Volume 28 Number 4
Chronic hepatitis C-related cryoglobulinaemic vasculitis manifesting as non-healing skin ulcers: a case presentation
Bonnie Fraser
Keywords cryoglobulinaemia, vasculitis, hepatitis C virus (HCV), skin ulcers
For referencing Fraser B. Chronic hepatitis C-related cryoglobulinaemic vasculitis manifesting as non-healing skin ulcers: a case presentation. Wound Practice and Research 2020; 28(4):181-188
DOI https://doi.org/10.33235/wpr.28.4.181-188
Abstract
Aim This paper presents a single case study of hepatitis C virus (HCV)-related cryoglobulinaemic vasculitis (CV) manifesting as non-healing skin ulcers (CV-SU). It discusses ulcer characteristics and management, and highlights the impact of quality of life (QoL) issues faced by the patient.
Background Mixed CV is an extrahepatic immune complex-mediated manifestation of HCV. Vasculitides (cryoglobulins) deposit in the endothelium of small to medium blood vessels in the skin and can lead to ischaemia, necrosis, and SUs or lesions. Cryoglobulins precipitate at temperatures below 37oC, generally in the lower extremities, and redissolve on rewarming. Clinical features include palpable purpura, arthralgia, asthenia and SUs. In severe cases, vasculitis may extend to the lungs, kidneys and nerves.
Methodology The methodology is a descriptive single case study of a 44-year-old male presenting with HCV-induced CV and refractory lower leg ulcers (CV-SU).
Conclusion The management of CV-SU is complex. Resolution requires eradication of HCV, suppression of B cell clonal expansion and cryoglobulin production, and symptom management. The patient’s story highlights the importance of understanding and appreciating the lived experience of the condition and the impact of their QoL.
Introduction
Cryoglobulinaemia (CG) is a condition where cryoglobulins (immunoglobulins) precipitate in the tissues at temperatures below 37°C, reversibly dissolving on rewarming1–6. Precipitation usually occurs in the lower extremities, with vasculitide deposition in the small to medium blood vessels resulting in cutaneous ischaemia and skin lesions or ulcers. The condition is referred to as cryoglobulinaemic vasculitis (CV). Cryoglobulins are associated with infections (e.g. hepatitis C), lymphoproliferative diseases and autoimmune diseases7. CG is characterised by a myriad of clinical manifestations including purpura, asthenia, arthralgia and variable organ involvement – kidney, nervous system, gastrointestinal system, interstitial lung involvement and endocrine disorders (diabetes)7. Diagnosis may include laboratory findings, histopathology and clinical presentation6.
Hepatitis C is associated with several extrahepatic manifestations, some of which affect the skin. The most prominent is CG-induced leukocytoclastic vasculitis or CV and skin ulcers (CV-SU)2. CV has a prevalence ranging from 15–50% in patients with chronic hepatitis C virus (HCV), with up to 80% of patients with CV having HCV1. CG has been identified as an independent factor in liver fibrosis in HCV patients and may be attributed to alcohol consumption and longer disease durations2.
The sequelae of HCV-induced CV-SUs and their impact on both health-related quality of life (HR-QoL) and quality of life (QoL), while unquantified, is likely to be significant8. The combination of infectious disease and an autoimmune disease may severely impact HR-QoL8. Chronic HCV with advanced liver disease alone can be physically and mentally debilitating, while treatment often carries significant physical side effects7,9. CV can have a profound impact on HCV patients, with pain, weakness and lethargy8 making activities of daily living difficult for some. Early and timely administration with appropriate medical therapy to treat HCV and timely re-treatment of CV if required may ameliorate symptoms of CV, preventing irreversible disability8.
Pathogenesis and aetiology of cryoglobulinaemic vasculitis
Several articles have identified a link between HCV advanced liver disease (fibrosis and cirrhosis) and the pathogenesis of mixed CG1–5,10. HCV may be implicated in the low clearance of CG due to the extent of liver damage adding to the severity of the disease10.
One proposed pathogenesis of CG occurs when dendritic bone marrow cells capture HCV particles and proteins and stimulate the release of B-lymphocytes via a family of B cell activating factors (BAFF)10. The B cells proliferate and synthesise large amounts of immunoglobulin M (IgM) with rheumatoid factor (RF) activity10,11. The IgM-RF molecules bind the HCV particles and proteins, producing immune complexes that precipitate when cold10,11. Immune complexes bind to endothelial cells of small to medium blood vessels, generating vasoactive peptides, stimulating inflammatory cell recruitment (neutrophils), and mediating leukocytoclastic vasculitis10,11.
CV presents as one of three types – type 1 (monoclonal immunoglobulin without the RF) and types II and III associated with RF-positive monoclonal antibodies (immunoglobulins and complements C3 and/or C4). Types II and III are referred to as mixed CV and are considered a true immune complex mediated vasculitis, with type II commonly associated with HCV3.
Pain management
HCV is often associated with advanced liver disease and cirrhosis/fibrosis, limiting pain management options. Pain associated with CV may involve both nociceptive and neuropathic pain pathways, both of which use the same neurotransmitters11. Nociceptive pain (pain caused by a noxious stimulus), however, is adaptive, having biological functions, whereas neuropathic pain does not11. Inflammatory processes recruit nociceptive pain receptors throughout the body, activating somatic pain involving bone, joint, muscle and connective tissue and, in some cases, deeper organs such as the kidneys and lungs11.
HCV treatment is recommended for the eradication of both HCV and CG. While the presence of cryoglobulins does not affect the response to antiviral treatment, antiviral treatment may sometimes be associated with immune-mediated adverse effects such as peripheral sensory/motor neuropathies with associated pain11. Analgesic treatment for CV and background SU pain, in addition to procedural pain, is critical for effective SU management, particularly in more severe non-healing ulcers12.
Pain management may involve a single drug therapy or a combination of therapeutic agents including biological, NSAIDS, steroidal, analgesic antidepressants, anticonvulsants and opioids11. Rituximab (a biological agent) has been shown to improve and resolve clinical symptoms of CV, including fatigue, skin manifestations (purpura and SUs), arthralgia and arthritis, and pre-peripheral neuropathies11. For more advanced symptoms of CV with SU involvement, steroids have shown to be effective11. Rituximab is profoundly immunosuppressive and initial management with steroids in combination with HCV treatment should be considered first. In advanced liver disease with cirrhosis and fibrosis, pain management may be limited as a large number of medications are metabolised by the liver. In cirrhotic patients without renal involvement, the clearance rates of many drugs is largely unknown11. With highly protein-binding drugs, low protein and serum albumin levels can lead to increased free drug levels with a concomitant increase in adverse effects and toxicity11. Proposed pain treatment plans are guided by the type of pain present (nociceptive, mixed or neuropathic) and on the liver and renal function11.
This case presentation is important as the literature on HCV-induced CV-SUs and their management is lacking. The overarching therapy and resolution of CV involves eradication of the active HCV. Available literature has identified this condition may significantly impact health and QoL in the long-term8. This patient’s story is poignant, demonstrating a deep insight into his own circumstance and highlighting QoL issues faced daily. The narrative also reflects how the therapeutic relationship helped the patient to stay resolute in both commencement and continuation of the therapy. Finally, this paper provides an opportunity to contribute to the existing knowledge of individualised treatment options, CV-SU presentation, histopathological findings and wound management.
Methodology
The methodology is a single case study of a 44-year-old male with HCV-induced CV and refractory lower leg ulcers (CV-SU). It is descriptive in nature with a unique focus on the patient’s story. Permission was obtained to write this article, to use digital imagery taken throughout the patient’s care, and to access their medical record for data relevant to the presentation. Data collection involved reviewing pathology results, reports from scientific investigations and biopsies, duplex scans and wound assessments. Data used was routinely collected and recorded in the patient’s electronic medical record as part of their ongoing care and medical management. All data has been de-identified and the patient’s anonymity maintained. As this is a single case study with patient permission no ethics approval was sought.
Case presentation
A 44-year old male was admitted to Hospital in the Home (HITH) in July 2019 with several deteriorating deep ulcers to the posterior left calf plus multiple superficial SUs over the remainder of the lower left limb. Known history at this stage was chronic HCV genotype 3a, inherited haemochromatosis (HC) (H63D), ex-intravenous drug use, severe alcohol abuse (currently a controlled alcoholic), advanced but compensated liver disease with hepatic portal hypertension and ascites. Skin integrity to the right limb was mostly intact; however, there was evidence of scratching secondary to significant pruritus (initially thought to be associated with advanced liver disease and/or venous stasis disease). The patient was diagnosed with the peripheral venous disease after duplex scans identified incompetent veins. There was no arterial compromise.
On examination of the lower limbs, the patient had what appeared to be a venous stasis rash over the dorsum of the right foot (Figure 1A) and bilateral lower limb oedema. No other evidence of dystrophic skin changes consistent with venous stasis disease was noted. The left limb was erythematous and very painful. The ulcers were atypical and not representative of venous stasis ulcers (Figure 1B). Initially, the exudate was dark red frank blood with a strong smell of metallic iron which may have been associated with haemosiderin deposition in the tissues or HC13,14. Haemosiderin is a by-product of the breakdown of the haem protein bound to red blood cells by the body’s immune system in the tissues13,14. Connection to mixed HCV-CV was not made at this time due to the atypical rash and diagnosis of venous stasis disease.
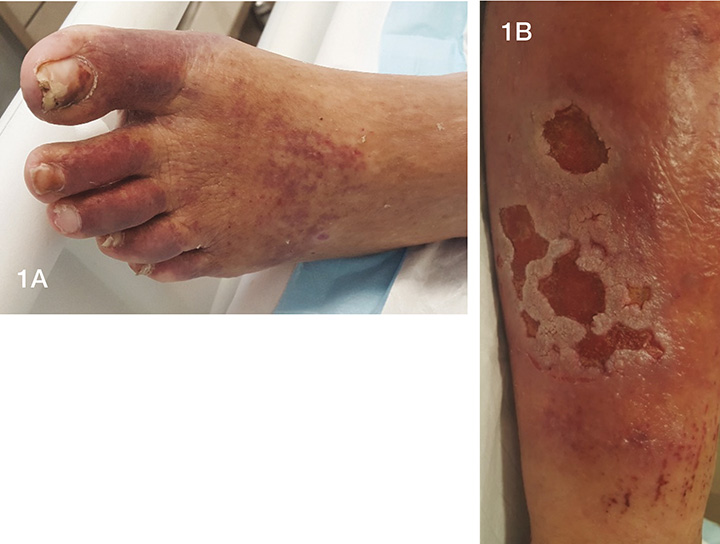
Figure 1. Patient’s right lower extremity at the time of initial presentation (photos used with permission).
1A. Rash to dorsum of right foot consistent with venous stasis disease.
1B. Left posterior calf showing deep cavity wounds with erythema and swelling. There is evidence of scratching due to pruritus on the lower aspect of the limb. Wounds after 6 weeks of NPWT.
Due to the severity of his ulcers and condition of the left lower limb, the patient had been told he may need to have a below knee amputation but was refusing to accept this as an option. Negative pressure wound therapy (NPWT) was applied to the deep ulcers on the posterior left calf with appropriate dressings applied to the remaining ulcers. Bilateral compression therapy was applied to manage the lower limb oedema and support the circulation.
In October 2019, the patient was rushed to the emergency department with bleeding oesophageal varices and haematemesis associated with a relapse of alcohol consumption. At this stage, the deep wounds had granulated sufficiently for NPWT to be ceased, and compression therapy became the mainstay of management in addition to local wound care. The patient was admitted to a neighbouring hospital for 3 weeks for management. On return to our service in November, laboratory work-up identified that the patient’s hepatitis C had mutated and re-activated, liver function enzymes (LFTs) were elevated, and the patient was anaemic (secondary to HC). Despite this, his wounds had improved considerably and were almost healed.
The patient was transferred to community nursing for ongoing wound care. The infectious disease (ID) physician remained involved in the management of the patient’s HCV and I was no longer involved in his care. Final diagnosis had not been made at this stage; however, on reflection (and in knowledge of the final diagnosis), CG can be initiated by the cold. Compression therapy may have aided in warming his lower limbs, facilitating the dissolution of cryoglobulins and potentially reversing the CV.
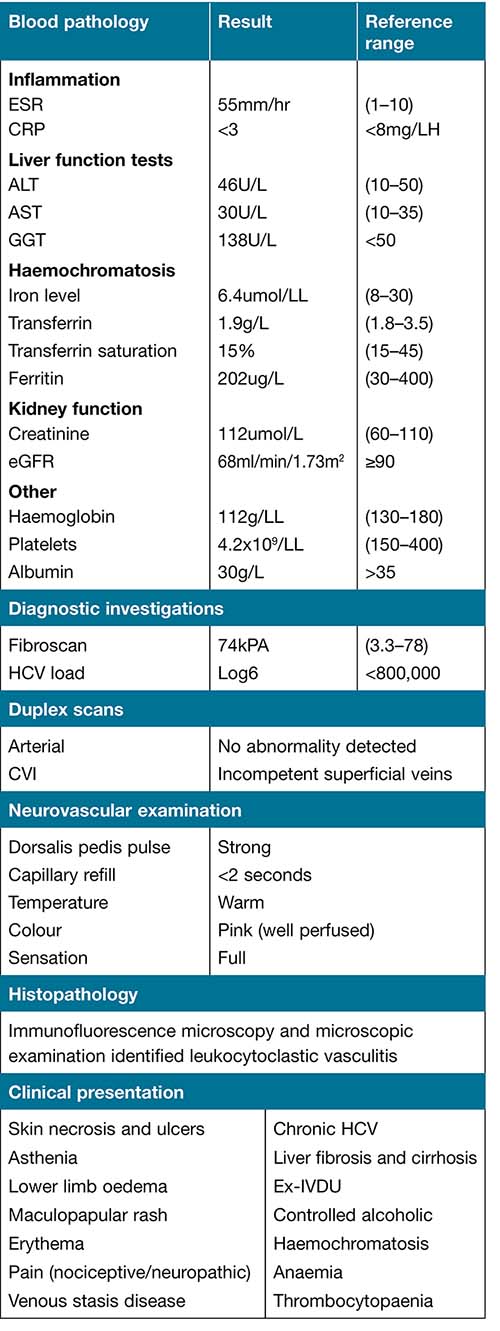
In February 2020, the patient was re-referred to the CNC wound management service. The patient presented with multiple ulcers to the left lower extremity, with moderate sanguineous exudate and a strong smell of iron. Ulcers were present on the right lower limb but not to the same extent nor severity as the left. Over the course of the next couple of months, the left limb swelled and turned a non-blanching deep red/purple colour. Laboratory work-up and clinical presentation are summarised in Table 1.
Table 1. Diagnostic work-up and clinical presentation
The ulcers were refractory not responding to any type of management (Figure 2). The wound bed consisted of a moist pitting granulation tissue with a diffuse yellowish necrotic tissue dispersed throughout. At the wound margin this necrotic tissue extended to the sub-cutis and adipose tissue. The wound margins were a deep purple colour with evidence of haemorrhaging under the skin, leading to the progression of a painful necrotic ulcer (Figure 2B). The patient complained of pain particularly in the right lower limb and hands, significant asthenia and weight loss (>20kg). In addition to wound pain, if the lower limbs were cold the pain would radiate to the patient’s abdominal area. The patient was admitted to HITH for a medical and ID physician review. On review, the patient had a diffuse maculopapular rash above the left knee and around the dorsum of the foot and there was skin necrosis to the left medial calf (Figures 2A & 2C).
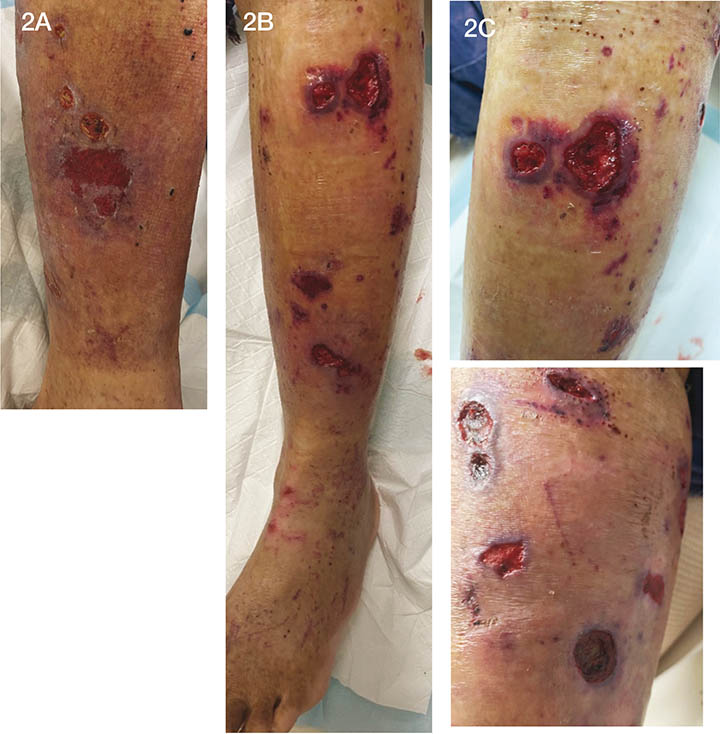
Figure 2. Patient’s lower extremities on second presentation (photos used with permission).
2A. Right lower limb with skin necrosis and ulceration.
2B. Left lower limb with skin ulcers.
2C. Wound presentation.
Initially, it was thought the wounds could be secondary to HC, but a literature search failed to find any causal relationship. On initial presentation, the patient presented with a confluent rash without any palpable purpura which appeared more like venous stasis disease. At this presentation the rash was more consistent with a vasculitic process having a diffuse maculopapular appearance. Differential diagnosis of HCV-induced CV manifesting as non-healing ulcers was made and a biopsy of the wounds, skin on the lower limb, and a papule was attended to confirm this diagnosis. Blood pathology was ordered to identify serum cryoglobulins; however, the test had to be cancelled due to the sample being grossly haemolysed.
Histopathology involving immunofluorescence microscopy was performed. The left thigh identified granular deposition of C3 (+) in the superficial dermal vessels and fibrinogen (trace) in the superficial and deep dermal vessels. There was a deposition of fibrinogen (+++) and occasional C3 in the superficial dermal vessels in the right leg. Microscopic examination showed necrosis at the base of the ulcers extending into the underlying dermal connective tissue, with areas of dermal capillary proliferation and haemosiderin deposition. Non-ulcerated skin in the dermis showed acute and chronic perivascular inflammation with focal leukocytoclasis. Fibrinoid necrosis was not seen. There was no evidence of granulomas, dysplasia nor neoplasia. These findings are consistent with vasculitis. The microscopic histopathology examination identified haemosiderin deposition in the tissues which may be due to venous stasis disease or HC or both.
The patient was commenced on topically applied betamethasone cream and oral administration of prednisone (25mg daily) for reducing inflammation and modulating immune properties to resolve the CG as per treatment recommendations. Since commencement of treatment, the ulcers present on the right lower limb have healed. The erythema and swelling to the left lower limb remain; however, several of the smaller ulcers have healed. Resolution of the larger ulcers is dependent on eradicating the patient’s HCV and achieving a sustained virological response. Wound maintenance to prevent infection and promote healing continues. In this presentation, full compression therapy and NPWT has not been an option due to lower limb pain. Lighter compression with tubiform is tolerated and assists in supporting the circulation and in keeping the lower limbs warm.
The HCV work-up included a fibroscan and determination of the HCV load. The fibroscan score was 74kPa (a measure of liver stiffness and degree of fibrosis/cirrhosis), equating to F4 on the metavir scale (a measure of the extent of inflammation and fibrosis/cirhosis of the liver in patients with HCV), with a 90% probability of both fibrosis and cirrhosis.15 The patient’s viral load was Log6 or 1000,000 viral particles. The patient has type 3aHCV with y98h mutation resistant to NS5A polymerase inhibitors. While the patient’s fibroscan score is very high, he still has compensated liver failure therefore HCV treatment remains a viable option. Wound management involves interactive dressings according to the condition of the wound bed and perilesional skin. The primary focus is to optimise skin integrity of good skin, protecting existing ulcers from deterioration, preventing infection and maintaining a warm moist healing environment conducive to wound healing. Keeping the lower limbs warm may also assist in reversing the patient’s CG.
The patient’s pain is localised to the lower limbs generally and wounds (on dressing changes); however, if the limbs become too cold, the pain progresses up the limbs into the patient’s abdomen. Warming cleansing solutions and maintaining room temperature when attending wound care helps to ameliorate this phenomenon. Current pain management is with Tapentadol 150mg modified release and Panadeine Forte. Tapentadol 100mg immediate-release plus topical EMLA cream 5% (5g tube) is used with good effect during wound care. Keeping the limbs warm also assists with pain management. The patient avoids exposing his lower limbs to the cold, wearing long pants and, while sitting or in bed, ensures his legs are covered with a lap blanket or light weight doona.
In this case, there was a missed opportunity to begin treatment for CV early as the rash in the original presentation was thought to be secondary to venous stasis disease. Even though the ulcers were not representative of the condition, the mainstay of management was compression therapy. The deep ulcers to the posterior calf were successfully managed with NPWT. With a definitive diagnosis, hopefully, we can work with this patient to eradicate his HCV, resolving his CV, and improving not only his HR-QoL but QoL generally.
After several discussions with the ID physician and the CNC wound management service, the patient has decided to go ahead with the HCV treatment. Inclusion of the patient in the decision-making process was pivotal if the proposed therapy was to be successful. Treatment commenced in August 2020 using the DOT (Directly Observing Therapy) care delivery model16. Treatment consists of a combination of direct-acting antiviral medication SOF/VEL/VOX (Sofasbivir/Velpatasvir/Voxilaprevi) plus Ribavarin9. Blood work-up for LFTs, FBC, EUC and ESR will be repeated in preparation for the treatment, and the blood will be closely monitored throughout the program. The patient’s CG and CV will initially to be managed with Prednisone 25mg daily.
Since commencing oral prednisone (August 2020) the majority of wounds have healed, with the exception of those located on the posterior left calf (Figure 3A–D). These wounds have progressively deteriorated and are not responding to steroid therapy. Objective wound assessments using MEASURE17 are conducted regularly in addition to digital imaging to monitor wound deterioration. A second biopsy (late September 2020) was attended to further investigate the nature the ulcers and to look for causative agents (fungi and microbacteria; acid fast bacilli) of the foreign body giant cell (FBGC) reaction identified in the first biopsy. However, no additional information was forthcoming other than to re-confirm a vasculitic process – skin tissue with ulceration, chronic inflammation, formation of granulation tissue, abundant haemosiderin pigment and FBGC reaction. There is infiltration of the inflammatory cells in the walls of the blood vessels of the sub-cutis (neutrophils, lymphocytes and plasma cells) that extends through the adipose tissue.
Figure 3. Photos of left posterior calf showing progressive deterioration of the wounds (photos used with permission).
3A. August 2020
3B. September 2020
3C. 2 October 2020
3D. 9 October 2020
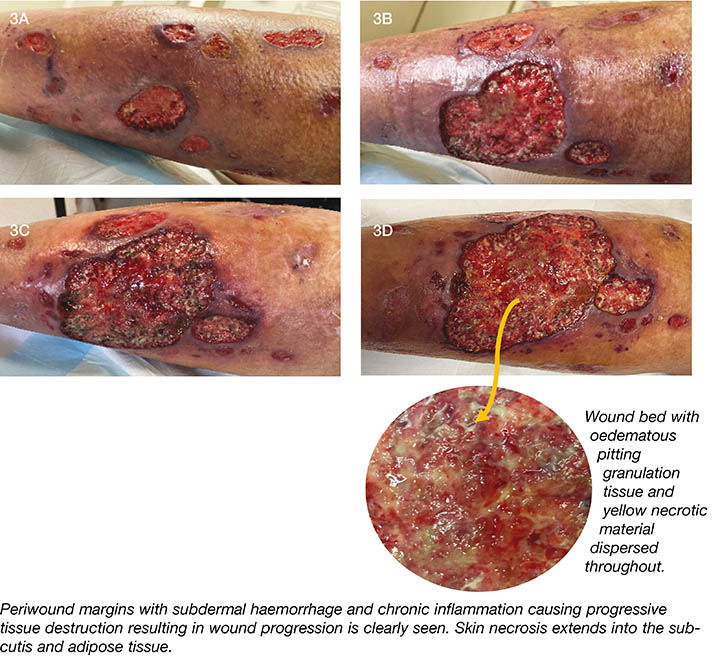
The presence of a FBGC reaction – caused by the clumping of monocyte-macrophages – may explain the progressive wound deterioration as this has been demonstrated to contribute to chronic inflammatory processes and progressive tissue destruction through the release of proteolytic degradative enzymes and excess reactive oxygen species (ROS)18. Wound management will be tailored to rebalancing these enzymes and reducing the levels of ROS to promote healthy granulation tissue growth.
The patient’s mental health has also deteriorated secondary to the steroid therapy. Eight weeks into the treatment to patient complained of experiencing unhealthy thoughts. These thoughts consisted of flash backs to unhappy lived experiences and forgotten memories or events that were addressed and locked away. The impact of these thoughts was such that the patient considered physically harming himself and was having suicidal tendencies. Medications such as steroids, while a first choice for treatment of CV, can be a confounding factor for poor mental health, interfering with thought processes having potentially significant deleterious side effects.
Due to the poor response of the wounds to steroid therapy and in light of the side effects of the medication on the patient’s mental health the plan is now to wean the prednisone and add Rituximab5,9,11,12,13 to the treatment regime. The patient is about to commence the final course of his HCV treatment.
I will continue to be involved in his care, monitoring treatment progress and hopefully see the eradication of his HCV and resolution of his CV and related SUs.
Patient’s story
The patient’s story is outlined below.
I have lived with chronic hepatitis for a long time. It’s my fault as I was an intravenous drug user and an alcoholic (still am but controlled). These ulcers on my legs have been there for about a year now and have caused me considerable grief. I am fortunate to have such strong support from the people who look after me on so many levels. They listen to me and don’t judge me. Without that support, I would not get through this.
The condition that has caused these ulcers and my HCV combined has severely impacted my health and quality of life. Some days I am unable to get out of bed due to fatigue and weakness. The pain in my lower legs and hands is very debilitating, and pain-relieving options are limited due to my advanced liver disease. At times I feel as though I need to increase my alcohol consumption to temper my anxiety and depression, especially on bad days but I know if I have another episode of oesophageal bleeding I may not survive.
I am the carer for my partner and stepson, both of whom have significant health and mental health issues. Trying to run a household and take care of them becomes impossible at times. Having hepatitis C, CV and advanced liver damage have depleted my capacity to live a healthy life. I am a controlled alcoholic and have lowered my alcohol consumption as low as I can possibly get it, even though it is hard sometimes not to have just one more drink. I understand that the Hep C treatment I am about to start could have serious side effects (more so if I don’t control my drinking) or fail but, if successful, it will send me into remission for a second time, giving me another chance to live a longer and healthier life. Not having to put up with the pain and fatigue caused by these ulcers and my hepatitis will be life-changing for me.
Conclusion
CG is a condition arising when cryoglobulins (immune complexes such as IgM-RF) deposit in small to medium blood vessels in the skin. The resulting vasculitis causes an inflammatory process characterised by infiltration of inflammatory cells (neutrophils, lymphocytes and plasma cells) leading to vascular damage and leucocytoclasis. While HCV-related CV and their management is well documented in the literature, information regarding the nature of associated skin lesions or ulcers and their management is limited. Holistic assessment and diagnostic work-up is pivotal to diagnosis. Assessment of the patient’s general health and wellbeing, HCV virology, histopathology of the SUs and papura, full blood work-up and wound assessment provides valuable information to guide management.
For this case presentation, overarching management has been the administration of direct-acting antivirals to eradicate HCV and steroids to manage the CV. Due to the poor response of the SUs to treatment and the deleterious side effect of the prednisone on the patient’s mental health, weaning has commenced, with the plan to add Ritixumab to the treatment regime. Ritixumab acts to deplete monoclonal antibodies (CD-20) causing the CG and treating the SUs not responding to therapy. Management of the SUs is tailored to the wound characteristics. Interactive dressings, debridement, control of inflammation and/or infection, and maintaining moisture balance are essential therapeutic goals. Assessment of background SU pain and procedural pain is essential to successful management. As the patient’s level of liver damage excludes many pain management options, keeping his lower limbs warm is a crucial element.
The patient’s story is poignant, highlighting the impact of the condition and its sequelae on both his HR-QoL and QoL. Chronic illness, pain, weakness and lethargy has profoundly impacted the patient’s mental health and limited his capacity to carry out daily activities. Medications such as steroids, while a first choice for treatment of CV, can be a confounding factor for poor mental health, interfering with thought processes and having potentially significant deleterious side effects. Treatment becomes a fine balance between achieving the desired therapeutic goal and maintaining the psychological and physical safety of the individual. This case presentation brings to light the complexity of managing both CV and CV-SUs. Treatment must be person-centred and tailored to the individual’s needs. Successful resolution of CV and CV-SUs is dependent on eradication of the underlying cause.
Acknowledgements
The author would like to acknowledge and thank the patient for their permission to write this paper and for their bravery and candour in sharing their story.
The author would also like to acknowledge the following people for their review and editing this manuscript: Dr Syed Haris Omar, PhD, Senior Lecturer in Pharmacology, Rural Clinical School, Faculty of Medicine, University of New South Wales; Jenny Price, Health Services Library, Manager, People & Culture Directorate, Murrumbidgee Local Health District, Wagga Wagga New South Wales; Michael Maw, RN NP MNursAP Adv Dip Bus Dip IMC (RCSEd) GradCertHlthHumSc, Clinical Skills and Simulation Educator, UNSW Rural Clinical School, Medicine, Wagga Wagga, New South Wales.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Patient consent
Obtained.
Author(s)
Bonnie Fraser
BSc, DipEd, BNURS, MWoundCare
Clinical Nurse Consultant Chronic and Complex Wounds, Wagga Wagga Base Hospital, NSW, Australia
Email Bonnie.Fraser@health.nsw.gov.au
References
- Ostojic P, Jeremic I. Managing refractory cryoglobulinaemic vasculitis: challenges and solutions. J Inflamm Res 2017; 10:49–54.
- Batsaikhan B, Huang C-I, Yeh M-L, et al. Association between cryoglobulinaemia and liver fibrosis in chronic hepatitis C patients. J Gastroenterol Hepatol 2018;33(11):1897–1903. doi:10.1111/jgh.14275.
- Quartuccio L, Isola M, Masolini P, et al. Validation of the classification criteria for cryoglobulinaemic vasculitis. Rheumatol 2014;53:2209–2213. doi:10.1093/rheumatology/keu271.
- Ferri C. Mixed cryoglobulinaemia. Orphanet J Rare Dis 2008;3(25):1. doi:10.1186/1750-1172-3-25.
- Dammacco F, Sansonno D. Therapy for hepatitis C virus–related cryoglobulinemic vasculitis. New Eng J Med 2013;369(11):1035–1045. doi:10.1056/NEJMra1208642.
- Gleason T, Ghimire S, Paladugu A. It’s not what it looks like: atypical rash in cryoglobulinaemic vasculitis. Br Med J 2017;1–4. doi:10.1136/bcr-2017-219468.
- Lunel F, Musset L, Cacoub P, et al. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterol 1994;106(5):1291–1300.
- Quartuccio L, Isola M, Masolini P, et al. Health-related quality of life in severe cryoglobulinaemic vasculitis and improvement after B-cell depleting therapy. Clin Experiment Rheumatol 2013;31(Suppl 75):S9–S14.
- Liu C-H, Su T-H, Liu C-J, Chen P-J, Chen D-S, Kao J-H. Sofosbuvir/velpatasvir/voxilaprevir plusribavirin for chronic hepatitis C patients with direct acting antiviral failures: implications for viral elimination in Taiwan. J Formosan Med Assoc 2020;1–5. doi:10.1016/j.jfma.2020.06.013.
- Roccatello D, Sciascia A, Rossi D, et al. The challenge of treating hepatitis C virus associated cryoglobulinaemic vasculitis in the era of anti-CD20 monoclonal antibodies and direct antiviral agents. Oncotarget 2017;8(25):41764–41777.
- Scarpato S, Atzeni F, Sarzi-Puttini P, et al. Pain management on cryoglobulinaemic syndrome. Best Practice Res Rheumatol 2015;29:77–89.
- Iannuzzella F, Valglio A, Garini G. Management of hepatitis C virus related mixed cryoglobulinaemia. Am J Med 2010;123(5):400–408. doi:10.1016/j.amjmed.2009.09.038.
- Caggiati A, Rosi C, Casini M, Petrozza V, Acconicia M.C, Zamboni P. Skin iron deposition characterises lipodermatosclerosis and leg ulcer. Eur J Vasc Endovasc Surg 2010;40(6):777–82. doi:10.1016/j.ejvs.2010.08.015.
- Zambi P, Lanzara S. Inflammation in venous disease. J Int Union Angiol 2008;27:361–369.
- Foucher J, Chanteloup E, Vergnio J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55(3):403–408. doi:10.1136/gut.2005.069153.
- McDermott C, Lockhart C.M, Devine B. Outpatient directly observed therapy for hepatitis C among people who use drugs: a systematic review and meta-analysis. J Virus Eradic 2018;4(2):118–122.
- Keast D.H, Bowering K.C, Evans W.A, et al. MEASURE: a proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen 2004;12(3 Suppl):S1–S17. doi:10.111/j.1067-1927.2004.0123S1.x.
- Sanchez M.C, Lancel S, Boulanger E, Neviere R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018;7(8):98. doi:10.3390/antiox7080098.
- Roccatello D, Fenoglio R, Sciasc S. The dilemma of treating hepatitis C virus associated cryoglobulinemia. Rheumatol 2019;31(5):500–504. doi:10.1097/BOR.0000000000000624.
- Muchtar E, Magen H, Morie A, Gertz M.A. How I treat cryoglobulinemia. Blood 2017;29(3):289–298. doi:10.1182/blood2016-09-719773.



