Volume 27 Number 1
A cluster-controlled clinical trial of two prophylactic silicone sacral dressings to prevent sacral pressure injuries in critically ill patients
Monica Stankiewicz, Jodie Gordon, Joel M Dulhunty, Wendy Brown, Hamish Pollock and Nicola Barker-Gregory
Keywords pressure injury prevention, Sacral Silicone Dressings, Cluster Control Trial
For referencing Stankiewicz M et al. A cluster-controlled clinical trial of two prophylactic silicone sacral dressings to prevent sacral pressure injuries in critically ill patients. WP&R Journal 2019; 27(1):21-26.
DOI https://doi.org/10.33235/wpr.27.1.21-26
Abstract
Objective Patients in the intensive care unit (ICU) have increased risk of pressure injury (PI) development due to critical illness. This study compared two silicone dressings used in the Australian ICU setting for sacral PI prevention.
Design A cluster-controlled clinical trial of two sacral dressings with four alternating periods of three months' duration.
Setting A 10-bed general adult ICU in outer-metropolitan Brisbane, Queensland, Australia.
Participants Adult participants who did not have a sacral PI present on ICU admission and were able to have a dressing applied for more than 24 hours without repeated dislodgement or soiling in a 24-hour period (>3 times).
Interventions Dressing 1 (Allevyn Gentle Border Sacrum™, Smith & Nephew) and Dressing 2 (Mepilex Border Sacrum™, Mölnlycke).
Main outcomes measures The primary outcome was the incidence of a new sacral PI (stage 1 or greater) per 100 dressing days in the ICU. Secondary outcomes were the mean number of dressings per patient, the cost difference of dressings to prevent a sacral PI and product integrity.
Results There was no difference in the incidence of a new sacral PI (0.44 per 100 dressing days for both products, p = 1.00), the mean number of dressings per patient per day (0.50 for both products, p = 0.51) and product integrity (85% for Dressing 1 and 84% for Dressing 2, p = 0.69). There was a dressing cost difference per patient (A$10.29 for Dressing 1 and A$28.84 for Dressing 2, p < 0.001).
Conclusions Similar efficacy, product use and product integrity, but differential cost, were observed for two prophylactic silicone dressings in the prevention of PIs in the intensive care patient. We recommend the use of sacral prophylactic dressings for at-risk patients, with the choice of product based on ease of application, clinician preference and overall cost-effectiveness of the dressing.
Introduction
Pressure injury (PI) prevention is paramount to safe and effective care within any health care facility. Patients in the intensive care unit (ICU) have increased risk of PI development due to critical illness, which predisposes a person to acute skin failure, and other factors including supine positioning and elevating the head of the bed to 45°, which exposes the skin of the sacrum and heel to greater pressure1. As a consequence, the ICU often has the highest reported rates of PI incidence within acute care facilities2-6. A recent systematic review of PIs in adult intensive care patients identified that sacral PIs were the most common PIs occurring in 27–48% of patients1. Prophylactic silicone dressings are one prevention strategy to maintain sacral skin integrity that has proved beneficial in reducing the incidence of PIs in the ICU3-8.
There is a growing body of evidence related to the effectiveness of silicone-based products to prevent sacral PIs3-8. A recent ICU study by Kalowes et al. showed that the onset of PIs in patients receiving standard care was 5.9% compared to 0.7% in patients where a prophylactic silicone dressing (Mepilex Border Sacrum™) was used4. A study of another prophylactic silicone dressing (Allevyn LifeTM) in an elderly patient group with hip fractures, found the adjunct use of a silicone-based dressing decreased the rate of PI development from 15.4% to 4.5%9. There is little comparative information, however, on various silicone-based products available in Australia for the prevention of sacral PIs. The aim of this study was to explore whether there was a difference in sacral PI prevention between two prophylactic silicone dressings used in the Australian ICU setting: Dressing 1 (Allevyn Gentle Border Sacrum™, Smith & Nephew) and Dressing 2 (Mepilex Border Sacrum™, Mölnlycke). Our null hypothesis was that there was no difference in PI onset with the use of either prophylactic dressing.
Methods
Study design
The two prophylactic sacral dressings (2PSD) study utilised a cluster-controlled clinical trial design to compare the effectiveness of two silicone dressings in a general adult ICU at Redcliffe Hospital10. Redcliffe Hospital is an outer-metropolitan public teaching hospital in Brisbane, Queensland, Australia. The ICU has 10 beds, seven of which are ventilated, with a case mix that includes all diagnostic categories, except neurosurgical, cardiothoracic, burns and major trauma.
Adult participants (>18 years) were enrolled into the study within 48 hours of their admission to ICU. Patients were excluded from the study if they had a PI present on admission to the ICU, had dislodged or soiled a sacral dressing more than three times in a 24-hour period or were unable to have a dressing applied for more than 24 hours. Recruitment for the study took place over a period of 18 months from 17 February 2016 to 17 August 2017. During this time, participants were allocated to one dressing type based on clusters of three monthly allocations of each product, alternating between Dressing 1 and Dressing 2, that is, three cycles each. This allocation strategy was chosen to minimise the potential impact of seasonal variation on patient admissions in the ICU. Participants recruited into one dressing group stayed within this allocated group during changeover periods. Prophylactic dressings were utilised in conjunction with standard PI prevention strategies in the unit, which includes 2–4 hourly repositioning for clinically stable patients, specialised low air-loss beds and the use of adjunct repositioning and turning aids. There were no changes in standard PI prevention strategies during the study period. A PI risk assessment using the Waterlow Score was conducted on admission to the ICU with a score >20 indicative of a very high risk of PI development4,11.
Study interventions
Two sacral dressings were compared. Dressing 1 is a five-layer product that has a silicone adhesive contact layer (silicone gel adhesive), a hydrocellular foam (polyurethane), an absorbent core (cellulose fibre and polyacylate particles), a protective masking layer (hydrophilic polyester yarn) and a breathable film with padding support (polyurethane film)10,12. Dressing 2 is five-layer silicone product that has a perforated silicone wound contact layer (Safetac® technology), an absorbent core made of three layers, a thin sheet of polyurethane foam, a piece of non-woven fabric and a layer of absorbent polyacrylate fibre on a polyurethane film10,13.
Prior to the commencement of the study, education was delivered to all ICU nursing staff on PI assessment. This was conducted by the principal investigator (MS), a clinical nurse consultant for the wound management service. The research assistant (WB) provided ongoing support and education throughout the study period to ICU staff and was responsible for data management. The confirmation of PI development and staging was conducted by the principal investigator (MS) or the wound management team (the clinical experts) within the hospital.
Study outcomes
The primary outcome was the incidence of a new sacral PI per 100 dressing days in the ICU. A new sacral PI was defined as a new localised injury to the skin or underlying tissue rated as stage 1 (non-blanchable erythema) or worse (tissue injury) commensurate with international definitions14. Secondary outcomes were the mean number of dressings per patient, the cost difference of dressings to prevent a sacral PI (cost-effectiveness) and product integrity. Cost-effectiveness was defined as the average cost of dressings per patient in each group divided by the proportion of patients without a sacral PI. The cost of product at the time of the trial was A$10.29 for Dressing 1 (dimensions 17.2 x 17.5 cm) and A$14.42 for Dressing 2 (dimensions 18 x 18 cm). Product integrity was defined as the product remaining intact in the appropriate location since the last inspection, as opposed to the need for reapplication of the product after skin inspection or due to rolling of edges, and reported as a percentage of the total inspections (assessed twice-daily). Skin integrity, including PI staging, was assessed each shift by clinical nursing staff and confirmed by the wound management service.
Statistical analysis
Categorical variables are reported as number and percentage; continuous variables are reported as mean and standard deviation (SD) or median and inter-quartile range (IQR) depending on whether or not data were normally distributed. Baseline differences were assessed by chi-square test or Fisher’s exact test (for categorical data) and a student’s t-test (normal data) or Mann-Whitney U test (non-normal data). The primary and secondary outcomes were evaluated using an intention-to-treat analysis of all eligible patients. The primary outcome was compared between the two groups using Poisson regression. The 95% confidence interval for the incident rate is reported. Secondary outcomes (mean number of dressing changes and cost-effectiveness) were compared between groups using a student’s t-test or Mann-Whitney U test, depending on whether or not normality assumptions were achieved. Product integrity was compared between groups using a Pearson chi-square test. A two-sided p-value < 0.05 was considered statistically significant.
A priori sample size calculation indicated 200 participants were required in each group to achieve 90% power to detect a difference in incidence rate of 4/100 dressing days versus 2/100 dressing days (a 0.5 hazard rate) with an alpha of 0.05. Statistical analysis was conducted using SPSS version 25 (IBM, Armonk, NY). MedCalc version 18 (Belgium) was used to compare PI incidence rates.
Ethical considerations
This study was approved by The Prince Charles Hospital Human Research Ethics Committee. The need for individual consent was waived as the use of silicone prophylactic sacral dressings is standard practice within the ICU.
Results
Participant demographics
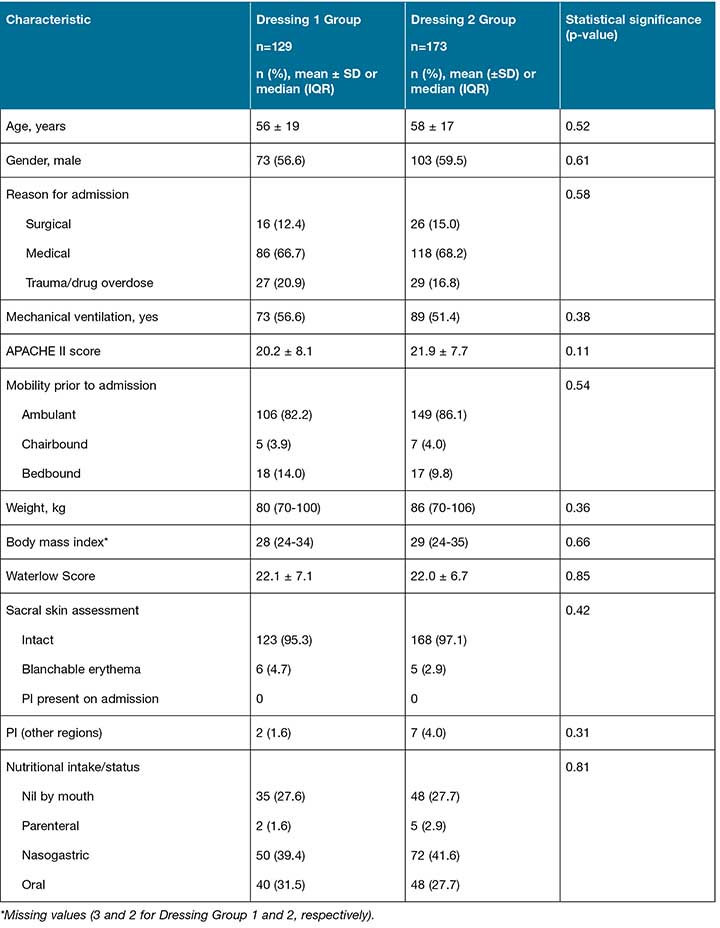
During the study period, 538 patients were assessed for eligibility with a total of 302 participants included in the analysis (Figure 1). There were 129 participants in the Dressing 1 group and 173 in the Dressing 2 group. There were no reported adverse effects from either prophylactic silicone dressing. Table 1 reports baseline characteristics for participants in the two dressing groups.
Figure 1: CONSORT flow diagram

Table 1: Baseline characteristics and pressure injury-related variables
Incidence of sacral pressure injuries
Two participants developed PIs in the Dressing 1 group (2/129) and three developed PIs in the Dressing 2 group (3/173). None of the participants who had a PI on other bodily sites at ICU admission (n=9) developed a sacral PI during the study. The number of dressing days in each group was 452 days and 677 days, respectively. The primary outcome, incidence of a new sacral PI per 100 dressing days, was not statistically different between groups (Table 2).
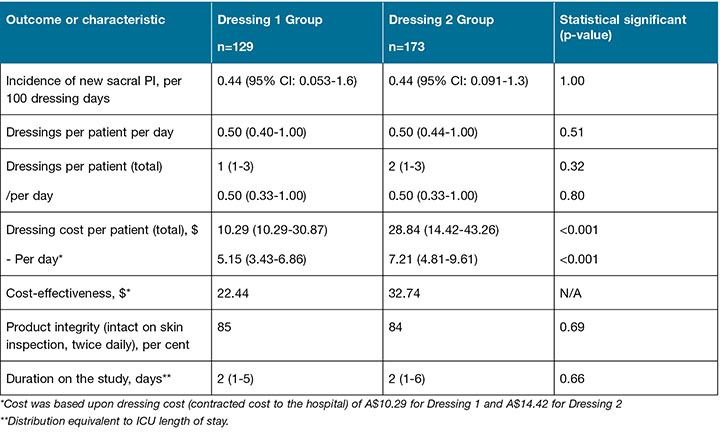
Table 2: Study and ICU outcomes
Secondary outcomes
Secondary outcomes are reported in Table 2. Of the 302 participants recruited to the study, the intervention was discontinued in 17.5% (n=53) due to the dressings being repeatedly soiled or not able to withstand loose stools. Intervention discontinuation frequency between Dressing 1 and Dressing 2 were similar (n=23 or 17.8% and n=30 or 17.3% respectively: Figure 1). There was a difference in the cost of dressings per patient, with no differences in the other secondary outcomes.
Discussion
This study found no difference in the primary outcome, the incidence of sacral PI development, between the two prophylactic silicone dressings investigated. We observed an overall low incidence of PI development during the study (0.44 per 100 dressing days) with a prevalence of 1.7% and 1.6% for the Dressing 1 and Dressing 2 group, respectively, supporting the effectiveness of both interventions. There was no difference between groups in terms of the number of dressings used per patient, intervention discontinuation due to soiling or product integrity. There was significant cost difference of A$18.55 per patient in favour of Dressing 1.
Our PI incident rate matched other study outcomes after prophylactic dressings were implemented. For example, a randomised controlled trial by Santamaria et al. showed the application of a multi-layer foam dressing (Dressing 2) to the sacrum and heels reduced the onset of new PI in the ICU by 10%, that is, 13.1% in the standard care group compared to 3.1% in the prophylactic dressing group5. Walsh et al. reported a 5.5% decline in sacral PIs following implementation of sacral silicone foam dressings (Dressing 2) from 12.5% in the year prior to silicone dressing implementation to 7% in the post-implementation year6. Chaiken similarly reported a 35-month prevalence rate for sacral PIs of 13.6%, which decreased to 1.8%, an absolute reduction of 11.8%, following the routine application of prophylactic silicone dressings (Dressing 2)8. In earlier investigations, Brindle reported on 93 ICU patients in a three-month period and observed that out of a group of 41 high-risk patients in whom prophylactic silicone foam was applied to the sacrum, no patients developed a PI7. During the same study period, three in the group without prophylactic sacral dressings developed PIs (3/52 or 5.8%) and three in the high-risk group (3/41 or 7.3%) developed PIs after the intervention was ceased.
The effectiveness of Dressing 2 against no dressing has been shown in five studies4-8. However, Dressing 1 has only recently been investigated for effectiveness3,12. Byrnes et al. conducted a prospective, non-randomised, quasi-experimental observational study of Dressing 1 in the ICU and found that prior to the implementation of the silicone dressing the PI incidence over a seven-month period was 6.98 per 1000 patient days. In the proceeding seven months with the implementation of the prophylactic silicone dressing, the PI incidence decreased to 3.40 per 1000 patient days. Our study contributes to the evidence base by comparing both dressings in a similar population of critically ill patients at high risk of PI development.
In regard to product integrity and protecting skin from soiling, we found that neither product was able to withstand loose stools. The reason for this was that stool would be trapped under the silicone dressings or would lead to removal of the product due to its loss of adhesion. The risk of leaving soiled dressings in situ would increase the risk of skin breakdown and development of incontinence-associated dermatitis (IAD). Furthermore the use of silicone dressing is not recommended in preventing IAD15-16. This study did not explore the relationship between prophylactic silicone dressing and IAD, nor the relationship between IAD and PI, thus we are unable to comment on this further.
There are a number of limitations of this study that deserve mention. We were not able to achieve the intended sample size due to a lower than expected recruitment rate and funding limitations. As a consequence, we were only able to achieve 76% of the intended sample size, which increases the chance of a type II error in terms of product effectiveness and performance. The baseline PI incidence was overestimated in sample size calculations, having a similar impact on decreasing the power to detect a difference in PI incidence if one did exist. Despite this, the high degree of concordance in findings suggest the likelihood of this is small. A further limitation of the study is that ICU staff contributed to data collection with the potential for missing data on the number of dressing changes. Therefore, this may have had some impact on secondary outcomes with the potential for results to be underestimated. However, the degree to which this occurred could not be estimated.
Finally, staff experience with use of both dressings was not systematically evaluated, therefore clinician preference could not be reported upon.
In conclusion, this study provides comparative evidence for two prophylactic silicone dressings used in Australia to prevent sacral PIs in the ICU population with no significant difference in overall performance observed, but an associated cost difference. Our study supports previous research reporting the benefits of prophylactic silicone dressings in conjunction with standard PI prevention strategies in preventing PIs. We recommend use of sacral prophylactic dressings for at-risk patients with the choice of product based on ease of application, clinician preference and overall cost-effectiveness of the dressing.
Acknowledgements
The authors would like to acknowledge Martin Christensen (Queensland University of Technology), Patrick Young (CIS Manager, ICU, Redcliffe Hospital) and all the ICU staff who ensured the commencement of the project and ongoing data collection was successful.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Monica Stankiewicz
RN, MAppSc (Research), MNSc (NP), Grad Dip WM, Grad Cert STN
Nurse Practitioner, Royal Brisbane and Women’s Hospital, Metro North Hospital and Health Service; Nurse Practitioner, Haut Dermatology, Spring Hill, Brisbane; Nurse Practitioner, Wound Therapies, Brisbane, QLD, Australia
Jodie Gordon*
RN, MClinSpec (WM), MHealthAdmin, Grad Cert STN
Clinical Nurse Consultant, Wound Management Stomal Therapy
Redcliffe Hospital, Metro North Hospital and Health Service, QLD, Australia
Email Jodie.Gordon@health.qld.gov.au
Joel M Dulhunty
MBBS, MTH, PhD
Director of Research & Medical Education, Redcliffe Hospital, Metro North Hospital and Health Service; Senior Lecturer, Faculty of Medicine, The University of Queensland; Adjunct Professor, School of Public Health and Social Work, Queensland University of Technology, QLD, Australia
Wendy Brown
RN, Cert ICN
Research Assistant, Intensive Care Unit,
Redcliffe Hospital, Metro North Hospital and Health Service, QLD, Australia
Hamish Pollock
BM, MRCP, FRCA, DICM(UK), FCICM
FANZCA, PGDipCU
Director, Caboolture-Redcliffe Intensive Care Unit, Redcliffe Hospital; Staff Specialist, Department of Anaesthesia and Perioperative Medicine, Royal Brisbane and Women’s Hospital, Metro North Hospital and Health Service;
Senior Lecturer, Faculty of Medicine,
The University of Queensland, QLD, Australia
Nicola Barker-Gregory
RN, Grad Cert ICU, Grad Cert Adult & Wkplace Ed, M Edu
Project Officer, Redcliffe Hospital, Metro North Hospital and Health Service, QLD, Australia
* Corresponding author
References
- Chaboyer W, Thalib L, Harbeck E et al. Incidence and prevalence of pressure injuries in adult intensive care patients: A systematic review and meta-analysis. Crit Care Med 2018;46(11):e1074–e1081.
- Edsberg LE, Langemo D, Baharestani MM et al. Unavoidable pressure injury: state of the science and consensus outcomes. J Wound Ostomy Continence Nurs 2014;41(4):313–334.
- Byrne J, Nichols P, Srocynski M et al. Prophylactic sacral dressing for pressure ulcer preventing in high-risk patients. Am J Crit Care 2016;25(3):228–234.
- Kalowes P, Messina V, Li M. Five-layered soft silicone foam dressing to prevent pressure ulcers in the intensive care unit. Am J Crit Care 2016;25(6): e108–e119.
- Santamaria N, Gerdtz M, Sage S et al. A randomised controlled trial of the effectiveness of soft silicone multilayered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J 2013; doi: 10.1111/iwj.12101
- Walsh N, Blanck A, Smith L et al. Use of a sacral silicone border foam dressing as one component of a pressure ulcer prevention program in an intensive care unit setting. J Wound Ostomy Continence Nurs 2012;39(2):146–149.
- Brindle T. Outliers to the Braden Scale: Identifying high-risk ICU patients and the results of prophylactic dressing use. WCET Journal 2010;30(1):11–18.
- Chaiken N. Reduction of sacral pressure ulcers in the intensive care unit using a silicone border foam dressing. J Wound Ostomy Continence Nurs 2012;39(2):143–145.
- Forni C, D’Alessandro F, Gallerani P et al.. Effectiveness of using a new polyurethane foam multi-layer dressing in the sacral area to prevent the onset of pressure ulcer in the elderly with hip fractures: A pragmatic randomised controlled trial. IMJ 2017;15:383–390
- Gordon J, Stankiewicz M, Pollock H et al. A trial of two prophylactic sacral dressings (2PSD) in the prevention of stage 1 sacral pressure injury in the critically ill patient: A study protocol. Wound Practice & Research 2017;25(2):82–86.
- Mahalingham S, Gao L, Nageshwaran S et al. Improving pressure ulcer risk assessment and management using the Waterlow Scale at a London teaching hospital. J Wound Care 2014;23(12):613–22
- Smith & Nephew Contract Briefing Document, Allevyn Life Sacrum. Retrieved 1 June 2014, from http://www.smith-nephew.com/allevynhome/our-products/allevynlife sacrum/
- STML Dressings Card, Mepilex Border Sacrum. Retrieved 1 June 2014, from http:www.dressings.org/Dressings/mepilex-border-sacrum.html
- Haesler E (Ed). National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Osborne Park, WA, Australia: Cambridge Media, 2014.
- Lee YC, Campbell J, Doubrovsky A et al. A descriptive exploratory survey of incontinence-associated dermatitis in the intensive care setting: The ADDrESS study. Wound Practice & Research 2018;26(4):170–181.
- Beeckman D et al. Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: moving prevention forward. Wounds International; 2015. Available from www.woundsinternational.com



