Volume 28 Number 3
Hyperbaric oxygen therapy in the treatment of radiation-induced wound complications of breast cancer
Yasantha Rajapakse and Glen C Hawkins
Keywords hyperbaric medicine, implant, prosthesis, reconstruction failure
For referencing Rajapakse Y & Hawkins GC. Hyperbaric oxygen therapy in the treatment of radiation-induced wound complications of breast cancer. Wound Practice and Research 2020; 28(3):133-136.
DOI https://doi.org/10.33235/wpr.28.3.133-136
Abstract
Reconstructive surgery is a very common procedure during breast cancer treatment. In the presence of adjunctive radiotherapy, failure rates are very high due to poor wound healing.
We present two case studies where expected significant surgery was avoided due to treatment with hyperbaric oxygen therapy (HBOT), particularly in the presence of a prosthesis.
Introduction
Current breast cancer treatment involves removal of the tumour by either a mastectomy or breast-conserving surgery (lumpectomy). In breast-conserving surgery, radiotherapy is often used as an adjunct treatment following surgery1.
A 2009 review reported the complication rates following mastectomy were more than twice as great when surgery was followed by radiotherapy2. Postoperative wound breakdown following radiotherapy is particularly problematic as it occurs in the presence of reconstructive procedures usually with an implant or tissue expander. Subsequent wound breakdown from radiation tissue injury in these situations can result in prosthetic, and thus reconstructive, failure, resulting in further surgery in a compromised tissue bed.
Radiation therapy results in progressive loss of small blood vessels in the irradiated field (hypovascularity) leading to hypoxia and cell death (hypocellularity)3. The tissue becomes increasingly fibrotic and, at a critical point, may break down to form a spontaneous ulcer, or may fail to heal following surgical intervention such as reconstruction with or without a prosthesis.
After radiotherapy, wound healing has been shown to be severely impaired for several reasons4, and has also been shown to increase the surgical complication rate of both autologous and implant-based reconstructions5,6. Hyperbaric oxygen therapy (HBOT) has been well described to treat radiation-induced tissue complications7. However, currently, there are few reports of its efficacy in the treatment of wounds secondary to radiation therapy of the breast, particularly in the presence of a prosthesis. Enomoto et al.8 describe a human case of a refractory skin ulcer that developed 26 years after an initial mastectomy followed by radiation therapy. This was successfully treated with 101 sessions of HBOT over 1 year. Snyder et al.9 report using 20 HBOT sessions pre-operatively and 10 postoperatively in five patients who had bilateral breast reductions after unilateral lumpectomy and radiotherapy. Complication rates were similar between the irradiated and non-irradiated breasts.
We report two cases of wound complications following radiotherapy, one with an early presentation and one late, both of which were successfully treated with HBOT. Both patients have given full written consent for publication of their clinical course and photographs of their wounds.
Case studies
Case 1
A 40-year-old woman with no other medical comorbidities presented in December 2018 for treatment of a wound following lumpectomy and radiotherapy. The patient was diagnosed with carcinoma of the right upper outer breast (T2N0M0 stage IIA) in June 2018. She underwent a lumpectomy and then, 4 weeks later, had external beam radiotherapy (50Gy over 25 fractions followed by a boost dose of 10Gy over 10 fractions). Tamoxifen was commenced after completion of radiotherapy.
One month after radiotherapy, a 5mm wound dehiscence occurred at the 9 o’clock margin of the right nipple. She underwent further surgery in October 2018 and the wound was debrided and re-sutured. Postoperatively, there was further dehiscence and the patient was referred for HBOT. At the time of presentation there was a 25mm x 25mm x 20mm deep wound (Figure 1) present for 2 months. There was no clinical evidence of infection. with all wound swabs negative for bacterial growth.
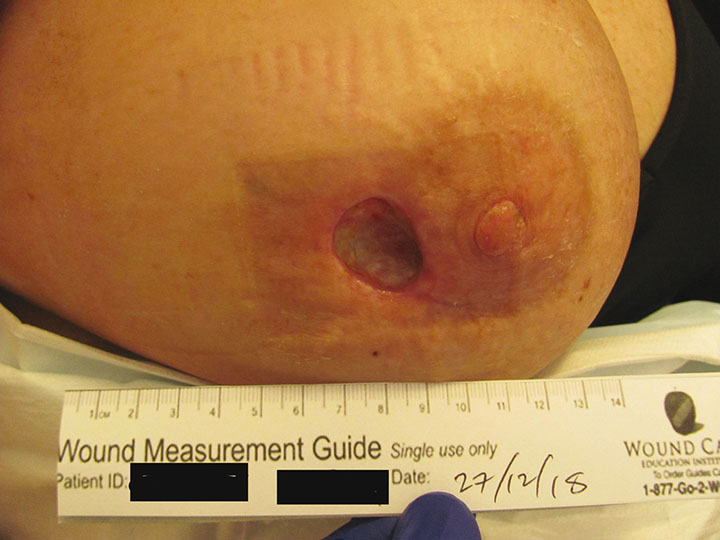
Figure 1. Right breast pre-HBOT treatment (probe depth 20mm)
Complicating the presentation was that she had undergone a cosmetic bilateral breast augmentation 10 years earlier with 400cc round silicone gel implants. There was a significant risk that if the wound breakdown worsened, this would result in implant exposure necessitating the unilateral removal of the implant and a subsequent cosmetic defect.
HBOT was prescribed with the specific aim of healing her wound while retaining the implant. She completed 60 treatments uneventfully over 3 months. Each HBOT treatment was conducted at 243kPa for 90 minutes, 5 days a week, in a Fink SL8 multiplace chamber. Figure 2 shows the wound at the end of treatment, 3 months after initial presentation. At 9 months after initial presentation her wound continues to be healed, requiring no further wound care. (Figures 3a and 3b).
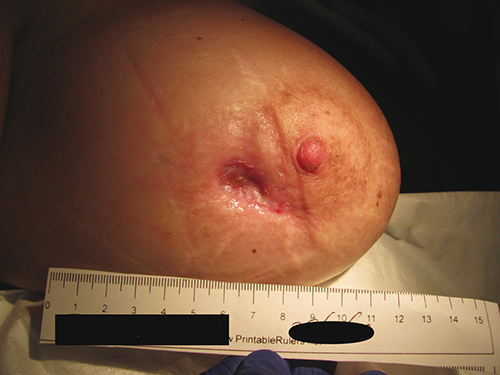
Figure 2. Wound at end of 60 treatments, 3 months after initial presentation
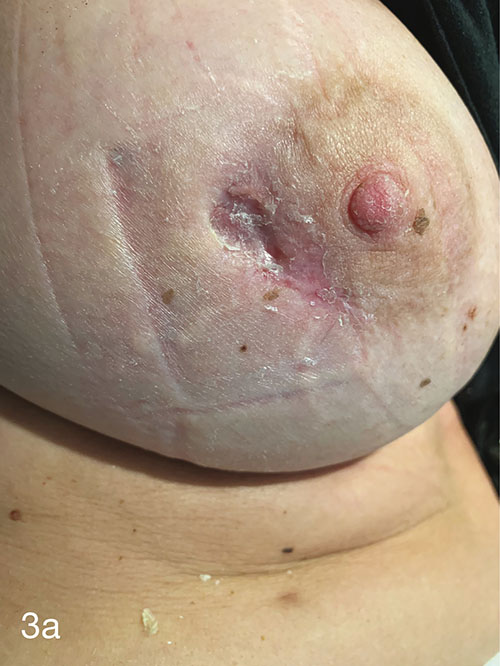
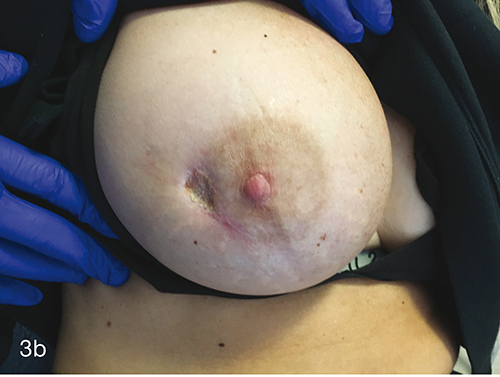
Figures 3a and 3b. 9 months after initial presentation
Case 2
A 76-year-old woman with no other medical comorbidities presented in March 2019 with a non-healing left chest wall ulcer after having a mastectomy with axillary clearance and chemo-radiotherapy for a left infiltrating ductal carcinoma (T3N1M0 stage IIIA) in September 2014. She received 50Gy to the left chest wall in 25 fractions and 50Gy to the left supraclavicular area in 25 fractions over 4 weeks.
She had no postoperative problems until November 2018, when she developed a seroma that required aspiration. A tissue biopsy at the time revealed no recurrence of the primary tumour. She developed a non-healing exudative ulcer at the biopsy site and presented to the hyperbaric facility in March 2019 when the ulcer measured 10mm wide x 10mm long x 20mm in depth (Figure 4). This wound was present for 5 months and was progressing in size despite frequent dressing by the district nurses.
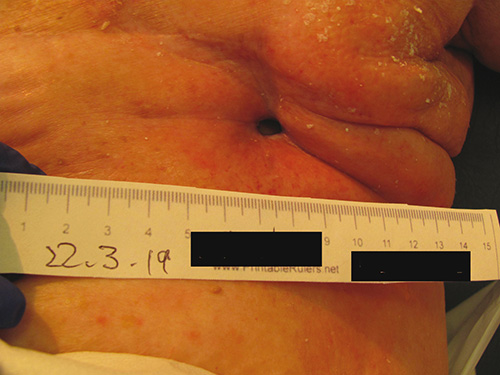
Figure 4. Left breast mastectomy site pre-HBOT treatment (wound located middle of chest – probe depth 10mm)
She commenced HBOT in April 2019 and had 60 treatments uneventfully over a period of 5 months. Each treatment was conducted at 243kPa for 90 minutes daily, 5 days per week in a Fink SL8 multiplace chamber. Due to personal, non-medical related circumstances, HBOT was interrupted for 2 months midway through her treatment course. At 8 months since her initial presentation, her wound continues to improve, requiring no further wound care (Figure 5).
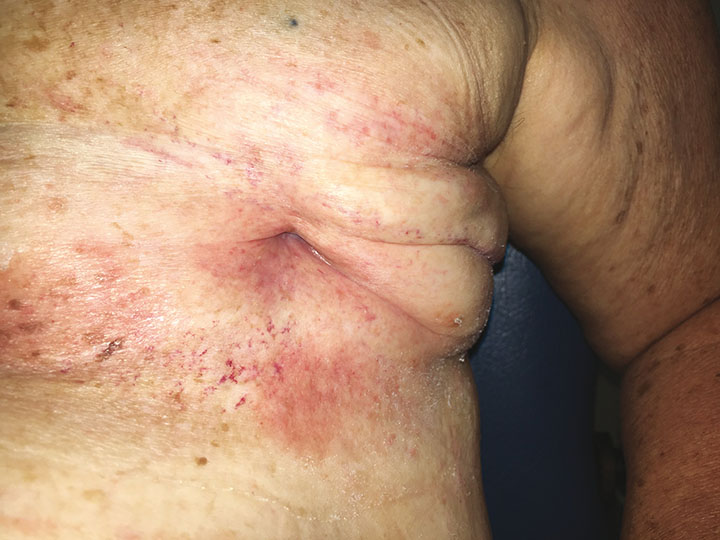
Figure 5. Left breast mastectomy site 8 months after initial presentation
Discussion
These two case studies presented with deteriorating wounds that were judged to potentially require significant surgical intervention. The aim of referring for HBOT was to avoid further surgical intervention in an irradiated field.
Post-mastectomy chemo-radiotherapy can more than double the risk of prosthesis loss when compared with chemotherapy alone2,6. However, there is no consensus about the optimal timing for radiotherapy. One series of two-stage reconstructions suggests that while the reconstructive failure rate is higher for patients who have radiotherapy at the time of tissue expansion compared to at the time of the definitive implant, the aesthetic results are better10.
The basis of HBOT is breathing pure oxygen at a partial pressure higher than atmospheric pressure leading to an elevated oxygen gradient being created in the irradiated field, triggering neoangiogenesis. Other beneficial mechanisms of the action of HBOT treatment are to increase tissue oxygen tension intermittently to optimise fibroblast proliferation and collagen synthesis as well as increasing the oxidative killing capacity of the white blood cells to help fight infection. The resulting neoangiogenesis and improved fibroblast function in the irradiated wound leads to improved wound healing3.
In practice, routine treatment pressure is between 206.6 and 243kPa (2.0–2.4 ATA). At our institution, the standard practice for treatment of radiation-induced wounds is 40 HBOT treatments, each at 243kPa. The wound is reviewed at 40 treatments and, if there is still potential for further improvement, treatment can be extended up to 60 treatments. This decision is made on a case by case basis, keeping in mind the potential complications of myopic shift, acute middle ear barotrauma and oxygen toxicity that can increase with prolonged numbers of HBOT sessions. During their treatments, neither patient had any complications nor side effects.
HBOT has been shown to be an effective treatment for late radiation tissue injury in a wide range of tissues including bone, rectum and bladder7,11. There has, however, been scant research on the effects of HBOT to prevent or treat complications of radiotherapy after breast surgery, particularly in the regular use of prostheses in breast reconstruction. A rat study in 201112 looked at the side effects of radiation therapy on breast implants and demonstrated that there was a statistically significant decrease in capsular thickness post-HBOT compared with control group, though this has not yet been confirmed in humans. A prospective study by Teguh et al.13 showed that persisting breast oedema, pain and erythema in some women who have had breast-conserving surgery and radiotherapy have all improved after HBOT. No evidence has been found of any beneficial effect of HBOT in the treatment of arm lymphoedema following breast surgery and adjuvant radiotherapy14.
Our experience with a wide range of late radiation tissue injuries suggested our two cases justified the use of a course of HBOT in an attempt to minimise or avoid further surgical intervention in these two patients. The first case, though strictly not an implant-based reconstruction, demonstrates the problems of radiation injury in the context of an implant. If wound breakdown had continued, there was a risk of implant exposure and the need for removal of the implant which would have resulted in a significant cosmetic defect. The successful outcome with the avoidance of further surgery, including removal of the implant, may be extrapolated to wound complications following implant-based reconstructions. We feel that, if it were not for HBOT intervention, this implant would certainly have had to be removed.
In the second case, the chronic sinus could have progressively worsened, requiring significant reconstructive surgery to correct. Subsequent surgical correction involving operating in an irradiated field was considered at high risk of further complications. This was successfully avoided with HBOT.
Even though the recent HOT2 study15 showed no improvement with HBOT in soft tissue radionecrosis of the bowel, our two cases suggest a clear improvement and implant salvage following HBOT in wounds that were otherwise likely to require major surgery.
Conclusion
Treatment of radiation-induced breast wounds can be difficult, especially if there is a risk of implant exposure. Prior to presentation, both patients had worsening wounds despite regular wound care. HBOT not only contributed significantly to wound healing but also had a great impact on the quality of life for both women by reducing the need for multiple dressing changes each week and avoiding the need for further significant reconstructive surgery.
These cases support an argument that HBOT has a role and should be considered in treating wound breakdown following either breast-conserving surgery or total mastectomy with or without reconstruction after radiotherapy. Although our numbers are small, the avoidance of serious disfiguring complications and lengthy surgery warrant a larger multi-centre prospective study looking at the efficacy of HBOT in implant salvage.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Copyright
All figures copyright of Hyperbaric Health are used with permission.
Author(s)
Yasantha Rajapakse*
MBChB FRACS
New Concepts Plastic Surgery, Bondi Junction, NSW
Email yosanta@yahoo.com
Glen C Hawkins
MBChB, FANZCA, DipAdvDHM
Hyperbaric Health, Mascot, NSW
* Corresponding author
References
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: breast cancer V.2; 2015. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg 2009;124(2):395–408. doi:10.1097/PRS.0b013e3181aee987. PMID:19644254
- Marx RE. Radiation injury to tissue. In: Kindwall EP, editor. Hyperbaric medicine practice, 4th ed. Flagstaff: Best Publishing; 2017. p. 727–776.
- Feldmeier JJ. Hyperbaric oxygen therapy and delayed radiation injuries (soft tissue and bony necrosis): 2012 update. Undersea Hyperb Med 2012;39(6):1121–39. PMID: 23242770
- Patel KM, Albino F, Fan KL, Liao E, Nahabedian MY. Microvascular autologous breast reconstruction in the context of radiation therapy: comparing two reconstructive algorithms. Plast Reconstr Surg 2013;132(2):251–7. doi:10.1097/PRS.0b013e31829586e2. PMID:23897324
- Lam TC, Borotkanics R, Hsieh F, Salinas J, Boyages J. Immediate two-stage prosthetic breast reconstruction failure: radiation is not the only culprit. Plast Reconstr Surg 2018;141(6):1315–24. doi:10.1097/PRS.0000000000004358. PMID:29750759
- Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev 2012(5):CD005005. doi:10.1002/14651858.CD005005.pub4.
- Enomoto M, Yagishita K, Okuma K, Oyaizu T, Kojima Y, Okubo A, et al. Hyperbaric oxygen therapy for a refractory skin ulcer after radical mastectomy and radiation therapy: a case report. J Med Case Rep 2017;11(1):5 doi:10.1186/s13256-016-1168-0. PMID: 28049509. PMCID:PMC5209955
- Snyder SM, Beshlian KM, Hampson NB. Hyperbaric oxygen and reduction mammaplasty in the previously irradiated breast. Plast Reconstr Surg 2010;125(6):255e–7e. doi:10.1097/PRS.0b013e3181cb67d0. PMID: 20517072
- Cordeiro PG, Albornoz CR, McCormick B, Hudis CA, Hu Q, Heerdt A, et al. What is the optimum timing of postmastectomy radiotherapy in two-stage prosthetic reconstruction: radiation to the tissue expander or permanent Implant? Plast Reconstr Surg 2015;135(6):1509–17. doi:10.1097/PRS.0000000000001278. PMID 25742523. PMCID:PMC5004350
- Tahir AR, Westhuyzen J, Dass J, Collins MK, Webb R, Hewitt S, et al. Hyperbaric oxygen therapy for chronic radiation-induced tissue injuries: Australasia’s largest study. Asia Pac J Clin Oncol 2015;11(1):68–77. doi:10.1111/ajco.12289. PMID 25382755
- Tumerdem-Ulug B, Kuran I, Ozden BC, Mete O, Kemikler G, Aktas S, et al. Does hyperbaric oxygen administration before or after irradiation decrease side effects of irradiation on implant sites? Ann Plast Surg 2011;67(1):62–7. doi:10.1097/SAP.0b013e3181e6cfa4. PMID 21301311
- Teguh DN, Bol Raap R, Struikmans H, Verhoef C, Koppert LB, Koole A, et al. Hyperbaric oxygen therapy for late radiation-induced tissue toxicity: prospectively patient-reported outcome measures in breast cancer patients. Radiat Oncol 2016;11(1):130. doi:10.1186/s13014-016-0700-0. PMID: 27682427. PMCID:PMC5041335
- Gothard L, Haviland J, Bryson P, Laden G, Glover M, Harrison S, et al. Randomised phase II trial of hyperbaric oxygen therapy in patients with chronic arm lymphoedema after radiotherapy for cancer. Radiother Oncol 2010;97(1):101–7. doi:10.1016/j.radonc.2010.04.026. Epub 2010 May 31. Erratum in: Radiother Oncol 2011 Feb;98(2):285. PMID 20605648
- Glover M, Smerdon G, Andreyev HJ, Benton BE, Bothma P, Frith O, et al, Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2). Lancet Oncol 2016 Feb;17(2):224–233. doi:10.1016/S1470-2045(15)00461-1. PMID: 26703894. PMCID: PMC4737893



