Volume 28 Number 1
Use of iodine-impregnated surgical drapes for prevention of surgical site infection: a systematic review and meta-analysis
Olivia Nicholson, Bernard Ho and Chun Chon
Keywords surgical site infection, iodine-impregnated drapes, surgery
For referencing Nicholson O et al. Use of iodine-impregnated surgical drapes for prevention of surgical site infection: a systematic review and meta-analysis. Wound Practice and Research 2020; 28(1):30-37.
DOI https://doi.org/10.33235/wpr.28.1.30-37
Abstract
Background Iodine-impregnated surgical drapes aim to protect the wound from bacterial re-colonisation and therefore prevent surgical site infection (SSI). Studies have produced conflicting results regarding the efficacy of this intervention.
Methods Ovid EMBASE, Ovid MEDLINE, Scopus and PubMed were searched for randomised control trials (RCTs) and cohort studies in which iodine-impregnated drapes were used to reduce SSI. The risk of bias was evaluated using the Cochrane Risk of Bias 2.0 tool for RCTs and the ROBINS-I tool for cohort studies. RevMan was used for meta-analysis. Additional sub-group analysis was performed for incision type.
Results Two RCTs and seven cohort studies inclusive of 4119 patients were included. The RCTs demonstrate a risk ratio (RR) for SSI in the intervention group of 0.92 (p=0.70), whereas the RR in the cohort studies is 0.45 (p=0.01). The number needed to prevent SSI in the cohort studies is 19.5. There is also a statistically significant reduction in SSI in the intervention group for clean-contaminated incisions, with SSI occurring in 3.8% of surgeries with an iodophor drape and 9.2% of surgeries without (RR 0.45, p=0.02).
Conclusion Our review suggests that iodine-impregnated drapes are beneficial in reducing postoperative SSI, particularly in clean-contaminated surgeries; however, the grade of evidence is poor.
Background
Surgical site infection (SSI) is defined by the USA Center for Disease Control (CDC) as an infection that occurs within 30 days of and is anatomically associated with a surgical procedure, involving either the incision site or an organ space1,2. The World Health Organization (WHO) states that SSI is the most common healthcare-associated infection and occurs in up to a third of patients who have undergone a surgical procedure3. In Australia, SSI occurs in around 3% of surgical procedures, resulting in patients remaining in hospital for an average of 20.3 extra days4.
Due to the high impact of SSI on health outcomes and costs, a number of strategies have been adopted to reduce their incidence. The use of plastic adhesive drapes was first investigated by Payne in 19565. Subsequently, there have been modifications to improve efficacy, including impregnating the drapes with the antiseptic iodine. The theory is that SSI is caused by skin flora due to re-colonisation which occurs during the surgery from deeper skin layers and hair follicles despite antiseptic skin preparation. Adhesive drapes act as a microbial barrier to prevent translocation of bacteria to the operative site6.
Although widely adopted, studies have demonstrated conflicting results about the efficacy of iodine-impregnated drapes. In 2010, a systematic review was performed on the effectiveness of iodine-impregnated drapes, evaluating seven studies7. However, a number of the studies included had significant limitations; two were observational studies, one did not include any operations with an iodine-impregnated drapes, and one evaluated the effectiveness of Ioban-1TM, which was subsequently discontinued due to the associated rate of adhesive lift8. In 2015, a systematic review was performed on the effectiveness of adhesive drapes but only included two studies with 1,113 patients that examined iodine-impregnated drapes9. In 2016, WHO released Global Guidelines for the Prevention of Surgical Site Infection which did not recommend the use of plastic adhesive incision drapes due to the low to very low quality of evidence3. However, this review only included four studies of 994 patients, in which one retrospective cohort study examined mesh infections rather than SSI and a pseudo-randomised cohort study was analysed as an randomised control trial (RCT) with regards to bias. A number of new studies on this topic have subsequently been published. These are all retrospective cohort studies and, although considered weaker evidence than an RCT, these should not be dismissed from the available evidence base.
The objective of this systematic review is to assess the effect of iodine-impregnated drapes used during surgery to prevent SSI compared with traditional liquid antimicrobial skin preparations or non-iodine-impregnated plastic drapes. Due to the low rate of SSI, it is estimated that, ideally, a sample size of at least 10,000 is required in order to confirm the efficacy of iodine-impregnated drapes7. Given that there are only small and limited systematic reviews available regarding a product that is widespread and may cause allergic reactions, this larger review, including relevant RCTs and retrospective cohort studies and sub-group analysis of incision types, is required to guide clinical practice.
Methods
Eligible studies
This review includes RCTs and cohort studies published between 1984 and 2019. The Ioban-2TM (3M Science, Minneapolis, USA) drape became widely available around 1984 after the discontinuation of the Ioban-1 drape.
Eligible participants
Any article studying patients who undergo surgery can be considered eligible for inclusion for this review. There are no age nor gender limitations.
Eligible interventions
The intervention to be examined in this study is iodine-impregnated drapes of any brand. These drapes can be used alone or in combination with other drapes or any antiseptic skin preparation. The comparison intervention is any liquid antimicrobial skin preparation or non-iodine adhesive drape.
Outcomes
The outcome of interest for this review is the rate of postoperative SSI.
Information sources
We searched Ovid MEDLINE, Ovid EMBASE, Scopus and Pubmed electronic databases on 10 September 2019. The Cochrane Central Register of Controlled Trials and Australia and New Zealand Clinical Trials Registry were searched to identify any unpublished data. The Ovid MEDLINE and EMBASE search strategy is outlined below; a similar strategy was employed for the other databases.
- surgical wound infection/
- surgical wound dehiscence/
- (surg* adj5 wound*).tw
- (surg* adj5 dehisc*).tw
- (surg* adj5 infection*).tw
- (wound* adj5 dehisc*).tw
- (wound* adj5 infection*).tw
- 1 or 2 or 3 or 4 or 5 or 6 or 7
- Ioban.tw
- (io* adj3 drape*).tw
- 9 or 10
- 8 and 11
Data collection and analysis
Selection of studies
The search results from each electronic database were examined and included based on the article’s title, abstract and the article itself.
Data collection and items
Data was extracted and imported into Review Manager 5 (RevMan)10 and included the type of study, number of participants, type of surgery, skin preparation used, pre-operative antibiotic usage, SSI definition used, and follow-up period.
Assessment of risk of bias
The authors assessed the quality and associated bias of eligible trials. RCTs included were analysed using the Cochrane Risk of Bias 2.0 tool11. Cohort studies were analysed using the ROBINS-I tool11. Funnel plots were created to assess the distribution and size of studies and the possibility of publication bias.
Summary measures
RevMan was used to perform a meta-analysis on the quantitative outcome, dividing the main analysis by study type. The relative risk (RR) and 99% confidence interval (CI) were calculated using a random-effect model. Significance was set at p<0.05. Heterogeneity was assessed by calculating the Chi2 statistic with significance set at p<0.10 and by calculating the I2 statistic. Additional subgroup analysis were performed for incision type.
Results
Study selection / search results
The initial search identified 59 articles. Nine studies were included in the review. A number of studies were excluded due to study type, being an animal study, or having a primary endo-point of wound bacterial colonisation. There were no new, unpublished studies nor data identified during the search. The study inclusion flowchart is demonstrated in Figure 1.
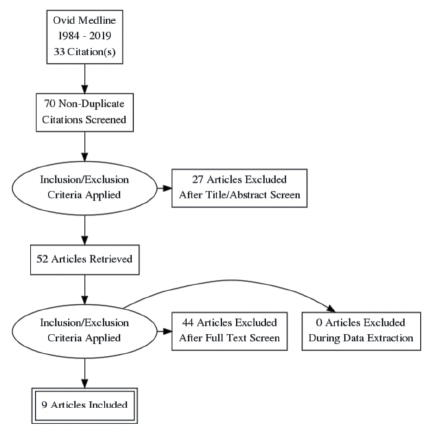
Figure 1. Study inclusion flowchart
Study characteristics
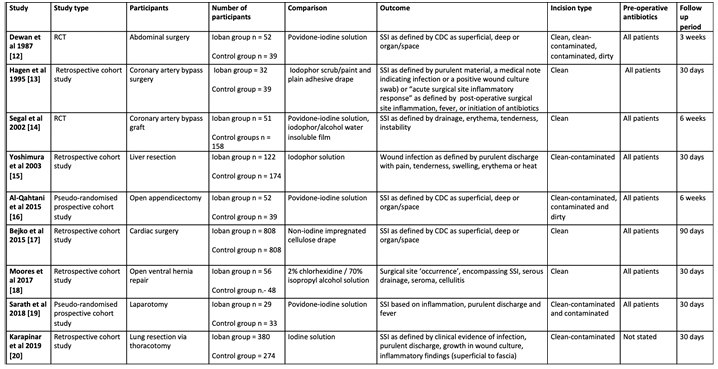
Two RCTs and seven cohort studies were included. The study characteristics are outlined in Table 1. The size of the studies ranges from 62 to 1,616 patients, and the type of surgery and incision classification varies widely between studies.
Table 1. Study characteristics
Effects of interventions
Pooled data analysis
Nine studies and 4,119 patients were included in the final analysis. There were 2,051 patients in the intervention (iodine-impregnated drape) group and 2,068 in the control (no iodine-impregnated drape) group. There were 245 incidences of SSI (5.95%); 82 of these occurred in the intervention group and 163 in the control group.
Overall, there was a statistically significant reduction in the rate of SSI in the intervention group when combining the results of both RCTs and cohort studies. In the intervention group, there was a 4.0% rate of SSI, compared to 7.9% in the control group (RR 0.53, 95% CI 0.32–0.88, p=0.01, see Figure 2).
Analysis of the RCTs demonstrates a reduction in SSI in the intervention group with an RR of 0.92 (95% CI 0.60–1.41); however, this is not statistically significant (p=0.70, see Figure 2). The number needed to treat is 111.1. Analysis of the cohort studies demonstrates a statistically significant reduction in SSI in the intervention group, with the RR of the patient in the intervention group developing an SSI being 0.45 (95% CI 0.25–0.72, p=0.01), hence a 55% reduction in the rate of SSI (see Figure 2). The number needed to treat is 19.5.
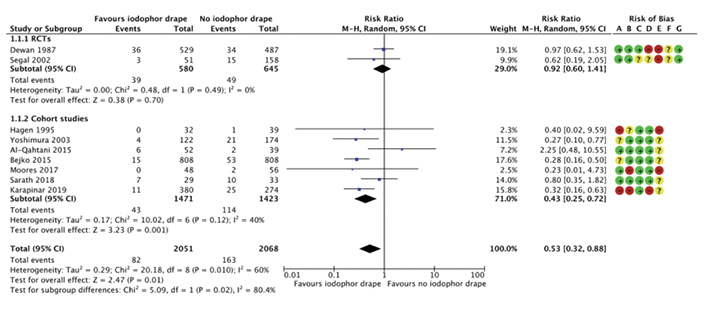
Figure 2. Forest plot of iodophor drape vs control group for the outcome of SSI by study type (RCT or cohort study), including risk of bias summary
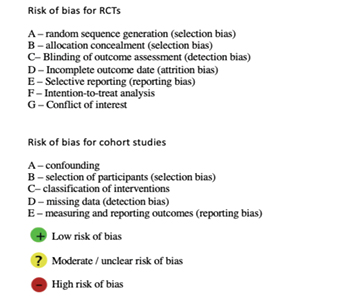
Legend for Figure 2
Subgroup analyses
Subgroup analysis was conducted for incision classification (see Figure 3). Sarath (2018)18 was excluded from this analysis as it was not possible to determine the distribution of incision classification. There is a statistically significant reduction in SSI when iodine-impregnated drapes are used in clean-contaminated incisions (see Figure 3), with 27 out of 712 cases with iodophor drape and 57 out of 622 without being complicated by SSI (RR 0.45 95% CI 0.24–0.87, p=0.02). There is a trend towards reduction in SSI when iodine-impregnated drapes are used in clean wounds, but this is not statistically significant (see Figure 3), with 30 out of 1,158 cases with iodophor drape and 81 out of 1,271 cases without being complicated by SSI (RR 0.52 95% CI 0.24–1.11, p=0.09). Iodine-impregnated drapes are not beneficial when used in contaminated or dirty wounds.
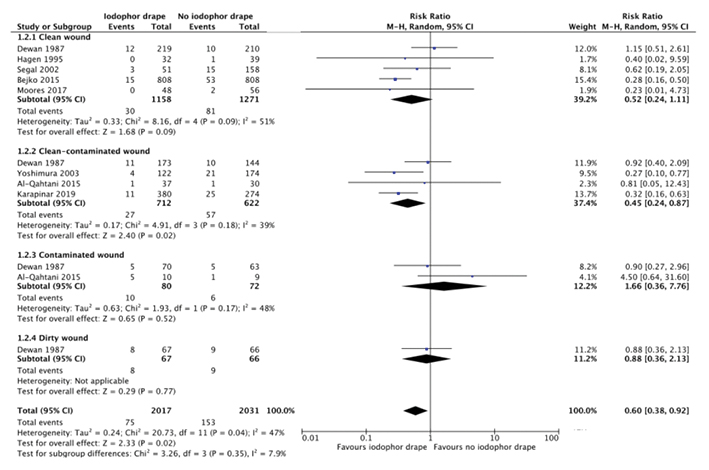
Figure 3. Forest plot of subgroup analysis by incision classification
Risk of bias in included studies – RCTs
Random sequence generation
Both RCTs have a low risk of bias as they state the method used to randomly allocate patients to the intervention or control group.
Allocation concealment
Both RCTs state that the allocation was concealed from the operating staff until just prior to the surgery. It is impossible for operative staff to be blinded to the patients’ assignment in RCTs performed on intra-operative interventions.
Blinding
In Dewan (1987)12, the assessors were blinded. However, in Segal (2002)14, it was not stated whether the assessors were blinded.
Attrition bias
Dewan (1987)12 has a high risk of bias because 86 of the 1,102 patients enrolled in the trial were not included in the final analysis due to incomplete records or follow-up. Segal (2002)14 does not state an attrition rate.
Selective reporting
It is likely that Dewan (1987)12 under-reports and Segal (2002)14 over-reports the rate of SSI due to follow-up periods of 21 days and 6 weeks respectively.
Intention-to-treat analysis
No group violations are reported.
Conflict of interest
No conflicts of interest are reported.
Risk of bias in included studies – cohort studies
Bias due to confounding
Karapinar (2019)20 and Hagen (1995)13 have a high risk of bias as the intervention and control occurred over different time periods and Hagen (1995)13 notes a significant increase in operative and cardiopulmonary bypass pump time in the control group.
Yoshimura (2003)15 has moderate confounding bias, as there was variation in the antiseptic skin preparation solution and the laparotomy incision approach. There were also significant patient demographic differences, and body mass index and smoking were also independent risk factors for SSI.
Bejko (2015)17 has a moderate risk of bias. Although the intervention group has significantly higher frequency of SSI risk factors compared to the control group, propensity scores were calculated to accommodate this. Secondly, the choice to utilise the iodine-impregnated drape depended on surgeon preference, so each surgeon’s baseline rate of SSI could have influenced the overall result.
Moores (2017)18 has a low risk of bias as it has well-matched control and intervention groups. The intervention and control surgeries occurred at different time intervals and sites; however, all the surgeries were undertaken by a single surgeon.
Al-Qahtani (2015)16 and Sarath (2018)19 have a low risk of bias as they are both pseudo-randomised prospective studies with well-matched participant characteristics.
Bias due to selection of participants
The intervention groups in each study are clearly defined and could not have been influenced by the outcome.
Moores (2017)18 and Karapinar (2019)20 have a high risk of bias due to the exclusion of patients with predisposition to SSI. Bejko (2015)17 has a low risk of bias because the investigators conducted a univariate regression analysis and calculated propensity scores in order to generate matched intervention and control groups. Al-Qahtani (2015)16 and Sarath (2018)19 have a low risk of selection bias due to being pseudo-randomised and prospective.
Bias in classification of interventions
All seven cohort studies clearly outline the control group and intervention.
Bias due to missing data (detection bias)
There is a high risk of bias in Karapinar (2019)20 due to exclusion of patients with missing data. The other studies demonstrate a low risk of bias.
Bias in measuring and reporting outcomes
The definition of SSI differs greatly between the studies, with only three using the CDC definition. Moreover, the follow-up periods also vary widely, with only five studies using the CDC definition of 30 days (see Table 1)1. The other studies likely over-report the rate of SSI due to longer follow-up periods.
Moores (2017)18 has a high risk of bias due to use of the term “surgical site occurrence”, encompassing a range of wound complications12. In particular, wound cellulitis is considered a separate complication, although the authors of this review would consider this to constitute SSI. Similarly, Hagen (1995)13 separates SSI from “acute surgical site inflammatory response” which the authors of this review would consider an SSI.
Segal (2002)14, Yoshimura (2003)15, Sarath (2018)19 and Karapinar (2019)20 have a moderate risk of bias as they clearly define SSI; however, they exclude infection of the deeper organ space, meaning SSI rates were likely under-reported.
Dewan (1987)12, Bejko (2015)17 and Al-Qahtani (2015)16 have a moderate risk of bias; although they clearly define the outcome of SSI according to the CDC criteria, the follow-up periods are longer than 30 days.
Publication bias
A funnel plot was created to assess the risk of publication bias (see Figure 4). The overall asymmetry is due to the differences in study size and effect measures. The asymmetry in the left lower aspect accommodates two studies, Hagen (1995)13 and Moores (2017)18. These are both small studies that demonstrate a reduced rate of SSI in the intervention group. The lack of studies in the right lower aspect may suggest publication bias. However, no unpublished data from conference abstracts nor clinical trial registries was identified during the literature search.
Discussion
This review of nine studies including 4,119 patients provides evidence that iodine-impregnated drapes can reduce the rate of SSI, particularly in clean-contaminated surgery. Overall, there is a 5.95% rate of SSI in the studies included in this review. The overall pooled effects favoured iodine-impregnated drapes, with a 47% reduction in the rate of SSI (RR 0.53, 95% CI 0.32–0.88, p=0.01). The incision classification sub-group analysis suggests that iodine-impregnated drapes are particularly effective when used in clean-contaminated wounds (RR 0.45; 95% CI 0.24–0.87, p=0.02). This correlates with the theory that the drapes reduce wound re-colonisation due to translocation of skin flora after sterile skin preparation. The drapes were less useful in clean wounds, likely as a result of the low incidence of SSI in this setting and therefore underpowered the nature of this review. The drapes were also less useful in contaminated and dirty wounds due to postoperative infection occurring due to direct contamination from the surgical site rather than bacterial re-colonisation of the wound.
Previous reviews by Kramer (2010)7 and Webster (2015)9 failed to demonstrate a statistically significant reduction in the rate of SSI in procedures using an iodine-impregnated drape. However, these included fewer studies and the Kramer review included studies with significant limitations. An analysis performed by WHO in 20163 also did not recommend the use of iodine-impregnated drapes; however, again, only four studies were included, one of which was excluded from our review, and Al-Qahtani16 was analysed as an RCT rather than a pseudo-randomised cohort study. This systematic review confirms the results of these previous reviews with the inclusion of new evidence but also offers additional information regarding the incision-type subgroup analysis, suggesting that iodine-impregnated drapes may be beneficial in clean-contaminated surgery.
The studies included in this review are of variable quality. The RCTs both have a moderate risk of bias. Four of the cohort studies have a low risk of bias, while three have a high risk. The three studies that demonstrate a statistically significant decrease in the rate of SSI in the iodophor-drape group have significant power in the final analysis, accounting for 2,566 of the 4,119 patients. Five studies have a non-statistically significant trend towards decreased rate of SSI in the iodine-impreganted drape group. Only one study demonstrates a higher rate of SSI in the intervention group and this is a study with a small number of patients. The fact that most of the studies trend towards favouring the iodine-impregnated drape provides some confidence in the individual trial results. However, due to the moderate bias of the RCTs and to most evidence arising from cohort studies, the grade of evidence regarding iodine-impregnated drapes is poor.
A strength of this review is that it is the largest and most up-to-date systematic review on this topic, including not only RCTs but also cohort studies. There are only two RCTs on this topic and, although considered a lower grade of evidence, the cohort studies are mostly of good quality with a low risk of bias and should not be dismissed from the evidence. Unlike previous systematic reviews, we have performed a sub-group analysis based on incision type, which is important because it demonstrates that there is only statistically significant evidence for iodine-impregnated drapes in clean-contaminated surgery. The main weakness of this review is the degree of heterogeneity between the studies, likely due to the differences in study method and size, surgery type, definition of SSI, and duration of follow-up. Moreover, most of the studies examine clean or clean-contaminated cardiothoracic or abdominal surgeries, making it difficult to extrapolate the data to other types of surgery.
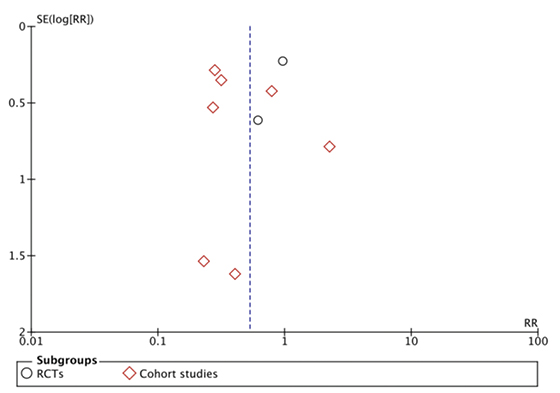
Figure 4. Funnel plot of included studies
Conclusion
Iodine-impregnated drapes appear to reduce the risk of SSI only when used in clean-contaminated wounds; however, the grade of evidence is poor. This is because we have included cohort studies which are graded as a lower grade of evidence despite many of these studies being good quality without a high risk of bias. Therefore, we do not recommend they be utilised as a first line approach to reducing SSI but suggest that larger RCTs could be performed to ascertain if there is a true benefit.
Acknowledgements
The protocol for this review was registered with PROSPERO on 13/12/2019 – registration number CRD42019147986. The authors of this review acknowledge the assistance of Professor Debra Nestel from the University of Melbourne and clinical librarians from the Health Sciences Library at Austin Health. There was no funding source for this review.
Disclosure statement
The authors declare no conflict of interest.
Author(s)
Olivia Nicholson* MBBS, GradDip Surg Anat
The University of Melbourne Faculty of Medicine,
Dentistry and Health Sciences
Melbourne, VIC, Australia
Email olivia.nicholson.29@gmail.com
Bernard Ho MBBS, GradDip Surg Anat
The University of Melbourne Faculty of Medicine, Dentistry and Health Sciences
Chun Chong MBBS
The University of Melbourne Faculty of Medicine,
Dentistry and Health Sciences
* Corresponding author
References
1. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection. Am Journal Infect Cont 1999 Apr;27(2):97–134.
2. Solomkin JS, Mazuski J, Blanchard JC, Itani KMF, Ricks P, Dellinger EP, et al. Introduction to the Centres for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee guideline for the prevention of surgical site infections. Surg Infect 2017;18(4):385–393.
3. World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection [Internet]. Geneva: WHO, 2016 [cited 2019 Aug 6]. 185 p. Available from https://www.who.int/gpsc/ssi-prevention-guidelines/en/
4. Australian Commission on Safety and Quality in Health Care. Healthcare-associated infections [Internet]. Canberra ACT: Commonwealth of Australia; 2018 [cited 2019 Aug 6]. Available from: https://www.safetyandquality.gov.au/sites/default/files/migrated/Healthcare-associated-infection-detailed-fact-sheet.pdf
5. Payne, JT. An adhesive surgical drape. Am J Surg 1956 Jan;91:110–112.
6. Fleischmann W, Meyer H, von Baer A. Bacterial recolonisation of the skin under a polyurethane drape in hip surgery. J Hosp Infect 1996 Oct;34(2):107–116.
7. Kramer A, Assadian Q, Lademann J. Prevention of postoperative wound infections by covering the surgical field with iodine-impregnanted incision drape (Ioban 2). GMS Krankenhhg Interdiszip 2010;5(2).
8. Alexander JW, Aerni S, Plettner JP. Development of a safe and effective one-minute preoperative skin preparation. Arc Surg 1985 Dec;120(12):1357–1361.
9. Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev 2015 Apr;(4).
10. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
11. Higgins JPT, Sterne JA, Savovic´ J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials in Cochrane Methods. Cochrane Database Syst Rev 2016;10(Suppl 1):29–31.
12. Dewan PA, Van Ru AM, Robinson RG, Skeggs GB, Fergus M. The use of an iodophor-impregnated plastic incise drape in abdominal surgery: a controlled clinical trial. Aus NZ J Surg 1987 Nov;57(11):859–863.
13. Hagen KS, Treston-Aurand J. A comparison of two skin preps used in cardiac surgical procedures. AORN J 1995 Sep;62(3):393–403.
14. Segal CG, Anderson JJ. Preoperative skin preparation of cariac patients. AORN J 2002 Nov;76(5):821–828.
15. Yoshimura SK, Kubo S, Ogawa M, Morimoto K, Shirata K, Kinoshita H. Plastic iodophor drape during liver surgery operative use of the iodophor-impregnated adhesive drape to prevent wound infection during high risk surgery. World J Surg 2003 Jun;27(6):685–688.
16. Al-Qahtani SM, Al-Amoudi HM, Al-Jehani S, Ashour AS, Abd-Hammad MR, Tawfik OR, et al. Post-appendectomy surgical site infection rate after using an antimicrobial film incise drape: a prospective study. Surg Infect 2015 Apr;16(2):155–158.
17. Bejko J, Tarzia V, Carrozzini M, Gallo M, Bortolussi G, Comisso M, et al. Comparison of efficacy and cost of iodine impregnated drape vs standard drape in cardiac surgery: study in 5100 patients. J Cardiovasc Transl Res 2015 Oct;8(7):431–437.
18. Moores N, Rosenblatt S, Prabhu A, Rosen M, Do iodine-impregnated adhesive surgical drapes reduce surgical site infections during open ventral hernia repair? A comparative analysis. Am Surg 2017 Jun;83(6):617–622.
19. Sarath RS, Umamaheswari V. A study to evaulate the effectiveness of iodine impregnated plastic adhesive drape during abdominal surgeries in preventing surgical site infections. IOSR-JDMS 2018 Aug;17(8):5–23.
20. Karapinar K, Kocaturk CI. The effectiveness of sterile wound drapes in the prevention of surgical site infection in thoracic surgery. Biomed Res [Internet]. 2019 Feb [cited 2019 Sep 10]. Available from: https://www.hindawi.com/journals/bmri/2019/1438793/ doi: 10.1155/2019/1438793



