Volume 28 Number 3
Management of venous leg ulcers with a two-layer compression bandage and a polyacrylate fibre dressing
Johnson Boey, Tjun Yip Tang and Emilio Galea
Keywords venous leg ulcers, multilayered compression, polyacrylate fibres, UrgoK2®, URGOClean®
For referencing Boey J et al. Management of venous leg ulcers with a two-layer compression bandage and a polyacrylate fibre dressing. Wound Practice and Research 2020; 28(3):127-132.
DOI https://doi.org/10.33235/wpr.28.3.127-132
Abstract
Venous leg ulcers (VLUs) are a common affliction in an ever ageing population. These wounds are challenging for clinicians to heal, but also affect badly the health-related quality of life of patients and their families. This article provides epidemiological information as well as discusses the etiopathogenesis of VLUs. Two VLU cases from Singapore that were managed using a two-layer compression bandage system (UrgoK2®) and a polyacrylate fibre dressing (UrgoClean®) are presented.
Introduction
Epidemiology
It is suggested that anywhere between 1.5 and 3.0 per 1,000 persons have active leg ulcers, with prevalence increasing with age to about 20 per 1000 persons older than 80 years; most leg ulcers are secondary to venous disease1. A raise in ulceration is attributed to an ageing population and it is suggested that this disease affects up to 3% of those aged over 60 years which increases to over 5% of those aged over 80 years2.
Annual incidence estimation in the UK, Switzerland and India ranges between 0.2 to 4.5 per 1000 inhabitants3–5. In the United States, it is estimated that patients with venous leg ulcers (VLUs) are using more medical resources than other patients, with an increase of their annual per-patient health expenditures up to US$7,030; employed individuals with venous ulcers are also missing 4 more days of work per year than others without venous ulcers6. Estimates indicate that leg ulcers affect up to 3.0 per 1000 of the Australian adult population, while in New Zealand an incidence varying between 393 and 839 per 100,000 population per year has been reported2,7.
Etiopathogenesis
Neumann et al. (2016)8 define venous ulceration as “a defect in pathologically altered tissue on the lower leg on the basis of chronic venous insufficiency”. It is a complex disease of symptoms and signs based on inadequate venous return, which leads to a decompensation of the venous and the microcirculatory function. Chronic venous ulceration is the severest manifestation of this disorder.
Clinical characteristics of venous insufficiency include skin changes that are caused by chronic venous hypertension such as oedema, visible capillaries around the ankle (corona phlebectatica), trophic skin changes comprising of hyperpigmentation caused by haemosiderin deposition, atrophie blanche, dermatoliposclerosis (induration of the skin and underlying tissue), stasis eczema and lipodermatosclerosis (painful, tight skin with hardened subcutaneous tissues just above the ankle due to infiltration of fibrin and inflammation)9. Veins can be damaged by surgery, trauma or DVT which causes a backflow of blood in the venous system at the point of damage. Venous ulcers most commonly occur in the gaiter region of the leg, most commonly around the medial malleolus. They are irregular with sloping margins, usually shallow and fibrinous, with a granulating base9.
Although vascular disease is associated with the most common occurrence of lower limb ulcers, a number of other causes, such as infectious diseases, immunological diseases, physical factors, skin tumours and other skin diseases that can lead to skin ulcerations are possible, which makes it important to make a correct aetiological assessment and diagnosis of the ulcer as the management of the different problems vary substantially10.
Managing VLUs with non-invasive methods
Harding et al. (2016)11 put forward the ABC model for leg ulcer management:
A. Assessment and diagnosis.
B. Best practice wound and skin management.
C. Compression therapy for active treatment and for prevention of recurrence.
Other therapy options such as surgery or alternatives, for example endovascular ablation or foam sclerotherapy and perforator vein interruption, may be an option, when indicated, to promote ulcer healing; however, conservative treatment using compression is more common and is still considered as the mainstay of VLU treatment12,13. Compression bandages, compression stockings, self-adjustable fabric hook and loop fastener devices, intermittent pneumatic compression pumps and hybrid devices are all utilised as conservative management of VLUs14.
Compression bandages are classified as inelastic or short-stretch bandages and elastic or long-stretch bandages12. In short-stretch bandages, the movement of the calf muscle provides the pressure which indicates that they are effective in ambulant patients while, in long-stretch bandaging, the elasticity of the bandage itself applies the pressure and may be more effective for immobile patients12. It is also suggested that bandage pressure is reduced by 25% after the first application of the short-stretch bandage, whereas the pressure in long-stretch bandaging is maintained for a longer period12.
Systems comprising of more than one-layer – multi-component systems – are available in kits which include at least a padding layer and bandages6. These vary from two- to four-layer systems; however, some patients have indicated that the more traditional four-layer system may be bulky, hot and painful, as well as loosen and slip very often15. It has been stated that “an increased proportion of patients with compression therapy are associated with an increase in the healing rate and interrelated cost savings”16.
Wound bed management by dressings in VLUs is primarily aimed at maintaining a moist environment and should be beneficial in ulcer treatment in conjunction with compression therapy10. The clinician needs to select the dressing that is best suited to the healing stage of the ulcer he/she is managing10.
Wound management modalities
This paper outlines a case report of two patients who had been suffering from long-standing VLUs that were not showing signs of healing despite compression and local management. This case report features two wound management modalities – a two-layer compression system (UrgoK2®) and UrgoClean®.
UrgoK2®: a two-layer compression system
This two-layer, high-compression bandage system was designed to ensure even distribution of pressure between the two dynamic bandages, namely, KTech (inelastic short-stretch bandage combining a viscose and polyester wadding with a polyamide and elastane knitted layer) and KPress (elastic cohesive elastic bandage made of acrylic, polyamide and elastane)17. It applies the therapeutic pressure (40mmHg) required to treat active and/or associated symptoms such as oedema in chronic venous insufficiency and the treatment and maintenance of lymphoedema17. The UrgoK2® system incorporates the PresSure® system which is a pressure indicator that is printed on each bandage to aid stretch and overlap – stretching the pressure indicator from an oval to a circle and overlapping enough to cover it is stated to provide optimal compression levels at each application18. The system was designed to help clinicians ensure safe application and consistent levels to achieve optimal levels of compression17,19. The evidence for this system is outlined below using the results of three studies.
The first is a non-comparative, open-label clinical trial to evaluate the efficacy, tolerability and acceptability of a two-layer bandage system (UrgoK2®) in 42 patients from 12 centres with VLUs – average size 7cm2; average duration 8 months; 62% recurrent20. A total of 86% of the patients improved or healed after 6 weeks of treatment (mean surface reduction 58.5%) and 24% achieved complete healing. There was an overall reduction in oedema of 83% of the treated patients, from 69% of patients at baseline to 12% at 6 weeks. Compared with compression therapy used previously, patients reported increased comfort, reflected by 100% concordance with UrgoK2®.
Two comparative clinical trials were also conducted comparing three systems. In the first trial18, the required therapeutic interface pressure was achieved by 85% of nurses with UrgoK2® (average pressure 40mmHg) compared to 69% with a four-layer system and only 25% with an inelastic system. A total of 25% of nurses applied high pressures (>50mmHg) with the four-layer system, while 75% applied sub-therapeutic pressures (<30mmHg) with the short-stretch bandage. Application time was also shorter for UrgoK2®. In the second study21, UrgoK2® maintained a similar level of sub-bandage pressure to the four-layer system after 7 days and was slightly better than the inelastic system; it was also considered to be more comfortable and tolerable than the other two systems.
A multi-centre, randomised controlled trial in France, Germany and the UK was conducted to evaluate of efficacy, tolerance and acceptability of the two-layer UrgoK2® system versus a four-layer bandage system in the management of 187 patients22. A total of 160 patients were followed until Week 12 or until complete re-epithelisation of their wound. Complete closure rates were higher in the UrgoK2® group (44% vs 39%, p=ns), although time to complete re-eplithelialisation was similar in both treatment groups. Local adverse event rate in the UrgoK2® group was 12%, and 16% in the comparative cohort. Pain between dressing changes was reported in 27% of the two-layer system and in 40% of the control group, and UrgoK2® was considered significantly easier to apply (p=0.038).
UrgoClean®
The UrgoClean® dressing traps and binds slough within its polyabsorbent fibres, and desloughing occurs when the dressing is removed from the wound23. It is available in a rope or pad format which facilitates gentle and pain-free desloughing24. The polyabsorbent fibres gel when in contact with exudate, which enables it to bind to and vertically absorb slough, trapping it and other non-adherent and devitalised material to the wound bed. UrgoClean®’s vertical absorption makes it suitable for use on all exuding wounds, with its absorbency having been found to be similar to that of hydrofibre25. The Technology Lipidocolloid Healing Matrix (TLC) technology which covers the pad promotes a moist environment that is conducive to healing and provides for atraumatic removal26. The evidence for this dressing includes two studies.
The first was a prospective, non-controlled open-label clinical trial to evaluate the efficacy and tolerability of UrgoClean® held in 21 investigating centres involving 44 adult patients presenting with either a VLU or a category III/IV pressure ulcer (PU) with more than 50% of the surface area covered with sloughy tissue, a duration of less than 24 months, and no clinical signs of infection27. Patients were followed over a 6-week period with weekly visits. Evaluations by the nursing staff and by the patients were made at each dressing stage. The median decrease of sloughy tissue observed was 75% for VLUs and 89% for pressure ulcers. Six wounds healed completely over 6 weeks. Dressing was rated as “very easy” to use, with “very good” conformability, and was widely reported as “painless” on removal, leading to good tolerability and acceptability of the dressing.
A non-inferiority European randomised controlled clinical trial was conducted in 37 centres on 159 patients presenting with venous or predominantly venous, mixed aetiology leg ulcers at their sloughy stage (more than 70% of the wound bed covered with slough at baseline)24. Patients were followed over a 6-week period and assessed weekly. The primary judgement criterion was the relative regression of the wound surface area after the 6-week treatment period and secondary endpoints were the relative reduction of sloughy tissue and the percentage of patients presenting with a debrided wound at the end of the treatment period. The two dressings had similar results for reduction in wound surface area and safety. However, UrgoClean® resulted in a higher reduction of sloughy tissue (p=0.013), and produced a higher percentage of debrided wounds (p=0.033).
Case studies
The following instances conducted at the Singapore General Hospital discuss two cases where clinicians utilised UrgoClean® and UrgoK2® in the management of patients suffering from VLUs.
Both subjects have provided consent for treatment and for the clinical images of their wounds to be used for research purpose. Their personal data have been deidentified for this publication. As the treatment does not involve new treatment and does not carry more than minimal risk to the patient, ethics approval from the IRB was not required.
Case 1
A 79-year-old male patient presented with a non-healing venous ulcer that was present for more than 3 months. Past medical history included hypertension, hyperlipidemia, thrombocytopenia, macrocytosis, bilateral chronic venous insufficiency (CVI), chronic ischemic heart disease (IHD), mild aortic stenosis, angina with mild exertion (CCS III). The patient had undergone a coronary artery bypass graft harvested from the right long saphenous vein. He presented with complaints of non-healing ulcerations over the anterior aspect of the right distal lower limb for a few months; he had a background of long-standing bilateral lower limb swelling with the right side affecting more than the left, gradual skin changes in the right lower limb, symptomatic pain on walking short distances, and leg restlessness. Physical examination revealed multiple ulcerations over the anterior aspect of the right distal lower limb with haemosiderin pigmentation observed over the surrounding skin. A duplex ultrasound revealed no reflux nor obstruction in the deep and superficial veins of the right lower limb.
CEAP classification28 was determined to be C6. The wound characteristic was classified according to the Society for Vascular Surgery (SVS) lower extremity threatened limb classification system, Wound, Ischemia and Foot Infection Classification (WIFI)29 and the Infectious Disease Society of America (IDSA) infection classification system; the WIFI score was 110 and it was non-infected. The wound failed to show improvement despite medical management with clopidogrel and pentoxifylline to improve blood flow, and a conventional four-layered compression bandage (Figure 1A).
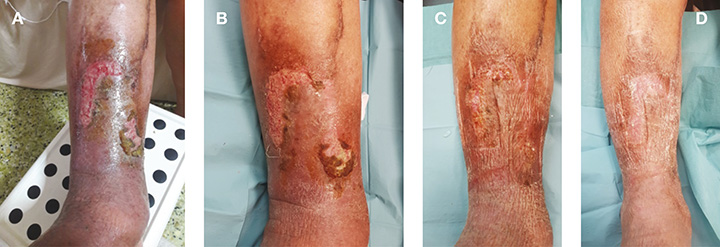
Figure 1. Clinical pictures of patient in Case 1 whose ulcer healed after 1 month of 2-layered compression bandages (UrgoK2®).
A: Initial presentation; B: 1 week after treatment; C: 2 weeks after treatment; D: 3 weeks after treatment
After assessment and consideration, the patient was treated with UrgoClean® and a UrgoK2® compression bandage [Laboratoires Urgo]. A conventional foam dressing was used as secondary dressing to lock the exuding fluid within the foam and prevent periwound maceration. The patient was reviewed in the clinic once per week for wound dressing changes for a month. One week after treatment, there was increased granulation tissue with reduced overlying adherent fibrin in both wounds. Pigmentation skin changes and peripheral oedema were lesser (Figure 1B). Two weeks after treatment, the anteromedial wound had achieved completed epithelisation, while the anterior wound had almost healed (Figure 1C). Three weeks after treatment, both ulcerations had epithelised. Lower limb swelling had regressed haemosiderin and pigmentation with the surrounding area of the erythema (Figure 1D). Throughout the entire treatment, there was no adverse events and the patient well-tolerated the compression bandage regimen.
Case 2
A 82-year-old Chinese male presented to clinic with a non-healing VLU of more than 3 years. He experienced symptoms of restlessness and occasional leg cramps, and there was frequent observation of soiled wound bandages due to excessive exudate discharge which was accompanied by malodour. Past medical history included chronic venous insufficiency, normocytic normochromic (NCNC) anaemia secondary to total iron (Fe) and folate deficiency, and mild chronic kidney disease. He underwent right great saphenous vein ablation and ultrasound-guided intravenous stenting in the common iliac vein (CIV) and external iliac vein (EIV) in 2015 and 2016 respectively. Physical examination revealed a 5cm x 5cm ulceration over the lateral malleolus of the right foot which consisted of a thick adherent layer of slough with minimal granulation tissue (Figure 2A). The peripheral wound edges were raised and rolled, with more than 2cm of erythema. Weepy skin lesions were noted over the entire dorsal midfoot. A recent venous duplex scan showed patency of both right CIV and EIV stent with normal colour doppler and waveform pattern.
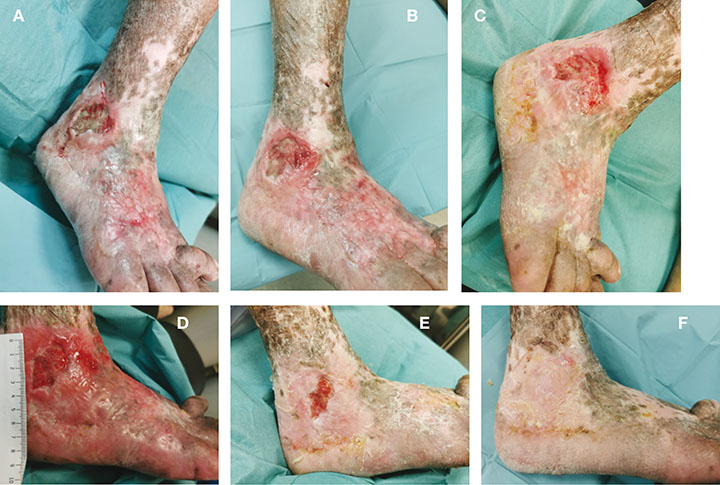
Figure 2. Clinical pictures of patient in Case 2 whose wound achieved full closure within 3 months of treatment.
A: Initial presentation; B: 2 weeks after treatment; C: 1 month after treatment; D: 6 weeks after treatment; E: 2 months after treatment; F: 3 months after treatment
CEAP classification28 was determined to be C6. The wound characteristic was classified in the same way as case study 1; the WIFI score was 101 with mild cellulitis around the wound site. A 1-week course of oral co-amoxiclav was prescribed to the patient to address local soft tissue infection. A decision was made to start with UrgoClean® and a UrgoK2® compression bandage [Laboratoires Urgo]. Conventional foam dressings were used as a secondary dressing to optimise wound exudate management. The patient was reviewed in the clinic once per week for wound dressing changes. Initial wound improvement started to show as early as the second week of treatment (Figure 2B). By the first month, there was a significant reduction in both the amount of adherent slough over the wound bed and wound exudation over the dorsum midfoot (Figure 2C). The wound continued to show signs of healing, with total resolution of excessive exudation in the dorsum midfoot (Figures 2D–E); full wound epithelisation was achieved within 3 months (Figure 2F). There were no adverse events during the duration of the intervention and the patient well-tolerated the compression bandage regimen.
Discussion
Exudate, slough, bioburden and pain are among the challenges in the effective management of chronic VLUs30. Wound fluid contains a mixture of pro-inflammatory cytokines, matrix metalloproteinase (MMPs) and cellular debris; their accumulation is detrimental to physiological process of wound healing31. A foam dressing may be chosen as a secondary dressing over the primary dressing to draw these wound fluids away from the wound, promote a moist and healthy wound bed environment and prevent peri-wound maceration32. Desloughing may be achieved using the sharp/surgical method; however, this is often associated with pain for patients with VLUs33. This limits the efficacy of adequate wound debridement and reduction of bioburden33. The action of the polyacrylate fibres in a UrgoClean® dressing attracts the slough within the wound bed which facilitates mechanical pain-free desloughing24.
Furthermore, notwithstanding the general belief that good compression plays a pivotal role, this treatment may be carried out by clinicians who have never been shown appropriate compression bandaging techniques and who may handle different systems incorrectly34. The PresSure® indicator system in UrgoK2® provides the clinician with easy guidance on how much to stretch the bandage material to an appropriate length while ensuring optimal compression levels with each overlapping layers, thus making it easy and safe to apply the correct pressures and to maintain the applied pressure for several days34. This is exemplified in these two cases where both patients reported a better experience with both the polyacrylate fibre dressings and the novel two-layer compression bandage than previous treatment. The decision to utilise these modalities for these two patients was taken after consideration of the evidence behind the two modalities which includes randomised control trials and in vitro and in vivo evaluations.
Conclusion
In this paper, the authors presented two cases of non-healing VLUs which did not respond well to previous treatment of multi-layered compression bandages. Although wound closure and a favourable patient and clinician experience were reported in both cases within the 3 months of evaluation and follow-up, it is deemed too early to determine if this novel model of treatment is better than its predecessor. Further cases need to be conducted to elucidate policy change to include these modalities as the standard of care in the facilities concerned.
Conflict of interest
Emilio Galea is an employee of URGO Medical which is the manufacturer of UrgoK2® and UrgoClean®. Johnson Boey and Dr Tang have no affiliations with the manufacturer nor conflict of interest.
Funding
No funding was obtained for this study however the bandages and dressings used in both case studies were supplied by the manufacturer Urgo Medical.
Author(s)
Johnson Boey*
Bachelor of Podiatry Medicine
Department of Podiatry, National University Hospital, Singapore
Email Johnson_CE_BOEY@nuhs.edu.sg
Tjun Yip Tang
MD, FRCS (gen)
Department of Vascular Surgery, Singapore General Hospital, Singapore
Emilio Galea
RN, MSc Tissue Viability
International Medial Director, URGO Medical, Singapore
* Corresponding author
References
- Nelson EA, Adderley U. Venous leg ulcers. Am Fam Physic 2017 May 15;95(10):662–3.
- Frade MA, Das PK. Chronic ulcers: updating epidemiology, physiopathology, and therapies. Ulcers 2013 Aug 1;2013.
- Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 2013 Apr 22;2013.
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009 Nov;17(6):763–71.
- Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care 2017 Apr 1;26(Sup4):S4–14.
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Medical Economic 2014 May 1;17(5):347–56.
- Australian Wound Management Association. Australian and New Zealand clinical practice guideline for the prevention and management of venous leg ulcers. Barton, ACT: AWMA; 2011.
- Neumann HA, Cornu-Thénard A, Jünger M, Mosti G, Munte K, Partsch H, Rabe E, Ramelet AA, Streit M. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatol Venereol 2016 Aug 1;30(11):1843–75.
- Franks PJ, Barker J, Collier M, Gethin G, Haesler E, Jawien A, Laeuchli S, Mosti G, Probst S, Weller C. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care 2016 Jun 1;25(Sup6):S1–67.
- Läuchli S, Bayard I, Hafner J, Hunziker T, Mayer D, French L. Healing times and the need for hospitalization for leg ulcers of different etiologies. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete 2013 Dec;64(12):917–22.
- Harding K. Challenging passivity in venous leg ulcer care: the ABC model of management. Int Wound J 2016 Dec;13(6):1378–84.
- Nair B. Compression therapy for venous leg ulcers. Indian Dermatol Online J 2014 Jul;5(3):378.
- Latz CA, Brown KR, Bush RL. Compression therapies for chronic venous leg ulcers: interventions and adherence. Chronic Wound Care Manag Res 2015 Jan 1;2:11–21.
- Partsch H. Compression for the management of venous leg ulcers: which material do we have?. Phlebol 2014 May;29(1suppl):140–5.
- Parks P. Advances in compression therapy for treatment of venous leg ulcers. Todayswoundclinic. 2017. Aug;11(8): Available from: https://www.todayswoundclinic.com/articles/advances-compression-therapy-treatment-venous-leg-ulcers
- Gutknecht M, Walzer S, Heyer K, Dröschel D, Shannon RJ, Lindsay F, Augustin M. Costs of compression therapy in venous leg ulcers in Germany and modelling of the economic effects of regional disparities in health care. Value in Health 2015 Nov 1;18(7):A372.
- Jones V, Grey JE, Harding KG. Wound dressings. BMJ 2006 Mar 30;332(7544):777–80.
- Benigni JP, Lazareth I, Parpex P, Gerard JL, Alves M, Vin F, Meaume S, Senet P, Allaert FA, Sauvadet A, Bohbot S. Efficacy, safety and acceptability of a new two-layer bandage system for venous leg ulcers. J Wound Care 2007 Sep;16(9):385–90.
- Benbow M. Efficacy and reproducible compression of KTwo. J Wound Care 2013 Nov;22(11 Suppl):S4–18.
- Young T, Connolly N, Dissemond J. UrgoKTwo® compression bandage system. Made Easy 2013 Feb; 4(1):1–6.
- Hanna R, Bohbot S, Connolly N. A comparison of inferface pressures of three compression bandage systems. Br J Nurs 2008 Nov 13;17(Sup9):S16–24.
- Junger M, Ladwig A, Bohbot S, Haase H. Comparison of interface pressures of three compression bandaging systems used on healthy volunteers. J Wound Care. 2009 Nov ;18(11): 474,476-90.
- Lazareth I, Moffatt C, Dissemond J, Padieu AL, Truchetet F, Beissert S, Wicks G, Tilbe H, Sauvadet A, Bohbot S, Meaume S. Efficacy of two compression systems in the management of VLUs: results of a European RCT. J Wound Care 2012 Nov;21(11):553–65.
- Grothier L. Three goals: deslough, manage exudate and promote healing. Clinical benefits of UrgoClean. Br J Nurs 2016;25(20 Suppl 2):S1–S32.
- Meaume S, Dissemond J, Addala A, Vanscheidt W, Stücker M, Goerge T, Perceau G, Chahim M, Wicks G, Perez J, Tacca O, Bohbot S. Evaluation of two fibrous wound dressings for the management of leg ulcers: results of a European randomised controlled trial (EARTH RCT). J Wound Care 2014;23(3):105–116.
- Meaume S, Teot L, Lazareth I, Martini J, Bohbot S. The importance of pain reduction through dressing selection in routine wound management: the MAPP study. J Wound Care 2004 Nov;13(10):409–13.
- Meaume S, Perez J, Rethore V, Sebbane G, Dompmartin A, Bressieux JM, Leguyadec T, Tacca O, Bohbot S. Management of chronic wounds with an innovative absorbent wound dressing. J Wound Care 2012 Jul;21(7):315–22.
- Moneta G. Classification of lower extremity chronic venous disorders. UpToDate, Eidt JF. Mills JL. ur. UpToDate [Internet]. 2017. Available from: https://www.uptodate.com/contents/classification-of-lower-extremity-chronic-venous-disorders
- Mills Sr JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G, Society for Vascular Surgery Lower Extremity Guidelines Committee. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIFI). J Vasc Surg 2014 Jan 1;59(1):220–34.
- Tuttle MS. Association between microbial bioburden and healing outcomes in venous leg ulcers: a review of the evidence. Adv Wound Care 2015 Jan 1;4(1):1–1.
- Löffler MW, Schuster H, Bühler S, Beckert S. Wound fluid in diabetic foot ulceration: more than just an undefined soup? Int J Low Extrem Wounds 2013 Jun;12(2):113–29.
- Dabiri G, Damstetter E, Phillips T. Choosing a wound dressing based on common wound characteristics. Adv Wound Care 2016 Jan 1;5(1):32–41.
- Briggs M, Nelson EA, Martyn‐St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2012(11).
- Partsch H. Foreword. Evidence review: efficacy and reproducible compression of the KTwo bandage system. J Wound Care 2013 Nov;22(11 Suppl):S3–.



