Volume 29 Number 1
Autologous adipose-derived stem cells (ADSCs) transplantation in the management of chronic wounds
T Nguyen Dung, V Dinh Han, G Nguyen Tien and H Quan Lam
Keywords Chronic wounds, structure, adipose-derived stem cells, ultrastructure
For referencing Dung TN et al. Autologous adipose-derived stem cells (ADSCs) transplantation in the management of chronic wounds. Wound Practice and Research 2021; 29(1):41-48
DOI https://doi.org/10.33235/wpr.29.1.41-48
Abstract
Objective Our aim was to characterise the chronic wound response to autologous adipose-derived stem cells (ADSCs) sheet transplantation.
Methods A pilot descriptive, longitudinal study was performed at the Wound Healing Center of the Vietnam National Burns Hospital from 1 July 2019 to 30 August 2020. Thirty patients with 38 chronic wounds were enrolled in the study and autologous ADSCs sheets were grafted on the wound beds. Wound edges, wound bed, wound size and structure were assessed using histology with ultrastructural changes visualised by transmission electron microscopy. Wounds were assessed at time of transplantation and at the first, second and third week of follow-up.
Results After ADSCs sheet transplantation, improved extracellular matrix (ECM) with neo-vascular structures was observed, and collagen fibres arranged side by side in the dermal layer. Fibroblast proliferation was observed with increased secretion of collagen. Keratinocytes were also observed proliferating and migrating into the epidermis layer. Neo-vascular cells, fibroblast proliferation and collagen production increased weekly however lymphocytes infiltration decreased. Wound size reduced significantly compared to before treatment commenced and wound beds appeared cleaner and filled with granulation tissue. Re-epithelialisation appeared at the wound edge and throughout the wound. Conclusion: ADSCs may have a beneficial effect on cutaneous regeneration and chronic wound healing.
Key points
- Most chronic wounds are related to comorbidities; patients with chronic wounds should therefore be taken care of by multidisciplinary experts.
- Almost all published trials about the effectiveness of adipose-derived stem cells (ADSCs) in skin regeneration are in vivo or in vitro studies; only a few clinical trials have been done.
- A descriptive longitudinal study including 30 patients with chronic wounds was conducted to demonstrate the effects of autologous ADSCs sheet transplantation in the management of chronic wounds.
- The autologous ADSCs promoted the wound healing process by increasing cell proliferation and improvement of the extracellular matrix (ECM) in the chronic wound site.
Introduction
Chronic wounds are breaks in the skin of greater than 6 weeks or with frequent recurrence. The most common aetiologies of chronic wounds include venous leg ulcers, pressure ulcers, diabetic neuropathic foot ulcers, and leg ulcers of arterial insufficiency.1 From the aetiologic classification alone, comorbid conditions are identified that have a significant impact on patient morbidity and mortality related to wounds.1 Chronic wounds present major challenges for the clinician and wound care specialist by consuming a great deal of healthcare resources.2 Despite the development of new therapeutic methods, the treatment of chronic wounds remains unsatisfactory, and more effective treatment strategies are needed.2 Adipose-derived stem cells (ADSCs) isolated from liposuction or lipectomy specimens are mesenchymal and multipotential stem cells.3 ADSCs are easier to isolate and are relatively abundant, which may make them better stem cell sources for wound repair and regeneration.3 Studies demonstrate that ADSCs are involved in the wound healing process by secretion of growth factors and cytokines to promote skin repair and regeneration, and differentiate into other lineages and cell types.4–7 However, the mechanism of how ADSCs promote skin regeneration is unclear.8 Most published trials about the effectiveness of ADSCs in skin regeneration are found in in vivo or in vitro studies;9–12 few clinical trials have been conducted.9–12
In Vietnam, there is only one wound healing centre, located at the Vietnam National Burns Hospital in the capital city of Hanoi. Every year, on average, our centre treats approximately 1,000 in-patients with acute and chronic wounds. The goal of the study was to evaluate the effects of autologous ADSC sheet transplantation by measuring clinical signs, structure and ultrastructure changes of chronic wounds.
Methods
A pilot, descriptive, longitudinal study at the Wound Healing Center at the Vietnam National Burns Hospital from 1 July 2019 to 30 August 2020 was conducted after institutional ethics committee approval was obtained.
Subjects
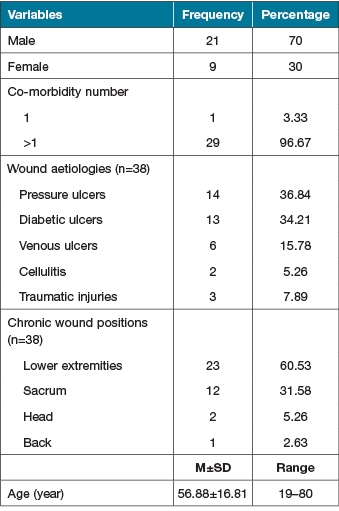
The study included 30 patients with a mean age of 56.88±16.81 years (range: 19–80 years). Of this group, most patients were men (male to female ratio: 3:1). Twenty-nine (96.67%) patients had more than one comorbidity. These 30 patients had 38 wounds with demonstrated wound aetiologies such as pressure ulcers (36,84%), diabetic ulcers (34.21%), venous ulcers (15.78%), cellulitis (5.26%) and traumatic injuries (7.89%), and wound positions such as lower extremities (60.53%), sacrum (31.58%), head (5.26%) and back (2.63%) (Table 1).
Table 1. Socio-demographic variables and clinical characteristics of chronic wounds
Study inclusion and exclusion criteria
All patients aged 18 years or older who presented to the clinic with a chronic wound as defined by less than 40% improvement after 6 weeks of wound care. Patients were asked to sign an informed consent form to participate in the study. Exclusion criteria included patients with Marjolijn’s ulcers or radiation ulcers and/or who were HIV positive or had hepatitis B and/or hepatitis C.
Procedure
On admission, all patients were evaluated for comorbid conditions and chronic wound beds were managed by the multidisciplinary wound care team. Based on examination results, these teams planned activities aimed at improving comorbidities and optimising wound bed preparation for cell therapy.
Isolation and culture of ADSCs in vitro
ADSCs were isolated from adipose tissue by an ADSC extraction Kit® which is manufactured by GeneWorld Co., Ltd (ISO 13485, 16128 management system). This Kit has been approved by the Ministry of Health to be circulated in Vietnam (permit number: 87/2016/BYT-TB-CT). Samples of human subcutaneous adipose tissue weighing 3–5g were acquired from the patients’ hypogastric or groin area by the surgical ablation of adipose tissue. The adipose tissue was digested with 0.075% collagenase type I in phosphate-buffered saline under gentle agitation for 30 minutes at 37˚C and centrifuged at 800×g for 10 minutes to obtain the stromal cell fraction. The pellets were resuspended, passed through a 100mm mesh filter, and cultured at 37˚C in 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum, 100mg/ml streptomycin, and 100UI/ml penicillin. The culture medium was replaced every 3 days. The primary cells were cultured until 80% confluence and were defined as passage 1. In this study, ADSCs were used at passage 5. ADSCs at passage 5 were seeded onto a polycarbonate membrane (Transwell®) with a density of 5x104 cells per cm2. The average time spent in isolation and culture of ADSCs in vitro was 12.63±3.78 days (range: 8–17 days).
Chronic wound management and autologous ADSCs sheet transplantation
While waiting for isolation and culture of ADSCs, wound beds were prepared in all patients following the TIME framework13 by the wound care team. Autologous ADSC sheet transplantations were only performed on wound beds of patients in a stable clinical condition. The wound beds themselves had no signs of necrosis or infection. In addition, bacterial culture results were negative or, if positive, had a median bacterial count in the wound site before grafting of less than 105 cfu/cm2.
The autologous ADSCs sheets were grafted on the surface of the wounds every 2–5 days until wounds were closed by re-epithelialisation and/or operation.
We assessed the wound edge and wound bed changes at the time of transplantation and at the first, second and third week of studied progress. This assessment was supported by photography. Wound size was calculated at every visit using Image Pro Plus 4.5 software.
Wound biopsies were taken before transplantation and at the first, second and third week to establish a baseline and follow the progress with assessment by H&E staining and transmission electron microscope (TEM).
Statistical analysis
The results before and after experiment were recorded and compared by using Stata 11.0 software. The Wilcoxon test was used for comparative studies. The value of p<0.05 was considered statistically significant.
Ethics statement
This study was executed in accordance with the recommendations and approved by the Scientific Council of the Vietnam Ministry of Health (decision number 338/QD-BYT on 22 January 2014) and the Ethics Committee of the National Institute of Burns (permit signed on 20 August 2013).
Results
Autologous ADSCs transplantation could enhance wound healing of chronic wounds
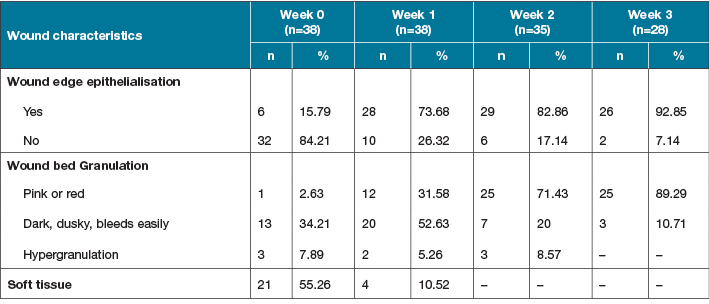
Out of the 38 wounds, before autologous ADSCs sheet transplantation (Week 0), 32 (84.21%) wounds had no epithelial signs along the wound edges, 21 (55.26%) had absence of granulation tissue in the wound bed, and 17 (44.73%) had unhealthy granulation tissue which manifested as dark and dusky and bled easily (13 wounds; 34.21%). Only one wound had red granulation tissue, and three (7.89%) wounds demonstrated hypergranulation tissue (Table 2). After autologous ADSCs sheet transplantation (Weeks 1, 2 and 3), the wounds were markedly improved as the treatment progressed. The proportion of wounds with epithelialisation at the wound edge and healthy granulation tissue increased significantly compared to before autologous ADSCs sheet transplantation; 82.86% of wounds at Week 2, and 92.85% at Week 3 had epithelialisation, 71.43% of wounds at Week 2 and 89.29% at Week 3 had healthy granulation tissue. Three wounds (after 2 weeks) and 10 wounds (after 3 weeks) were closed by epithelialisation alone or with skin grafts or skin flaps (Table 2).
Table 2. Wound characteristics before and after autologous ADSCs sheet transplantation
Autologous transplantation of ADSCs sheets was used to treat chronic wounds with areas ranging between 23.72±19.85cm2 (range: 3.01–81.38cm2). The wound measurements were recorded weekly. The results before and after experiment were compared by the Wilcoxon test. Our quantitative data were significantly different at the second and third weeks after starting treatment (Week 2: 12.8±11.56cm2, range: 1–47.42cm2, p<0.05; Week 3: 7.44±5.68cm2, range: 0.45–20.10cm2, p<0.001) (Figure 1), indicating the autologous ADSCs treatment enhanced the healing of chronic wounds.
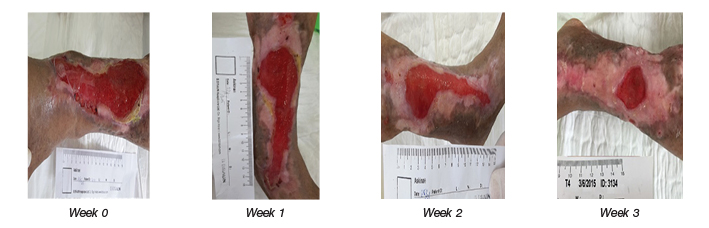
Figure 1. Chronic wound treated by autologous ADSCs sheets: representative images of a venous ulcer shown at Weeks 0, 1, 2 and 3.
Structure and ultrastructure of chronic wounds after autologous ADSCs sheet transplantation
In the 30 patients, 38 chronic wounds were biopsied for H&E staining and TEM. Before autologous ADSCs transplant, the chronic wounds were characterised by hyperproliferative epidermis. In addition, the presence of hyperkeratosis – a thick cornified layer – and parakeratosis were additional hallmarks portraying epidermal keratinocytes of chronic wounds. The capillary and fibroblast density were low. In the epidermis and dermis layer, the principal leukocytes were polymorphonuclear leukocytes and macrophages (Figure 2, A1). In ultrastructure imaging, the tissue was swollen. Collagen fibres were deranged and not bound. There were many polymorphonuclear leukocytes and lymphocytes in the ECM (Figure 2, B5 & B9 a, b, c, d).
At Week 1 post-autologous ADSCs sheet transplantation, wound beds were cleaner and had filled with granulation tissue. Re-epithelialisation appeared at wound edge. Neo-vascular cells and fibroblasts proliferated and collagen fibres were arranged side by side in the dermis layer. There were many active rough endoplasmic reticulum in the fibroblast. Increased pro-collagen production and collagen secretion was observed with collagen fibres arranged in compact, parallel lines and alongside fibroblasts (Figure 2, A2, B6 & B10 e, f, g).
At Week 2 post-autologous ADSCs sheet transplantation, keratinocytes proliferated and migrated to the epidermis layer. However, this epidermis layer was still thin; it only had a stratum basale layer, a stratum spinosum layer and a partial stratum granulosum layer. Connective tissue was densely infiltrated with lymphocytes. Images of neo-vascular cells and fibroblasts and collagen were very clear in the dermis layer (Figure 2, A3). In ultrastructure images, the epithelial cells of stratum basale layer were close to each other, without intercellular space (Figure 2, B7). In the ECM, fibroblasts proliferated strongly and showed increased secretion of collagen (Figure 2, B11).
At Week 3 post-autologous ADSCs sheet transplantation, epithelial cells covered most of the wound surface. The structure of the epidermis layer gradually returned to the epidermis structure of healthy skin. Neo-vascular, fibroblast proliferated at an increased rate compared to the previous weeks. Fewer lymphocytes infiltrated the connective tissues (Figure 2, A4). In ultrastructure imaging, fibroblasts replaced myofibroblast (Figure 2, B8 ). The ECM significantly improved as seen with the neo-vascular structures which have thin walls and large endothelial cells (Figure 2, B12).
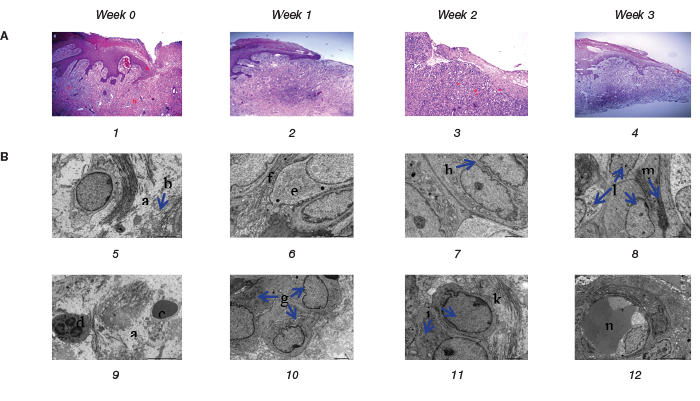
Figure 2. Histology of representative chronic wound before and after autologous ADSCs sheet transplantation:
(A) Chronic wound histological images (original magnification x100, H&E stain).
(B) Chronic wound ultrastructure images (original magnification x3000)
A1, B5 and B9 show the structure and ultrastructure before the autologous ADSCs sheet transplantation. A2, B6 and B10, A3, B7 and B11, A4, B8 and B12 are the structures and ultrastructure of the chronic wound after 1 week, 2 weeks and 3 weeks of autologous ADSCs transplantation resepectively.
Treatment result and side effects of autologous ADSCs sheet transplantation
After autologous ADSCs sheet transplantation, out of the 38 wounds 23 (60.53%) were closed by epithelialisation alone (Figure 3, 4), 15 (39.47%) wounds were closed by skin grafts or skin flaps. The average length of stay in hospitals was 51.47±15.48 days (range: 29–89 days) (Table 3). A total of 32 (84.21%) wounds had no side effects; three (7.89%) had infection and showed hypergranulation.
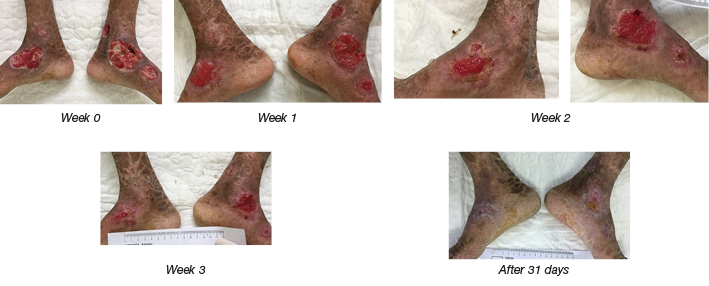
Figure 3. After autologous ADSCs sheet transplantation: the wound in the lower extremities of the patient with ichthyosis was closed by epithelialisation.
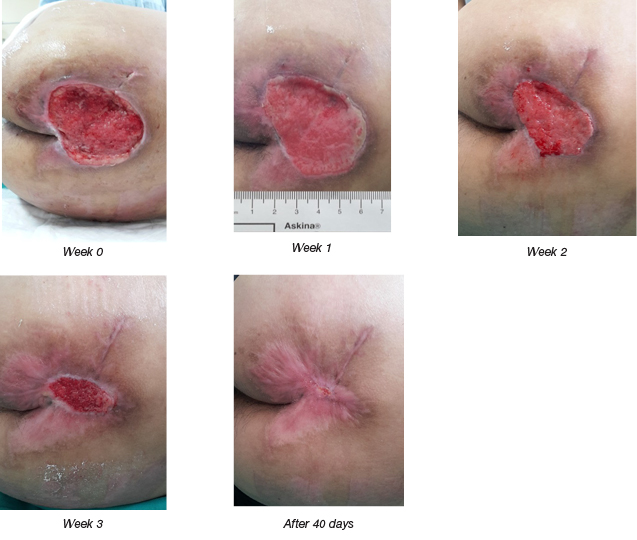
Figure 4. After autologous ADSCs sheet transplantation: a stroke patient with a sacral pressure injury.
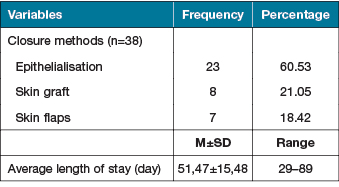
Table 3. Closure methods and the average length of stay in hospital
Discussion
While not all wounds in this pilot study closed primarily through ADSC, all wounds demonstrated an improved ECM. Chronic wounds lack an ECM that promotes wound healing.2,14 Several therapies exist to promote chronic wound healing; however, the lack of a healthy ECM limits wound healing. For instance, negative pressure wound therapy (NPWT) promotes wound healing by improving oxygenation, cellular proliferation, granulation and reducing bacterial load and inhibitory cytokines.15 Another therapy option is low temperature plasma (LTP) following surgical debridement;16,17 this is considered to be fine-tuning the debridement after removing non-visible necrotic tissue with LTP.16 Platelet rich plasma (PRP) is another method demonstrated in a meta-analysis that promotes the healing process in chronic ulcers.17 PRP is rich in growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), platelet factor-4, and epidermal growth factor (EGF) which causes cellular proliferation, angiogenesis, reduced inflammation and epithelialisation to stimulate wound healing.16–18 Studies have demonstrated that ADSCs are involved in the wound healing process by secretion of growth factors and cytokines to promote skin repair and regeneration while differentiating into other lineages and cell types.4–7
In this study, among the 30 patients who were included in this study, 29 (96.67%) had more than one comorbidity. Before hospitalisation at our centre, their chronic wounds failed to respond to presently available treatment plans which included dressings or skin grafts. When these patients were admitted to our centre they were evaluated by our multidisciplinary teams to manage comorbidities, then grafted with the autologous ADSCs sheets on the wound bed.
Epithelialisation is an essential component of wound healing. Epithelialisation occurs by an orderly series of events whereby keratinocytes migrate, proliferate and differentiate to restore the covering function of skin.19 Granulation tissue is new connective tissue and microscopic neo-vascularisation that forms on the surface of a wound during the healing process. The healthy granulation tissue will create favourable conditions for epithelialisation, skin grafting or local flap surgeries at the wound site.20 In this study, before autologous ADSCs sheet transplantation, almost all wounds had unhealthy granulation which was expressed as dusky, necrotic tissue (13 wounds; 34.21%). Only one of the wounds had healthy, red granulation tissue, while three (7.89%) had signs of hypergranulation (Table 2). One week following ADSC, the proportion of wounds with unhealthy granulation tissue decreased and was replaced by healthy granulation tissue. We also observed that after 1 week, epithelialisation appeared at the edge of wounds in 73.68% of wounds. The proportion of the wounds with epithelialisation rose at Week 2 (82.86% of wounds) and, by Week 3, 92.85% of wounds showed improved epithelialisation.
Addressing wound size, after autologous ADSCs transplantation, wound dimensions decreased significantly compared to before transplantation. In a study about the effect of secretory factors of ADSCs on human keratinocytes, Kyoung Mi Moon et al. showed that ADSCs contained various growth factors, making it an attractive raw material for healing.20 Advanced ADSCs protein extract contained growth factor/cytokine such as hepatocyte growth factor (HGF), fibroblast growth factor-1 (FGF-1), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), inteleurkin-6 (IL-6), vascular endothelial growth factor (VEGF), and transforming growth factor-β3 (TGF-β3). More keratinocyte wound healing and migration was shown in the experimental group.4 In another study, Won-Serk Kim and colleagues directly compared ADSC treatment with collagen gel solution without any transplantation, verifying that ADSCs in collagen gel solution are effective in wound healing in vivo.21 To confirm the practical role of ADSCs, Kim and colleagues also pointed out that treatment of collagen gel solution and that of ADSCs in the collagen gel solution is valid.21 ADSCs treatment significantly accelerated wound healing after 7 days with faster healing in the ADSCs-treated group than in the control group.21 Closure was almost achieved within 14 days.21
Regarding the structure and ultrastructure of chronic wounds after autologous ADSCs sheet transplantation, in preparation for autologous ADSCs sheet transplantation, while waiting for ADSCs culture and to seed them onto a polycarbonate membrane of Transwell® with a density of 5x104 cells per cm2, as well as the treatment of the patients’ comorbidities we managed chronic wounds by dressing changes with antimicrobial agents (BETAplastTM Silver, Aquacel® Ag) and debridement. Our aim was to prepare a clean receiving bed that was without signs of inflammation. In this study, bacterial culture results were negative or positive, with the median bacterial count in wound site before grafting less than 105cfu/cm2. However, before autologous ADSCs sheet transplantation, we still saw evidence of the chronicity of wounds in terms of structure and ultrastructure as determined by the presence of hyperkeratosis – a thick cornified layer – and parakeratosis. In addition, capillary and fibroblast density were very low. In the epidermis and dermis layer, the principal leukocytes were polymorphonuclear leukocytes and macrophages. Collagen fibres were degraded and there were many polymorphonuclear leukocytes and lymphocytes in the ECM.
The effects of ADSCs on wound healing and skin regeneration has been proven in both in vivo and in vitro studies. Seung Ho et al. found that the proliferation of HaCaT cells and fibroblasts was increased by ADSCs in the viability assay.8 ADSCs promoted in vitro wound healing of HaCaT cells and increased the contraction of the fibroblast-populated collagen lattice. Transcription-polymerase chain reaction showed that the transcription of the type I procollagen α1 chain gene in fibroblasts was upregulated by conditioned medium of ADSCs (ADSCs-CM). ADSCs promoted human dermal fibroblast (HDF) proliferation, not only by cell-to-cell direct contact, which was confirmed by co-culture experiment, but also by paracrine activation through secretory factors, resolved by Transwell® co-culture and culturing with conditioned medium of ADSCs. ADSCs enhanced the secretion of type I collagen in HDFs by regulating the mRNA levels of ECM proteins – up-regulation of collagen type I, III and fibronectin and down-regulation of matrix metalloproteinase-1 (MMP-1).19 Moreover, ADSCs-CM showed a stimulatory effect on the migration of HDFs in in vitro wound healing models.19 As an in vivo comparison study, the impact of human ADSCs sheet transplantation into mice with full-thickness excision wounds, Cerqueira et al. found that ADSCs promoted neo-tissue vascularisation at Days 14 and 21.22
In this study we observed improved wound healing rates and structure of the wound similar to that seen in both in vitro and in vivo studies.18–22 The neo-vascular, fibroblast proliferation and collagen fibres’ arranging side by side at the dermis layer improves wound healing.18–22 Fibroblast increased pro-collagen production and collagen secretion; collagen fibres were arranged in compact, parallel bundles beside the fibroblasts at Day 7. Neo-vascular, fibroblast proliferation and collagen production was very clear in the dermis layer. The epithelial cells of stratum basale layer were close to each other, without intercellular space.
Currently, a number of investigators have used ADSCs in wound healing studies.6,7,23–27 Some studies demonstrated trans-differentiation of ADSCs into keratinocyte-like cells6 and epithelial and endothelial lineages7; others reported improved wound healing parameters including increased wound closure rate (re-epithelialisation), increased healthy granulation tissue, and improved wound vascularity and perfusion.23–27 However, the mechanism of how ADSCs promote skin regeneration is unclear.4 Our study is a descriptive longitudinal case series study limited by the absence of a control group. Nevertheless, we feel that the longitudinal nature of our study adds significance to the attractiveness of autologous ADSCs sheet transplantation as a supportive therapy. These observations are preliminary and therefore not conclusive, as they require further studies allowing comparisons with a control group and evaluating changes of ADSCs after transplantation on the wound bed.
Summary
This study demonstrates statistically significant improved wound healing rates related to the effects of ADSCs in cutaneous regeneration. ADSCs sheet transplantation promotes the wound healing process through cell proliferation and improved structure of the ECM in the chronic wound site.
Acknowledgements
We would like thank all staff at the Vietnam National Burns Hospital for helping us to collect data.
Conflict of interest
The authors declare no conflict of interest.
Author(s)
T Nguyen Dung*
Wound Healing Center, National Burn Hospital
Vietnam Military Medical University
Email ntzung_0350@yahoo.com
V Dinh Han
Wound Healing Center, National Burn Hospital
Vietnam Military Medical University
G Nguyen Tien
National Burn Hospital, Vietnam Military Medical University
H Quan Lam
Histology Department, Vietnam Military Medical University
* Corresponding author
References
- Markova A, Eliot NM. US skin disease assessment: ulcer and wound care. Dermatol Clin 2012;30(1):107–11.
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4(9):560–82.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–95.
- KM, Park Y-H, Lee JS, et al. The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J Mol Sci 2012;13:1239–57.
- Mazini L, Rochette L, Admou B, et al. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci 2020;21(4):1–19.
- Chavez-Munoz C, Nguyen KT, Xu W, et al. Trans-differentiation of adipose-derived stem cells into keratinocyte-like cells: engineering a stratified epidermis. PLoS ONE 2013;8(2):e80587.
- Nie C, Yang D, Xu J, Si Z, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2001;20(2):205–16.
- Lee SH, Jin SY, Song JS, et al. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 2012;24(2):136–43.
- Marino G, Moraci M, Armenia E, et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 2013;185(1):36–44.
- Han S-K, Kim H-R, Kim W-K. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen 2010;18(4):342–48.
- Chopinaud M, Labbé D, Creveuil C, et al. Autologous adipose tissue graft to treat hypertensive leg ulcer: a pilot study. Dermatolol 2017;10.1159/000478009.
- Holm JS, Toyserkani NM, Sorensen JA. Adipose-derived stem cells for treatment of chronic ulcers: current status. Stem Cell Res Therapy 2018;9:142.
- Schultz G, Sibbald G, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:1–28.
- Pai DR, Madan SS. Techniques in chronic wound management: review of the literature and recent concepts. J Nov Physiother 2013;3(2):134. doi:10.4172/2165-7025.1000134
- Plikaitis CM, Molnar JA. (2006) Subatmospheric pressure wound therapy and the vacuum-assisted closure device: basic science and current clinical successes. Expert Rev Med Devices 2006;3:175–184
- Kramer A, Hübner NO, Weltmann KD, Lademann J, Ekkernkamp A, et al. Polypragmasia in the therapy of infected wounds: conclusions drawn from the perspectives of low temperature plasma technology for plasma wound therapy. GMS Krankenhhyg Interdiszip 2008;3:Doc13.
- Villela DL, Santos VL. Evidence on the use of platelet-rich plasma for diabetic ulcer: a systematic review. Growth Factors 2010;28:111–16.
- Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med 2010;83:1–9.
- Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014;3(7):445–64.
- de Oliveira Gonzalez AC, Fortuna Costa T, de Araújo Andrade Z, et al. Wound healing – a literature review. An Bras Dermatol 2016;91(5):614–20.
- Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24.
- Cerqueira MT, Pirraco RP, Santos TC, et al. Human Adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules 2013;14(11):3997–4008.
- Manning CN, Martel C, Sakiyama SE-E, et al. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Therapy 2015;6(74).
- Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue–derived stromal cells. Arterioscler Thromb Vasc Bio 2005;25:2542–47.
- Alexaki VI, Simantiraki D, Panayiotopoulou M, et al. Adipose tissue-derived mesenchymal cells support skin reepithelialization through secretion of KGF-1 and PDGF-BB: comparison with dermal fibroblasts. Cell Transplant 2012;21:2441–54.
- Mendez JJ, Ghaedi M, Sivarapatna A, et al. Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials 2015;40:61–71.
- McLaughlin MM, Marra KG. The use of adipose-derived stem cells as sheets for wound healing. Organogenesis 2013;9(2):79–81.



