optimal design to study incidence. We used a combination of data-collection methods, including medical record documentation for the pre-, peri- and post-operative periods as well as direct skin observation and assessment for outcome assessment 24 and 48 hours after surgery. To ensure consistency of reporting, RAs were trained in skin assessment, PI staging and medical record data collection. In addition to capturing PI incidence, this study also reported evidence-based processes of care along the surgical patient journey.
Study limitations include, firstly, that it was conducted at one large inner-city hospital and the results may not be generalisable to other health facilities, particularly in rural areas. Secondly, only elective surgical patients were recruited and these patients may have been healthier than surgical patients admitted via the emergency department. However, the study sample had comparable general characteristics to those documented in other studies and was representative of patients who undergo surgery requiring a 48-hour stay at the study site facility13,23,34. Thirdly, we only followed patients for 48 hours post-operatively and it may be that PIs developed after this period, particularly for patients that were identified as being at very high-risk of developing a PI and it may have been useful to continue to follow up these patients to observe any PI development. However, it is debatable whether PIs developed more than two days post-operatively could be directly attributable to the surgical procedure.
Conclusions
Nurses along the health care continuum play an important role in preventing the development of PIs in surgical patients by conducting risk assessments, monitoring skin integrity and implementing preventive strategies peri-operatively and in the post-operative period until patients are independent and able to reposition themselves and mobilise. Even where the incidence of PIs is very low, improvements are needed in terms of documenting and instituting appropriate PIP for high to very high-risk patients before, during and following surgery. In particular, an understanding of how nurses interpret and use the information from PI risk assessments to make decisions about, and for informing a PIP plan for those at high risk, would be of value for improving practice.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study was funded by a multidisciplinary patient-focussed grant provided by the St Vincent’s Clinic Foundation. The funding source had no role in study design, data collection, analysis, or interpretation of the data.
Acknowledgements
The authors would like to acknowledge the SPPII Advisory group. Dr James Southwell-Keelv, Plastics, Visiting Medical Officer; Dr Maroun Mallat, Anaesthetist, St Vincent’s Hospital (SVH); Ms Levina Saad, Clinical Nurse Educator, SVH; Ms Hilary Kloczko, SVH Peri-operative and interventional services (PAIS) Peri-operative recovery, SVH; Ms. Dominique Christian, Physiotherapy, SVH; Ms Meredith Gimblett, Nurse Educator, PAIS, Peri-operative Scrub Team; Mrs Dominique Pennington, Nurse unit Manager, PAIS, Day Procedure Centre; Mrs Kristel Alken, Clinical Nurse Educator, PAIS, Day Procedure Centre; Mr Gavin Brunker, Nurse Unit Manager, SVH Admissions Centre; Ms Margaret Butler Nurse Educator, PAIS, Peri-operative Scrub Team; Flavia Darwell, SPPII study Research Assistant, Nursing Research Institute; Deanna Rowe, SPPII study Research Assistant, Nursing Research Institute and Dr Oyebola Fasugba Research Officer, Nursing Research Institute.
Author(s)
Cintia M Martinez-Garduno
PhD
Nursing Research Institute, St Vincent’s Health Australia (Sydney) and Australian Catholic University, NSW, Australia
Jane Rodgers
MS Nursing
St Vincent’s Hospital Sydney, NSW, Australia
Rosemary Phillips
BA
Nursing Research Institute, St Vincent’s Health Australia (Sydney) and Australian Catholic University, NSW, Australia
Anoja W Gunaratne
PhD
Nursing Research Institute, St Vincent’s Health Australia (Sydney) and Australian Catholic University, NSW, Australia
Peta Drury
PhD
School of Nursing, Midwifery and Paramedicine Australian Catholic University, NSW, Australia
Elizabeth McInnes*
PhD
Nursing Research Institute, St Vincent’s Health Australia (Sydney) and Australian Catholic University, NSW, Australia
Email liz.mcinnes@acu.edu.au
* Corresponding author
References
- Australian Institute of Health and Welfare. Australian Hospitals: 2014–15 at a glance. Canberra, 2016.
- Association of Perioperative Registered Nurses (AORN). AORN Position Statement on Perioperative Pressure Ulcer Prevention in the Care of the Surgical Patient. AORN J 2016;104:437–8.
- Fawcett D. Prevention of positioning injuries. In: Watson D (Ed). Perioperative Safety. St Louis, MO: Elsevier Health Sciences, 2010, 167–78.
- Nilsson UG. Intraoperative positioning of patients under general anesthesia and the risk of postoperative pain and pressure ulcers. J Perianesth Nurs 2013;28:137–43.
- Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J Wound Ostomy Continence Nurs 2016;43:585–97.
- Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5.
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Osborne Park, Australia: Cambridge Media, 2014.
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Aust Health Rev 2015;39:329–36.
- Bliss M, Simini B. When are the seeds of postoperative pressure sores sown? Often during surgery. BMJ 1999; 319:863–4.
- Gefen A. How much time does it take to get a pressure ulcer? Integrated evidence from human, animal, and in vitro studies. Ostomy Wound Manage 2008;54:26.
- Hayes RM, Spear ME, Lee SI et al. Relationship between time in the operating room and incident pressure ulcers: a matched case-control study. Am J Med Qual 2015;30:591–7.
- Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: An analysis of bedding materials. Res Nurs Health 1994;17:333–9.
- Schoonhoven L, Defloor T, van der Tweel I, Buskens E, Grypdonck MH. Risk indicators for pressure ulcers during surgery. Appl Nurs Res 2002;15:163–73.
- Franks PJ, Winterberg H, Moffatt CJ. Health-related quality of life and pressure ulceration assessment in patients treated in the community. Wound Repair Regen 2002;10:133–40.
- Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs 2007;57:494–504.
- Gorecki C, Brown JM, Nelson EA et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 2009;57:1175–83.
- Lumbley JL, Ali SA, Tchokouani LS. Retrospective review of predisposing factors for intraoperative pressure ulcer development. J Clin Anesth 2014;26:368–74.
- Heslop L, Lu S. Nursing-sensitive indicators: a concept analysis. J Adv Nurs 2014;70:2469–82.
- Griffiths P, Jones S, Maben J, Murrells T. State of the art metrics for nursing: a rapid appraisal. Kings College London, 2008.
- Australian Commission on Safety and Quality in Health Care. Safety and Quality Improvement Guide Standard 8: Preventing and Managing Pressure Injuries. Sydney: Australian Commission on Safety and Quality in Health Care, 2012.
- Miles S, Fulbrook P, Nowicki T, Franks C. Decreasing pressure injury prevalence in an Australian general hospital: a 10-year review. Wound Practice & Research 2013;21:148.
- Waters TM, Daniels MJ, Bazzoli GJ et al. Effect of Medicare’s nonpayment for hospital-acquired conditions: lessons for future policy. JAMA Intern Med 2015;175:347–54.
- Webster J, Lister C, Corry J, Holland M, Coleman K, Marquart L. Incidence and risk factors for surgically acquired pressure ulcers. J Wound Ostomy Continence Nurs 2015;42:138–44.
- Australian Commission on Safety and Quality in Health Care. Hospital-Acquired Complications Information Kit. Sydney: Australian Commission on Safety and Quality in Health Care, 2018.
- Sutherland-Fraser S, McInnes E, Maher E, Middleton S. Peri-operative nurses’ knowledge and reported practice of pressure injury risk assessment and prevention: A before-after intervention study. BMC Nurs 2012;11:25.
- Ganos D, Siddiqui A. Operating room. Washington, DC: National Pressure Ulcer Advisory Panel, 2012.
- Ursi ES, Galvão CM. Occurrence of pressure ulcers in patients undergoing elective surgeries. Acta Paul Enferm 2012;25:653–9.
- Chen HL, Chen XY, Wu J. The incidence of pressure ulcers in surgical patients of the last 5 years: a systematic review. Wounds 2012;24:234–41.
- Gunningberg L, Mårtensson G, Mamhidir AG, Florin J, Muntlin Athlin Å, Bååth C. Pressure ulcer knowledge of registered nurses, assistant nurses and student nurses: a descriptive, comparative multicentre study in Sweden. Int Wound J 2015;12:462–8.
- Engels D, Austin M, McNichol L, Fencl J, Gupta S, Kazi H. Pressure ulcers: factors contributing to their development in the OR. AORN J 2016;103:271–81.
- Scott SM. Progress and challenges in perioperative pressure ulcer prevention. J Wound Ostomy Continence Nurs 2015;42:480–5.
- Walton-Geer PS. Prevention of pressure ulcers in the surgical patient. AORN J 2009;89:538–48.
- Lyder C, Ayello E. Pressure ulcers: a patient safety issue. In: Hughes R (Ed). Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville: Agency for Healthcare Research and Quality (US), 2008.
- Bulfone G, Marzoli I, Quattrin R, Fabbro C, Palese A. A longitudinal study of the incidence of pressure sores and the associated risks and strategies adopted in Italian operating theatres. J Perioper Pract 2012;22:50–6.
- Schoonhoven L, Defloor T, Grypdonck MH. Incidence of pressure ulcers due to surgery. J Clin Nurs 2002;11:479–87.
- European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: Quick Reference Guide. Washington DC, 2009.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276–82.
- Moore Z, Johansen E, Etten Mv et al. Pressure ulcer prevalence and prevention practices: a cross-sectional comparative survey in Norway and Ireland. J Wound Care 2015;24:333–9.
- lLewicki LJ, Mion L, Splane KG, Samstag D, Secic M. Patient risk factors for pressure ulcers during cardiac surgery. AORN J 1997;65:933–42.
- Papantonio CT, Wallop JM, Kolodner KB. Sacral ulcers following cardiac surgery: incidence and risks. Adv Wound Care 1994;7:24–36.
- Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN J 1999;70(3):434–49.
- Tschannen D, Bates O, Talsma A, Guo Y. Patient-specific and surgical characteristics in the development of pressure ulcers. Am J Crit Care 2012;21:116–25.
- Vermillion C. Operating room-acquired pressure ulcers. Adv Skin Wound Care 1990;3:18–31.
- Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. J Wound Ostomy Continence Nurs 1999;26:130–6.
- Kemp MG, Keithley JK, Smith DW, Morreale B. Factors that contribute to pressure sores in surgical patients. Res Nurs Health 1990;13:293–301.
- Rao AD, Preston AM, Strauss R, Stamm R, Zalman DC. Risk factors associated with pressure ulcer formation in critically Ill cardiac surgery patients: a systematic review. J Wound Ostomy Continence Nurs 2016;43:242–7.
- Shen WQ, Chen HL, Xu YH, Zhang Q, Wu J. The relationship between length of surgery and the incidence of pressure ulcers in cardiovascular surgical patients: a retrospective study. Adv Skin Wound Care 2015;28:444–50.
- Liu P, He W, Chen HL. Diabetes mellitus as a risk factor for surgery-related pressure ulcers: a meta-analysis. J Wound Ostomy Continence Nurs 2012;39:495–9.
- Feuchtinger J, Halfens RJ, Dassen T. Pressure ulcer risk factors in cardiac surgery: a review of the research literature. Heart Lung 2005;34:375–85.
- Barakat-Johnson M, Lai M, Wand T, White K. A qualitative study of the thoughts and experiences of hospital nurses providing pressure injury prevention and management. Collegian 2018.
- McInnes E, Jammali-Blasi A, Bell-Syer SEM, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2015; Art. No.: CD001735. doi: 10.1002/14651858.CD001735.pub5.
- McInnes E, Chaboyer W, Murray E, Allen T, Jones P. The role of patients in pressure injury prevention: a survey of acute care patients. BMC Nurs 2014;13:41.
- Whitty JA, McInnes E, Bucknall T et al. The cost-effectiveness of a patient centred pressure ulcer prevention care bundle: Findings from the INTACT cluster randomised trial. Int J Nurs Stud 2017;75:35–42.



