Volume 29 Number 1
The importance of nutrition in wound management: new evidence from the past decade
Rochelle Kurmis, Michael Woodward, Hayley Ryan and Jan Rice
Keywords wound healing, pressure injuries, diabetic foot ulcers, nutrition, arginine, glutamine
For referencing Kurmis R et al. The importance of nutrition in wound management: new evidence from the past decade. Wound Practice and Research 2021; 29(1):18-40
DOI https://doi.org/10.33235/wpr.29.1.18-40
Abstract
Malnutrition is known to contribute to wound development and impair wound healing through reduction in the availability of nutrients to maintain optimal cell maintenance and repair.
This review examines studies from the last decade identified via a search of PubMedTM and systematic review databases to identify evidence for the effectiveness of nutritional interventions in wound healing. Studies reported identified via the search included 61 primary studies and six systematic reviews.
Generally, single nutrient interventions were found to be less effective than interventions utilising multiple nutrients. Immune modulating supplements containing arginine (Arg) were shown in 13 studies to result in significant improvements in at least one outcome measure for the intervention groups. There was also support for the use of arginine combined with anti-oxidant nutrients in malnourished individuals with pressure injuries (PI), and this intervention was found to be cost effective. The administration of glutamine (Gin) via the enteral nutrition (EN) route appears to convey a beneficial effect, particularly in burns and trauma patients, compared to parenteral nutrition (PN) administration. Omega-3 fatty acids were found to improve healing of diabetic foot ulcers. Encouraging further large-scale, multi-centre, prospective nutritional intervention research in areas of evidence deficiency is recommended.
Introduction
Wound healing is an important focus of care across all settings, not limited to any particular condition or age group. Achieving optimal healing requires an understanding of nutritional requirements, and these need to be adapted to the setting and incorporated into a care plan.
The economic burden of wounds across various healthcare setting is not fully defined. This is due to a lack of centralised incidence and cost data. Chronic wounds such as pressure injuries (PIs) (also called pressure ulcers) are recognised as one of the more challenging wounds to manage for a multi-disciplinary team1. In Australia, PIs alone have been reported to cost an estimated A$983 million for the 2012–13 fiscal year, equating to 1.9% of public hospital expenditure or 524,661 bed days from 121,645 cases2. The 2017 New South Wales Pressure Injury Point Prevalence Survey reported that 7.7% of inpatients had a PI, with 4% of these being hospital acquired3. In residential aged care facilities, 7.8% of residents had a PI, and 9.3% of community or outpatient participants reported a PI in this survey3. In addition, acute wounds such as postoperative wound breakdown or surgical wound dehiscence (SWD) are often under-reported and contribute significantly to the economic burden of care4. In the US, non-healing infected surgical wounds were the most common and costly wound type, equating to US$13.1 billion in Medicare benefits in 20144. These are not only managed in the hospital setting, increasing average hospital length of stay (LOS) in the US by 9.4 days, but also in the community setting4. It has been reported in the UK that over 57% of SWD healing by secondary intention were managed in the community setting4.
Risk factors for the development of wounds are complex and multi-factorial. It is recognised that unintentional weight loss is a predictor for wound development; however, this is also complicated by health co-morbidities as well as individual circumstances5. Elderly people living on their own are at higher risk of malnutrition. Whilst meal supports may be available, this may not supply an adequate full day’s required nutritional intake, and comes at an economic burden to the individual that may be a deterrence. Additionally, physical functioning may be impaired, limiting ability to optimally prepare meals and subsequently decreasing intake. Confinement to a bed or chair is a known contributor to PI and increased mortality risk5.
Malnutrition is known to contribute to wound development and impair wound healing through reduction in the availability of nutrients to maintain optimal cell maintenance and repair. Due to the decrease in sub-cutaneous adipose tissue in undernourishment, cushioning afforded over bony prominences is reduced, compromising the tissues ability to cope with pressure, friction and shear1. In addition, immunity is decreased in the undernourished, allowing infection4. Malnourished patients are twice as likely to develop PI (relative risk (RR) 2.1; 95% CI, 1.1–4.2)5. Whilst malnutrition is not commonly thought of as a condition prevalent in western countries, the Nutrition Care Day Survey completed in 2010 indicated that, from 56 participating hospitals across Australia and New Zealand, representing 3122 patients, 32% of patients were malnourished, with a further 41% identified as “at risk of malnutrition”6.
Addressing nutrition in wound healing is a recognised part of the multi-disciplinary management required to achieve optimal healing outcomes1,4. Differences do exist between the nutritional management of acute and chronic wounds; however, there are many similarities1,4. Nutrition for wound healing is often described in terms of the provision of macronutrients, micronutrients and fluid (water). Macronutrients are probably the most commonly known group of nutrients and are considered as important for their role in wound healing, with the three pillars of protein, fats and carbohydrates falling under this umbrella term. Protein as a whole is considered the ‘building block’ of muscle or lean tissue for the body, as well as for cells required for optimal immune function (lymphocytes, leukocytes, phagocytes, monocytes and macrophages) and the wound healing protein collagen7. As part of normal digestion it is broken down into amino acids, of which some are non-essential (able to be produced by the body), some are essential (required to be provided through nutritional intake in adequate amounts), and some are conditionally essential8.
Conditionally essential amino acids are of particular interest in wound healing. Conditionally essential amino acids are those which, under normal physiological circumstances, are available in adequate volumes within the body to achieve healthy homeostasis; however, in periods of stress, additional exogenous sources are required to maintain optimal function9. Such examples regarding wound healing in the reported literature are Arg, Gln and methionine. Arg is a precursor for proline, glutamate and polyamine synthesis10. It has been demonstrated to promote wound healing, stimulate insulin, insulin-like growth factor-1 and pituitary human growth hormone. In-vitro studies have also demonstrated that it has a role in the promotion of T-cell proliferation10. Gln acts as a direct source of cellular energy to assist with metabolic functions as a nitrogen shuttle. Gln stimulates immune function and wound healing through acting as a fuel source for lymphocytes, macrophages and fibroblasts10. Importantly, Gln preserves gut integrity through acting as a primary fuel source for the enterocytes and colonocytes within the gastrointestinal tract which may prevent translocation of pathogenic bacteria across the intestinal lumen, in turn preventing systemic infections10. Gln is an important nutrient in the support of anti-oxidant function through its role as a precursor for glutathione and potentially reduces insulin resistance10.
During times of physiological stress, synthesis of nucleotides is down-regulated in the body, resulting in decreased replication of rapidly dividing cells required for wound healing and immunity, such as GI mucosa, lymphocytes and macrophages11. One area of research relating to wound healing is the exogenous supplementation of RNA nucleotides in combination with other active nutrients11.
Upon intake, fat is broken down into smaller components known as fatty acids which, similar to protein, are regarded as essential and non-essential, and cholesterol8. Fatty acids themselves are essential in the body to form the lipid bi-layer of all cell and organelle membranes as well as the membranes that insulate nerve axons. Fats also provide a source of cellular energy via beta oxidation during catabolic states8. Similar to protein, certain subgroups of fatty acids have been researched with specific interest related to wound healing. For example, the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are metabolised to comparatively less inflammatory and less immunosuppressive metabolites than omega-6 fatty acids11 and this in turn may aid wound healing. Carbohydrates, specifically glucose, are the preferred substrates for cellular energy, especially for brain and erythrocytes. Current recommendations for a healthy diet are that 45-65% of energy be provided through carbohydrates (Australian Dietary Guidelines, available from: https://www.health.gov.au/resources/publications/the-australian-dietary-guidelines).
Micronutrients is the group terminology for nutrients that are present in the body in minute amounts but contribute to essential function and optimal homeostasis. This grouping is made up of vitamins, minerals and trace elements. Micronutrients provide co-factors for many necessary enzymatic processes in the body. Some micronutrients, especially fat soluble nutrients, have good reserves in the body when dietary intake is adequate in a healthy state; however, water soluble nutrients are not stored in the body and regular intake is essential for healthy functioning. During periods of stress, including that of wound healing, their intake becomes more essential.
Ascorbic acid, or Vitamin C, is possibly the most well recognised micronutrient in regards to is contribution to wound healing and immunity. Vitamin C acts as an anti-oxidant as well as being essential for collagen production in wound healing through its role as a co-factor during collagen synthesis. Similarly to Vitamin C, zinc is well regarded for its role in immune functions and wound healing12. Deficiency of zinc leads to suppression of cell proliferation as it is a co-factor for many enzymes required for the synthesis of RNA, DNA and proteins13,14.
Iron also has an essential role in wound healing as an essential part of haemoglobin which is required for oxygen transport to the regenerating wound tissue12,15. In addition, iron is a co-factor in the enzymatic process required for synthesis of collagen15,16. Emerging areas of research regarding wound healing include vitamin D, calcium b-hydroxy-b-methylbutyrate (CaHMB), probiotics, bioflavonoids and folate; however, their roles are currently less well understood17–22.
Nutrients investigated for their role in wound healing have traditionally been supplemented as an individual or single nutrient supplementation strategy or in addition to other nutrients which may convey their own beneficial effect on wound healing as a combined nutrient supplementation strategy. Alternate nutritional interventions have also been reported where interventional strategies employed are not able to be assigned to either of the first two categories.
The aim of this review is to summarise the readily accessible evidence from the past decade that addresses nutritional interventions in wound healing.
Methods
A literature review was conducted using the online database PubMed™ to identify studies published in the past 10 years that evaluated the effect of nutritional interventions on wound healing outcomes. PubMed™ was utilised due to its easy and free accessibility for clinicians, regardless of organisational affiliations. This interface searches the same Medline content as Ovid; however, it does not require subscription and includes all references as soon as they are added to the US National Library of Medicine (NLM) without delay. Search terms included nutrition, vitamin, mineral, protein, amino acid, arginine, glutamine, fat, carbohydrate, zinc, iron and wound. In addition, to identify any systematic reviews related to this topic, a search was conducted of systematic review databases including The Cochrane Database, Joanna Briggs Institute Database of Systematic Reviews and Implementation Reports as well as PubMed™. Search parameters were limited to human trials, English language articles and publication date (defined for the purpose of this review as 1 January 2010 to 17 January 2020). Studies published earlier to this date range were included for presentation only where they were presented as part of a systematic review published within the specified date range for the search.
Interventions considered for inclusion were nutritional interventions including parenteral (PN), enteral (EN) or oral nutrition (ON) strategies. Outcome measures of interest to this study included wound healing, anastomosis integrity, LOS and mortality. Where included systematic reviews reported on alternate outcomes of interest than this review, this data was excluded from reporting. For the purpose of this review, studies investigating topically applied nutrients/dressings, preventative administration of nutrition prior to radiotherapy, nutritional strategies to prevent wound development, study protocols, radiotherapy-induced skin/mucous membrane conditions (dermatitis, mucositis), and pharmaceutical nutritional adjuncts (such as human growth hormone and anabolic steroids) were excluded. All citations retrieved from database searches were exported into the bibliographic citation management software EndNote® X9 (Thomson Reuters). After removal of duplicates and screening of titles and abstracts against eligibility criteria for the review, potentially relevant full text articles were retrieved and assessed for their suitability for inclusion in the review.
Results
The full process of study selection is detailed in Figure 1. The results are broken down into three categories – single nutrient supplementation strategies, combined nutrient supplementation strategies, and alternate nutritional intervention strategies. These are considered in detail, in particular relation to their role in wound healing.
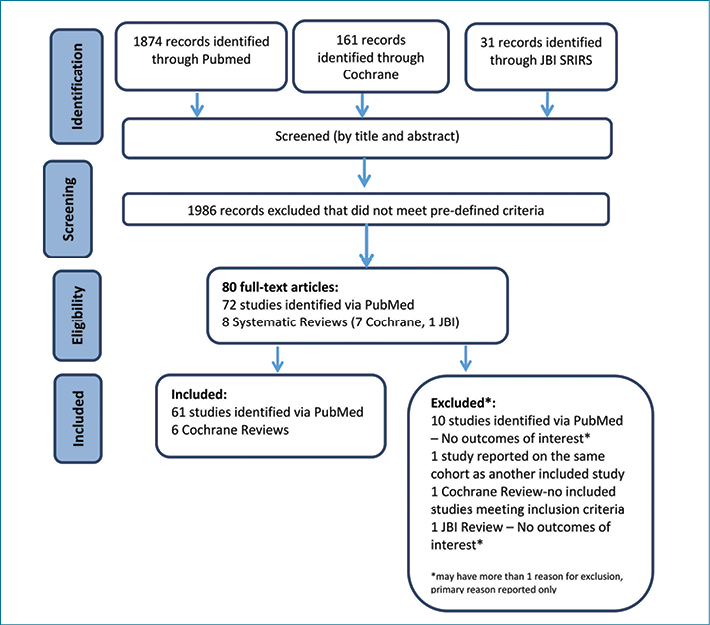
Figure 1. Flow chart of literature review process (adapted from PRISMA flow diagram23)
Single nutrient supplementation strategies and their role in wound healing
Arginine
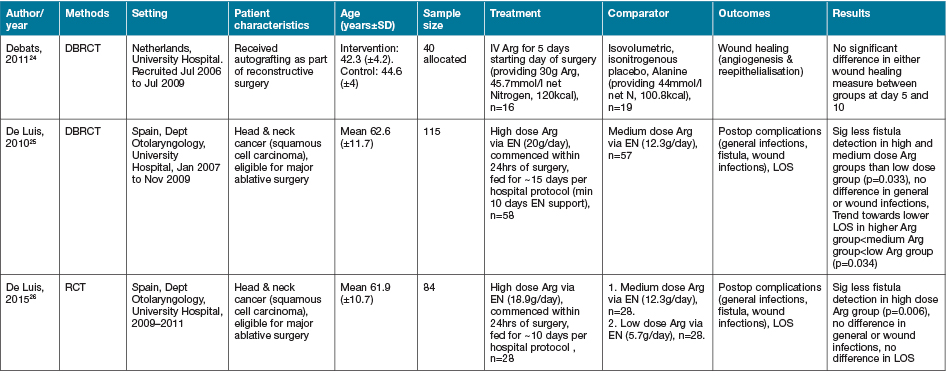
Three studies identified via our search strategy investigated the amino acid as a single nutrient intervention strategy in wound healing (Table 1)24–26. Debats and colleagues24 investigated the use of 30g intravenous (IV) Arg supplementation for 5 days post-autograft reconstructive surgery vs an isonitrogenous control and demonstrated no significant difference on wound healing outcomes24. Two studies from Spain investigated the effect of EN supplementation of high dose Arg (20g and 18.9g per day) compared to lower dose Arg (12.3g per day) supplementation on wound healing outcomes following head and neck cancer surgery25,26. Both of these studies demonstrated significantly less wound fistula formation in the high dose intervention groups (p=0.033 & p=0.006, respectively); however, no significant differences were seen in the rate of wound infections nor LOS25,26.
Table 1. Arginine: characteristics of included primary studies
One systematic review identified27 included one study assessing the effectiveness of arginine butyrate administered parenterally on leg ulcer healing in patients with sickle cell disease. There was a non-significant difference in incidence of complete ulcer closure reported in the Arg butyrate group (30%, 11 of 37 ulcers) versus the control group (8%, two of 25 ulcers) (p=0.056). This study also reported an improved rate of healing as determined by the mean decrease in pressure ulcer size with Arg butyrate administration compared to the control; however, the relative effect was not estimable in the systematic review and this study was assigned a very low quality of evidence due to inconsistencies with randomisation of its small cohort (62 ulcers) and its lack of statistical power to determine an effect.
Glutamine
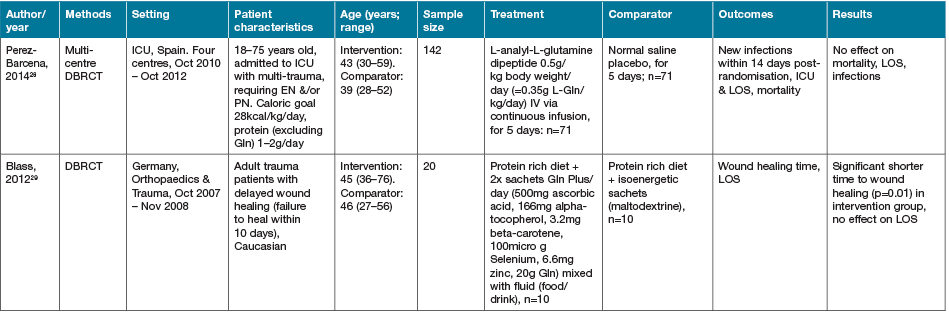
There were two studies identified investigating the supplementation of Gln as a single nutrient intervention strategy (Table 2)28,29. Perez-Barcena and colleagues28 investigated the use of IV Gln for 5 days in the intensive care unit (ICU) following multi-trauma admission. No effect of supplementation was demonstrated on mortality, LOS nor infectious complications28. In contrast, the study reported by Blass and colleagues29 investigated EN supplementation of Gln, in addition to other anti-oxidant nutrients, and a high protein diet in trauma patients who had demonstrated delayed wound healing. This study demonstrated a significant improvement in time to wound healing (p=0.01); however, no effect on LOS was seen29.
Table 2. Glutamine: characteristics of included primary studies
In the systematic review conducted by Tan and colleagues30 investigating the effects of immunonutrients following burn injury, seven studies were identified that investigated the supplementation of Gin vs a control or placebo. All of these included studies were published between 2001 and 200430. Three of the included studies reported on all-cause mortality following supplementation and, when pooled in the systematic review, showed a significant decrease (RR 0.25 (95% CI 0.08 to 0.78) p=0.02)30. All of the seven included studies reported on LOS, representing 255 participants30. When pooled, these results demonstrated a significant decrease in LOS (RR –5.65 (95% CI –8.091,31 to 3.22) p=<0.0001)30. It should be noted that these analyses included studies where Gin supplementation was via the EN or PN routes, and results may differ for these methods of administration.
This systematic review also included three studies investigating the effects of ornithine α-ketoglutarate (a precursor for Gin and Arg) vs soy protein or placebo30. All three studies, representing 155 participants, reported on mortality and, when pooled, results demonstrated no significant effect of supplementation (RR 0.93 (95%CI 0.37 to 2.36) p=0.88)30. One included study (n=48) reported on LOS and failed to demonstrate a significant decrease (RR –4.21 (95% CI –18.87 to 10.45) p=0.57)30.
Omega-3 fatty acids
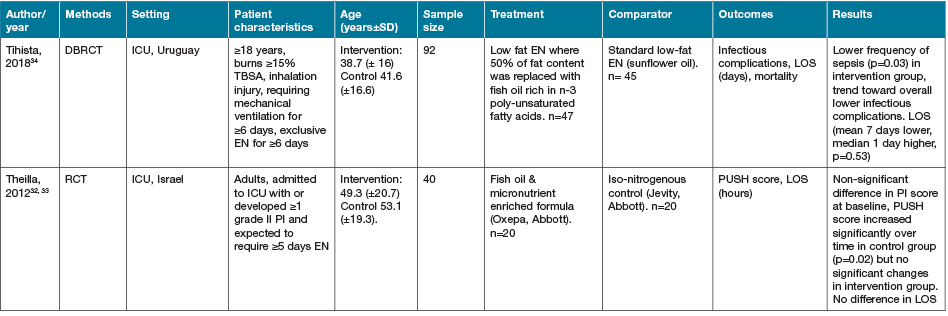
Three studies were identified that investigated the supplementation of omega-3 fatty acids from fish oil sources32–34, although two of these studies32,33 reported on different outcomes from the same cohort of study participants so have been combined for the presentation of outcomes in Table 332–34. Two study cohorts were administered EN enriched with fish oil compared to standard EN formulas32–34. The study by Tihista and colleagues34 in burn injury patients requiring mechanical ventilation demonstrated a significant decrease in sepsis (p=0.03); however, no difference in LOS was seen34. The cohort presented by Theilla and colleagues32,33 investigated PI patients in the ICU. This group demonstrated a deterioration in PI state in the control group, whilst the intervention group PI severity did not change significantly.
Table 3. Omega-3 fatty acids: characteristics of included primary studies
An additional double blind, randomised controlled trial (DBRCT) by Soleimani and colleagues35 investigated the supplementation of omega-3 fatty acids derived from flaxseed oil (100mg/day, give twice daily for 12 weeks), versus a placebo in 60 patients with grade III diabetic foot ulcers. They reported that supplementation significantly reduced ulcer length (p=0.03), width (p=0.02) and depth (p=0.01) compared to the placebo35.
In the systematic review identified by Tan and colleagues30, only one study investigating the effect of omega-3 fatty acids following burn injury was included. This study was published in 1995 and included 25 participants. No significant effect was seen on mortality (p=0.41); however, a decrease in LOS was reported (RR –21.0 (95% CI –41 to –0.97) p=0.04)30.
Zinc
One small primary study was identified that investigated the role of zinc supplementation as a single agent on wound healing outcomes in a cohort of 58 patients36. Momen-Heravi and colleagues36 supplemented patients with grade III diabetic foot ulcers with 220mg zinc (50mg elemental zinc) daily for 12 weeks in a DBRCT, placebo controlled study. They reported a significant increase in serum zinc levels (p<0.001), and a significant decrease in ulcer length (p=0.02), width (p=0.02), but not depth (p=0.05) in the intervention group compared with the placebo group36. Significant improvements in serum insulin (p=0.009), HbA1c (p=0.01), total anti-oxidant capacity (p<0.01) and total glutathione status (p=0.006) were also reported benefits with supplementation36.
Two additional studies identified investigated the supplementation of a carnosine zinc complex (Polaprezine) in adult patients with PIs37,38. Both studies reported significant improvements with Polaprezine supplementation at 150mg/day compared with no supplementation on wound healing from baseline until week 8 of supplementation (p=0.00937 and p<0.00138). Neither study was blinded nor randomised, and both studies consisted of small cohorts and were conducted by the same authors at the same site37,38.
Two systematic reviews were identified that included the investigation of zinc sulphate supplementation on PI39 or arterial and venous leg ulcer40 healing. Only eight trials were identified between the two reviews, representing 217 total participants, all conducted prior to 1980. All included studies were identified as having risk of bias. No beneficial effects of supplementation were identified by either review39,40.
Vitamin D
Three studies identified as part of this review supplemented with vitamin D versus placebo20,41,42, whilst a fourth study provided a nutritional supplement fortified with vitamin D, calcium b-hydroxy-b-methylbutyrate (CaHMB) and protein versus a standard diet19.
Razzaghi and colleagues41 investigated the supplementation of 50,000 IU of vitamin D every 2 weeks versus placebo, over a 12-week period in 60 participants with grade III diabetic foot ulcers. This study reported an overall positive effect on wound healing with supplementation by demonstrating a significant decrease in the length (p=0.001), width (p=0.02) and depth (p<0.001) of the ulcers41.
Burkiewicz and colleagues20 investigated the supplementation of 50,000 IU of vitamin D every week for 2 months versus no supplementation in 52 vitamin D deficient participants with chronic venous leg ulcers. A non-significant trend towards improved healing with supplementation was reported (p=0.0676)20.
In the DBRCT presented by Gottschlich and colleagues42 vitamin D supplementation (100 IU/kg/d vitamin D2 in 18 patients or 100 IU/kg/d vitamin D3 in 15 patients) was compared to a control group (17 patients) following severe paediatric burn injury. No difference in LOS, number of surgical procedures, nor mortality was identified42.
The randomised control trial (RCT) reported by Ekinci and colleagues19 investigated the use of a diet supplemented with specialised wound healing supplements (enriched with 3g CaHMB, 1000 IU vitamin D, and 36g protein) vs a standard diet alone in 75 postoperative hip fracture patients. Supplementation was reported to significantly decrease wound healing time (p=0.037); however, no differences in LOS were observed between groups (p=0.76)19.
Vitamin C
One study identified by Li and colleagues43 investigated the supplementation of oral vitamin C for 7 days post dental implant surgery in 128 participants on wound healing outcomes compared to no supplementation. They demonstrated that patients who received dental implants supported with a guided bone regeneration (GBR) technique and implants for chronic periodontis had significantly improved wound healing outcomes (p<0.002) compared to no supplementation43. Patients who underwent implants and Bio-Oss collagen grafts and dental implants without grafts or periodontis did not demonstrate any significant benefit from supplementation43.
Magnesium
Two studies identified investigated the effect of magnesium supplementation either alone or combined with other nutrients on wound healing outcomes44.
In the DBRCT conducted by Razzaghi and colleagues44, 70 participants with grade III diabetic foot ulcers were administered either 250mg/day of magnesium or placebo. Supplementation was reported to significantly decrease ulcer length (p=0.01), width (p=0.02) and depth (p=0.003)44.
Also investigating the effects of nutritional supplementation of grade III diabetic foot ulcer healing, Afzali and colleagues45 supplemented 57 participants with either 250mg/day of magnesium combined with 400 IU vitamin E/day or placebo for 12 weeks. This intervention was also reported to significantly reduce ulcer length (p=0.003), width (p=0.02), and depth (p=0.02) compared to the placebo group, although the mix of nutrients makes it difficult to determine if this effect was related to either nutrient alone or the combination45.
Combined nutrient supplementation strategies and their role in wound healing
The largest grouping of supplementation type identified as part of this review was the supplementation of Arg in conjunction with other macro and micronutrients as part of immunonutrition regimens, delivered via various EN and ON preparations, with 16 studies identified (Table 4)46–61.
Table 4. Combined Arg containing supplements with other active nutrients: characteristics of included primary studies
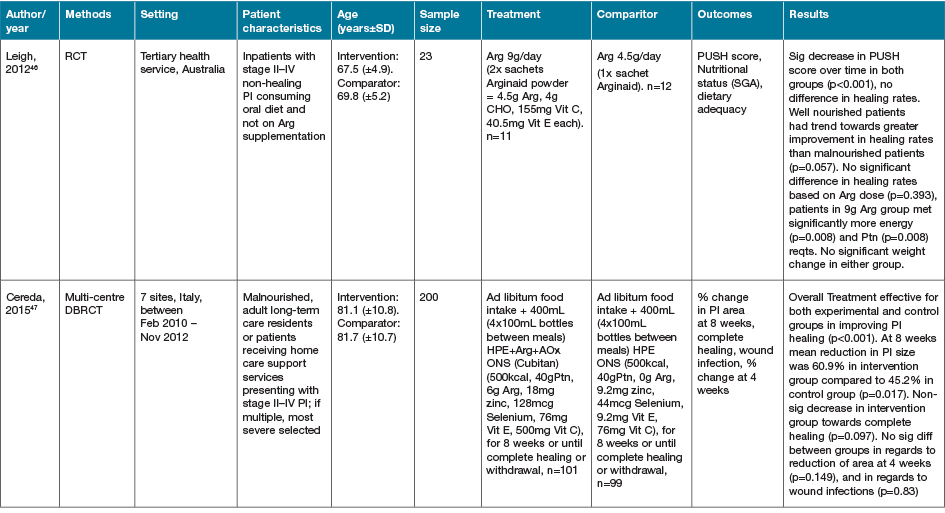
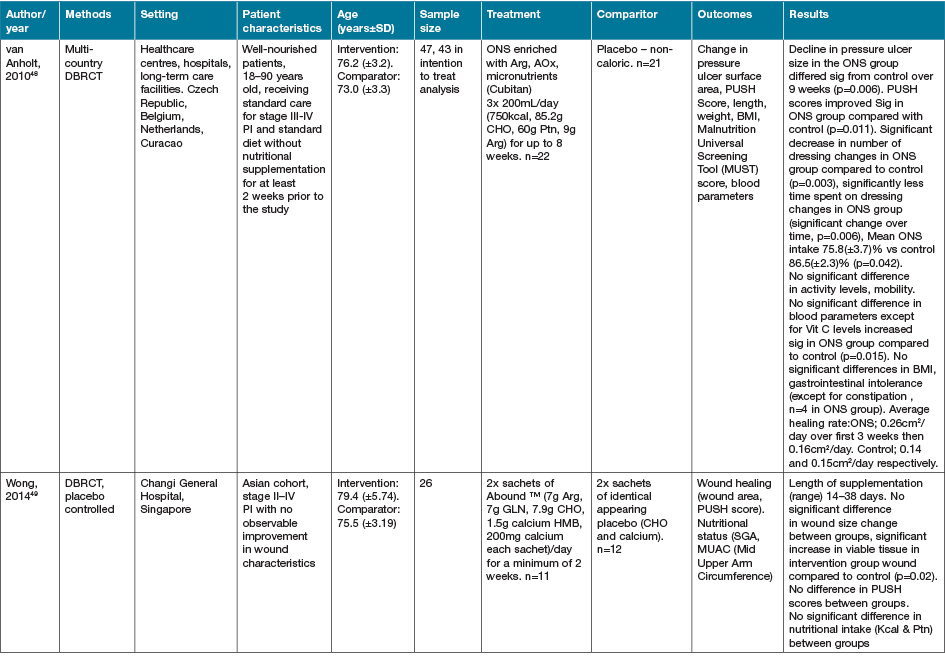
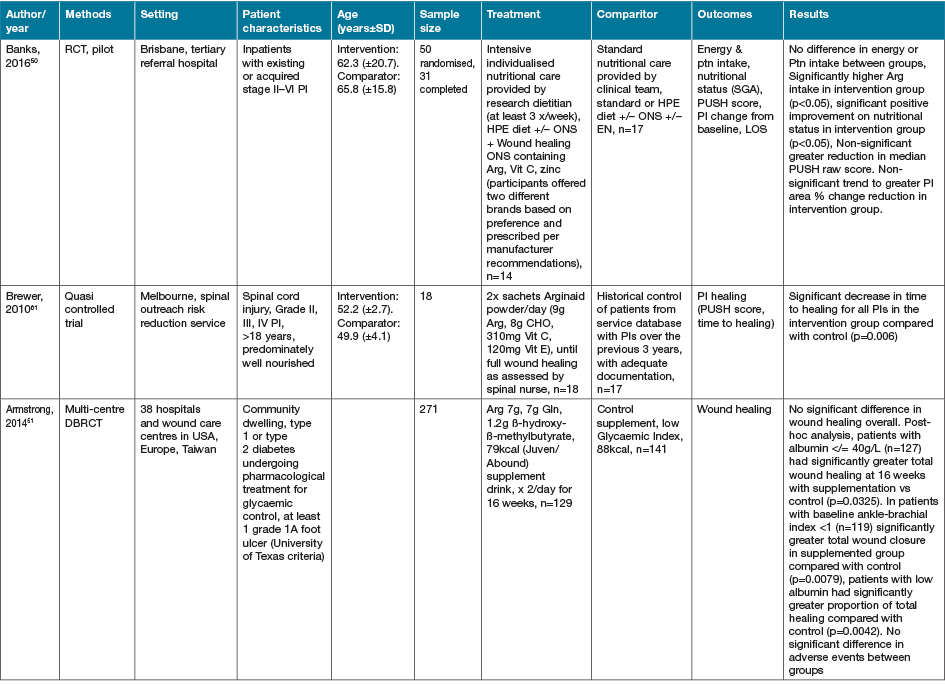
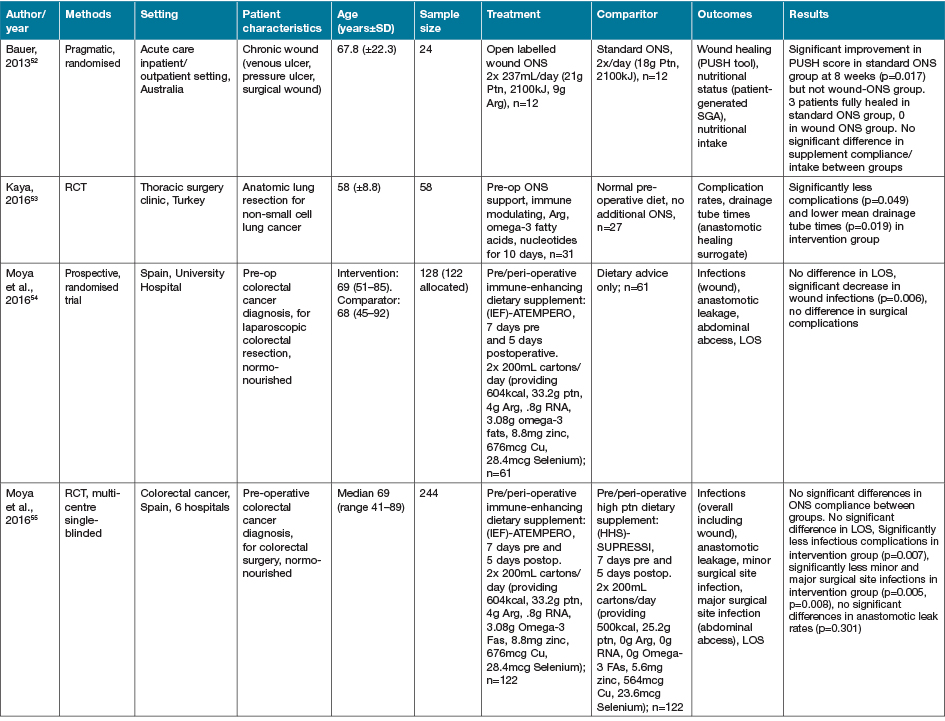
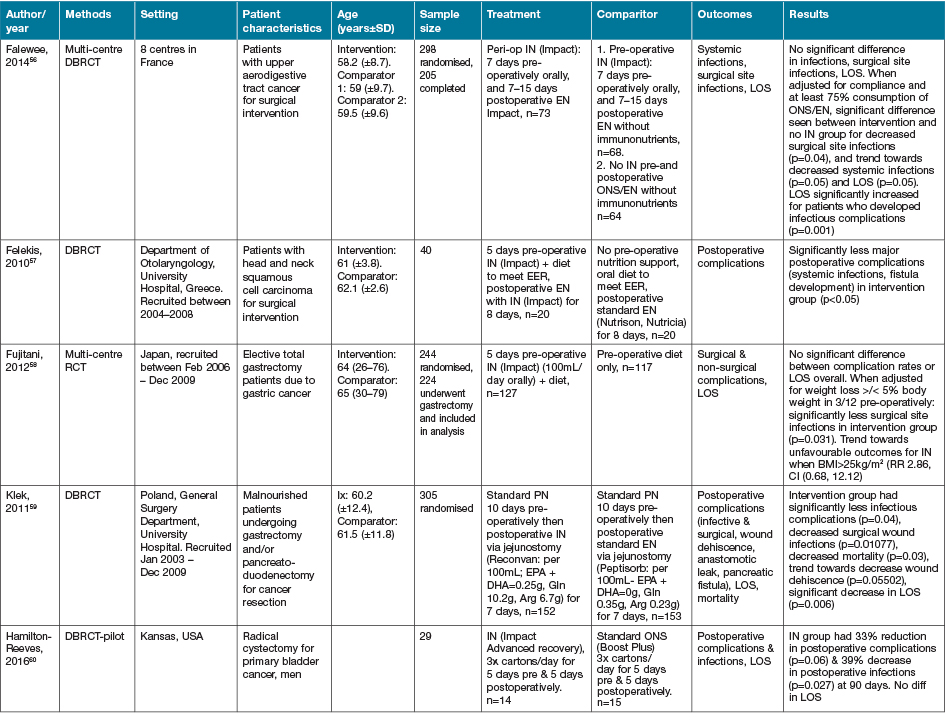
AOx = anti-oxidant; CHO = carbohydrate; EER = estimated energy requirements; HPE = high protein & energy; IN = immunonutrition; ONS - ON support; Ptn = protein; SGA = subjective global assessment
Eight of the studies investigating immune-nutrition strategies identified studied cohorts of patients presenting with PI, venous ulcers or diabetic ulcers46–52,61. The remaining eight studies investigated outcomes for patients undergoing surgery for various malignancies53–60.
In 13 studies where an immune modulating supplement containing Arg was compared to a standard or placebo control with no or very low levels of Arg, significant improvements were seen in at least one outcome measure for the intervention groups47–50,52–57,59–61. In two studies where no effect was seen in the whole cohort, when adjusted for nutritional parameters, patients with poorer nutritional indices (severe weight loss or hypoalbuminaemia) demonstrated significant improvements in outcome measures compared with controls51,58. This effect was not seen in the study by Leigh and colleagues46. This study compared a high dose of Arg (9g/day) supplementation with a moderate dose Arg (4.5g/day) supplementation in non-healing PI patients. Both groups demonstrated significant improvements in wound healing outcomes with supplementation, whilst well-nourished patients demonstrated a trend towards improved healing rates compared to malnourished patients (p=0.057)46. The high dose supplementation group did meet significantly more of their energy (p=0.008) and protein (p=0.008) intake compared with the moderately supplemented group, with no difference in weight change seen over the study period46.
The cohort of 200 malnourished adults with stage II–IV pressure ulcers in long-term and home care services presented by Cereda and colleagues47 provided a mixed nutritional supplement containing Arg and anti-oxidant nutrients compared to an isocaloric, isoitrogenous control. Supplementation demonstrated a significant decrease in pressure ulcer area (60.9%) compared to control (45.2%) (p=0.017). Both intervention and control demonstrated significantly improved wound healing (p<0.001)47. Interestingly, this group provided a later economic evaluation of this study62. This demonstrated that although the intervention supplement was significantly more expensive that the comparator (p<0.001), the intervention resulted in significant savings overall from the non-nutritional costs of care (p<0.001) [nursing p=0.001, dressings p=0.024], and significantly lower costs of PI care overall (p=0.013)62.
In the systematic review conducted by Langer and Fink39, seven studies (including the studies by van Anholt et al.48 and Ohura et al.63 identified in this literature search, with the remainder published prior to our search date criteria) were identified investigating mixed nutritional supplements compared to other nutritional interventions, and four trials (again including van Anholt et al.48) were identified comparing Arg-enriched mixed nutritional supplements against a standard hospital diet. When pooled, Arg-enriched mixed nutritional supplements improved Pressure Ulcer Scale for Healing (PUSH) score when compared to the standard hospital diet (p=0.0001). This analysis was limited by the small number of participants in the three individual studies included (n=80). Two studies were pooled regarding the outcome measure of ulcer size, representing 71 participants. This analysis favoured the effect of supplementation on ulcer size; however, the overall effect was not significant (p=0.14). This lack of significance is likely due to the small sample sizes in both included studies, identified in the review as being statistically underpowered, as well as their large confidence intervals.
In the systematic review conducted by Tan and colleagues30, four studies (published prior to 2010) were identified that investigated the effects of combined immunonutrients vs multi-nutrient supplementation or placebo following burn injury. All four studies, with a total of 163 participants, reported on mortality as an outcome measure30. When pooled, there was no significant effect of supplementation seen (RR 1.1 (95%CI 0.47 to 2.6) p=0.83)30. Three of the included studies reported on LOS; when pooled, these also failed to demonstrate a significant effect with supplementation (RR 1.93 (95%CI –4041 to 8.28) p=0.55)30.
Probiotics
Three studies identified through the review search strategy investigated the effect of probiotic administration on wound healing outcomes.
The DBRCT conducted by Kotzampassi and colleagues21 investigated supplementation with a probiotic regimen on outcomes following elective open colonic resection with primary anastomosis for colorectal cancer. Participants (n=164) were assigned to a probiotic regimen consisting of a pre-operative loading dose of four capsules followed by one capsule twice daily orally for 15 days or placebo21. The probiotic capsules contained four active strains consisting of Lactobacillus acidophilus 1.75x 109cfu, Lactobacillus plantarum 0.5x 109cfu, Bifidobacterium lactis 1.75x 109cfu, Saccharomyces boulardii 1.5x 109cfu21. Probiotic supplementation was reported to significantly decrease overall complications (p=0.01), infectious complications (p=0.009), anastomotic leak (p=0.031), and LOS (although this data was not provided by authors)21.
In the DBRCT, placebo controlled study conducted by Mohseni and colleagues17, the effect of a probiotic regimen was investigated on wound healing outcomes in 60 grade III diabetic foot ulcer patients. A probiotic capsule containing L. acidophilus, L. casei, L. fermentum, B. bifidum (2x 109cfu/g each) or placebo was provided daily for 12 weeks17. Supplementation was reported to significantly decrease in ulcer width (p=0.02), length (p=0.01), and depth (p<0.02)17.
Mayes and colleagues64 in their RCT administered Lactobacillus rhamnosus GG (15 billion CFU/dose), or placebo twice daily to their cohort of 20 paediatric acute burn patients requiring feeding tubes, until 95% wound closure was achieved. They reported trends towards lower requirements for operative excision/grafting procedures (p=0.23) and time to complete healing (p=0.23) with supplementation; however, no difference in medical LOS. It should be noted, however, that this small study was designed to evaluate the safety, not the efficacy, of supplementation with probiotics following burn injury and, as such, this study was underpowered to determine statistical effects on outcomes of interest to this review.
Bioflavenoids
Two studies were identified that Bioflavenoids alone18 or combined with anti-oxidant nutrients65. In the study presented by Serra and colleagues18 in 83 patients with venous leg ulcers for more than 6 weeks, 8 months’ supplementation was shown to have an improved healing rate at 12 months (83.8%) compared to the comparator group (60.56%)18. In contrast, in the small cohort of 20 superficial to partial thickness adult burn injury patients reported by Raposio et al.65, supplementation of bioflavonoids combined with anti-oxidants demonstrated no differences on LOS (p=0.63)65.
Alternate nutritional intervention strategies
Five additional studies identified investigated alternate or novel strategies not categorisable to the groupings above22,66–69. Fifteen studies identified as part of the search strategy investigated heterogenous early EN, PN or ON support regimens in various medical conditions63,70–83. Characteristics of these studies are summarised in Table 5. Despite population groups, early EN and ON interventions appeared to have positive effects on outcomes of interest, especially when compared to PN interventions.
Table 5. Early EN, PN and/or ON support strategies: characteristics of studies
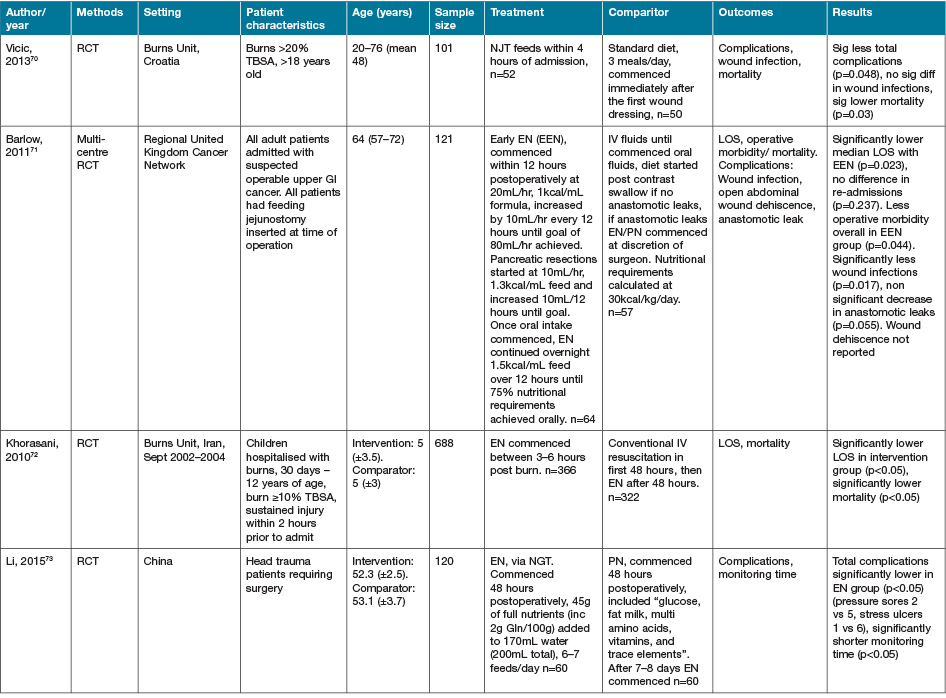
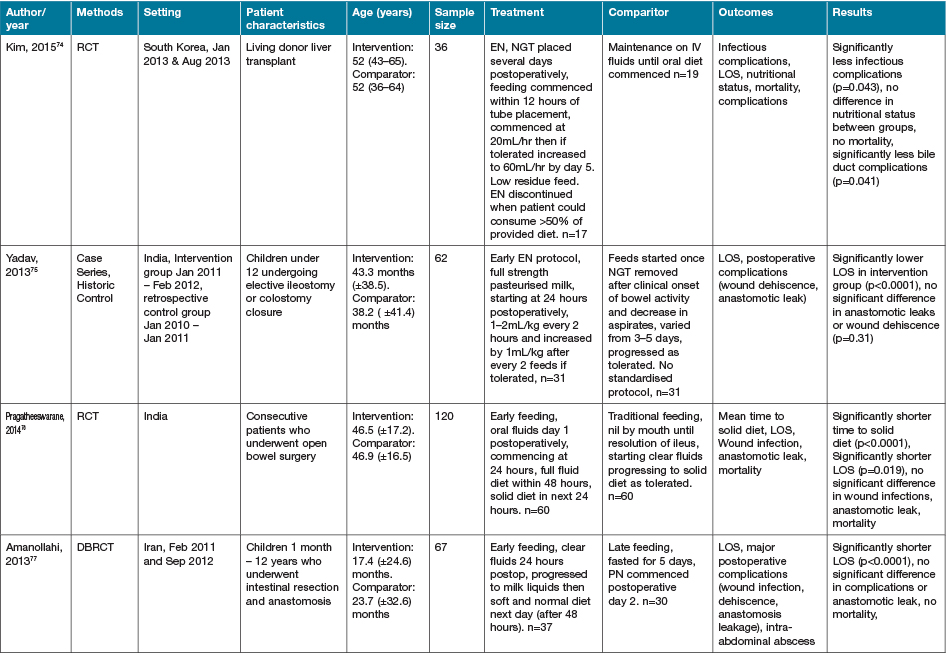
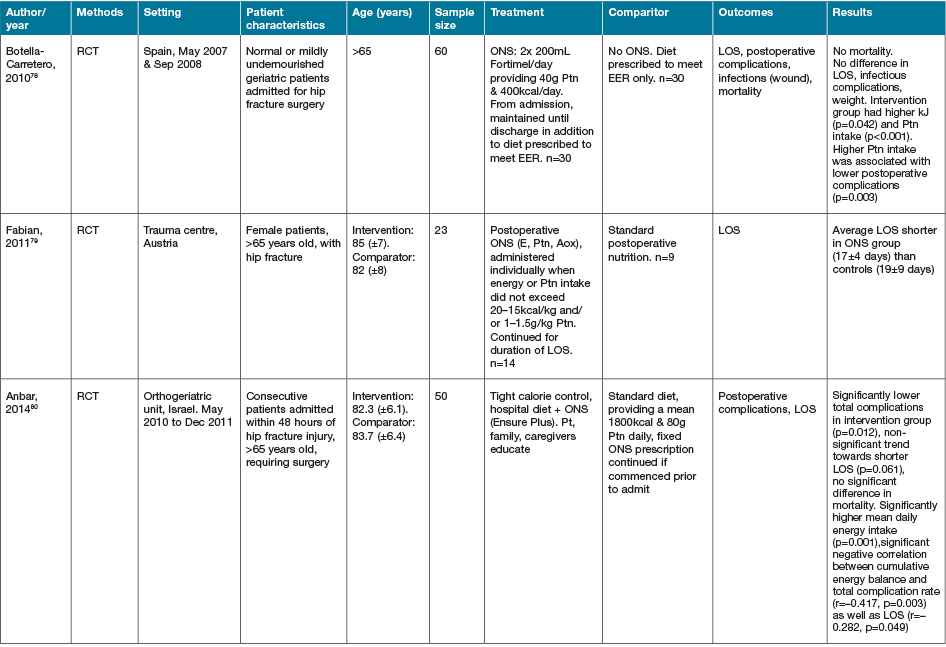
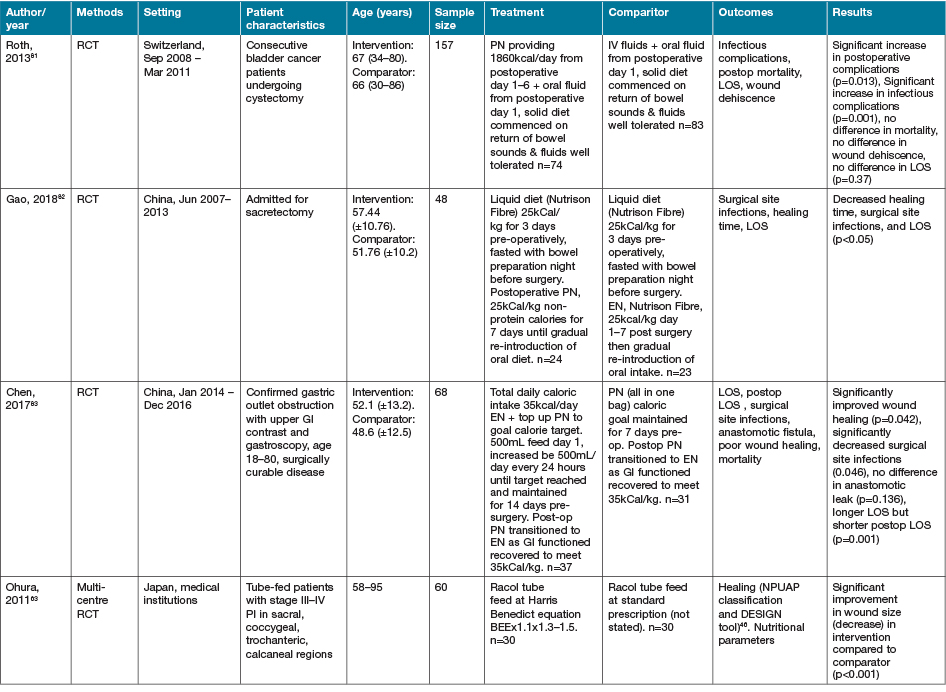
AOx = anti-oxidant; BEE = basal energy expenditure; EER = estimated energy requirements; GI = gastrointestinal; NGT = nasogastric tube; NJT = naso jejunal tube; ONS = ON support; Ptn = protein
In the RCT presented by Najmi and colleagues67, 100 patients with 10–20% second degree burns were provided an oral diet consisting of 20% protein, 60% carbohydrate and 20% lipid until discharge. The intervention group received their lipid from olive oil sources, whilst the control group received their lipid from sunflower oil67. The provision of lipid from olive oil was reported to significantly decrease the duration of wound healing (p=0.01) and LOS (p=0.05)67. The DBRCT, parallel group study by Babajafari and colleagues69 investigated the effect of isolated soy protein supplementation with and without flaxseed oil as a functional food versus a wheat flour and corn oil food (cookie) comparator in 73 patients with 20–50% total body surface area (TBSA) burn injuries. Significant improvements were seen in wound healing in the isolated soy protein groups compared with the control at days 22 and 25 (p<0.05)69. All groups demonstrated a significant reduction in wound size from baseline over the 3-week study period (p<0.001); however, there was no significant difference between groups overall (p=0.7)69.
An open label, parallel group study conducted by de Franciscis and colleagues22 investigated the supplementation of folic acid (1.2mg/day) for 12 months in 87 patients with chronic venous ulcers who had hyperhomocysteinaemia (HHcy) versus chronic ulcer patients without HHcy and not supplemented with folate22. This study demonstrated a significantly higher rate of healing in the folic acid group than the comparator (p<0.05)22.
The RCT conducted by Fujita and colleagues68 investigating the rate of anastomotic leaks following thorascopic esophagectomy for cancer resection, provided their intervention group with a continuous, warmed intra venous infusion of 18 amino acids for 30 minutes prior to and during surgery68. They reported a significant decrease in surgical site infections (p=0.029) and no difference in anastomotic leaks (p=0.76) in their cohort of 130 participants68.
A prospective, controlled, before and after comparative interventional study conducted by Bell and colleagues66 investigated two differing nutritional care models on outcomes for 116 patients who had sustained hip fractures that required surgical intervention66. The treatment group received a multidisciplinary nutritional care model, whilst the comparator group received individualised nutrition care66. Whilst the intervention was shown to increase intake of energy (by 210%) and protein (by 207%), recruitment numbers were insufficient to determine an effect on pressure areas, surgical site infections, or mortality66.
One additional systematic review was also identified that investigated nutrition strategies not elsewhere reported84. In the systematic review presented by Masters and colleagues84, two studies with a total of 93 participants, published prior to the year 2000, were included84. One study investigated the use of two different high fat EN feed preparations versus an high carbohydrate EN feed84. The second study compared a high fat EN feed control group to two high carbohydrate EN feed groups, one supplemented with omega-3 fatty acids84. Due to the low participant numbers and heterogeneous nature of the included studies, no solid recommendations could be offered by the review authors regarding optimal fat to carbohydrate ratios in the EN feed provision to burn injury patients84.
Discussion
Despite the recognised importance of nutrition in wound healing, this review has highlighted the paucity of high quality evidence generated in this field over the past decade and the difficulty in its interpretation. One major limitation with interpreting much of the literature in this field is the combined nutrient strategies. This prevents the active nutrients and optimal dosage from being deduced. Another limitation of these studies is the lack of nutritional intake or status (pre- and/or post-intervention) being reported or taken into consideration with the study design, such as in those reported by Serra et al.18, Raposio et al.65, and Fujita et al.68 amongst others reported in this review. This is of particular importance in determining the effect of a nutritional intervention on wound healing, especially given the difference seen in nutritional interventional effectiveness by many studies that have included nutritional status as part of their outcome analysis. Although one would assume than malnourished patients may have better wound healing outcomes than well-nourished patients, this effect was not observed in the study conducted by Leigh et al., where well-nourished patients showed a trend towards improved healing rates compared to malnourished participants when supplemented with Arg for non-healing PIs46. In addition to this complexity in literature interpretation, some studies explicitly measured and reported on compliance with ON supplementation and accounted for this in their statistical analysis or reporting46–48,50,61, whilst other studies failed to report on compliance53,55,60. Such distinctions in nutritional status and compliance is important for clinicians to apply the correct nutrient prescriptions to their populations for optimal efficacy and fiscal justifications.
Another limitation in interpreting and comparing efficacy of nutritional intervention strategies reported in the current literature is the lack of standardisation in wound healing outcome measures used. For example, the fairly homogenous wound group of PIs, multiple tools such as the PUSH, overall percentage size, change in surface area, and time to healing were all reported for this outcome46–48. Whether effectiveness seen in chronic wounds is translatable to acute wounds, and vice versa, is also unknown.
Despite the limitation of this review only searching one database representing all Medline listed investigations, the majority of primary studies identified were single centre and small in sample size. This likely reflects the complexity in conducting high quality nutrition intervention research given the limitations of population type and numbers. One example of this issue is the comparatively large study conducted by Falewee et al.56. Despite having a strong study design including eight recruitment centres, their pre-determined sample size was not met due to inadequate recruitment in the allocated study timeframe56. The majority of studies identified were also conducted in adult populations, with only five identified being conducted in paediatric participants – three in burn injury, one in ileostomy/colostomy closure, one in intestinal resection64,72,75,77,85. This paucity in paediatric wound healing literature is of concern given the importance of adequate nutrition for optimal growth and development in this population86. This may be reflective of the small numbers of paediatric wounds presenting comparatively to the adult population or complexities in establishing or conducting research in this setting. This lack of specific evidence for the paediatric population potentially hampers the delivery of optimal nutrition support for wound healing in this setting.
Health professionals endeavour to base their recommendations on the available evidence, and guidelines aim to assist translation of evidence into practice1. This review identified that the more recent evidence for some of the key recognised macro and micronutrients involved in wound healing is, in many instances, weak, and often this is due to research design and reporting limitations. Encouraging further research in specific areas of deficiency, such as that identified in the paediatric population, is recommended. That being said, health professionals should always be recommending adequate amounts of highly nutritious food and fluids for general wellbeing in accordance with the guidelines for healthy eating86. When it comes to aiding wound healing in complex wounds, nutrition is an integral strategy to complement good wound hygiene practice and care1.
Nutrition as an important consideration for best outcomes has been included in the care of complex burns for decades87, so why is it not an automatic consideration in slow to heal wounds, chronic wounds, dehisced surgical wounds? It seems that because there is no specific evidence for each wound type and nutrient intervention, the nutrition aspect has often given less importance. Nevertheless, some of these studies provide sufficiently strong evidence to influence clinical practice. For example, arginine administered as a single interventional agent appears to convey some benefits for wound healing outcomes in the surgical oncology patient populations, at a higher administered doses (12–20g/day) for at least 10 days. Evidence in other populations remains insufficient to support its routine use. It also must be noted that there are recommendations for caution with its administration in the ICU setting. The Canadian Critical Care Guidelines, considering unpublished evidence showing possible increased mortality when initiated in severely septic medical ICU patients (however, not in patients who are already established on Arg who later become septic), make this recommendation88.
The combination of arginine with other anti-oxidant or immune-modulating appears to offer benefit in malnourished patients with chronic wounds such as PIs, and has been shown to convey cost benefits to care47,62. The lack of effect seen in the systematic reviews identified as part of this search strategy are not surprising39. This area of research is diverse in its inclusion criteria, doses and nutrients administered, outcome measures and often of small scale. This is prohibitive to the accurate pooling of data to determine effects, as well as the ability to ascertain the key nutrients providing benefit. Regarding the management of chronic wounds, dosage of 4.5–9g/day administered orally appears effective. To strengthen this area of research, future studies should also employ cost benefit analysis to assist with determination of efficacy and translation of evidence into clinical practice for these types of nutritional strategies10.
The administration of Gln via the EN route appears to convey a beneficial effect, particularly in burns and trauma patients, compared to PN administration10,30. This may be due to its role as a primary metabolite for gut enterocytes, improving gut integrity and preventing known sources of sepsis such as bacterial translocation of the intestinal tract10. To elucidate this effect it is important to calculate Gln dosages in addition to total protein requirements. Similarly to Arg it is sometimes administered as part of combined immune-nutrition regimens which complicates the ability to determine the most beneficial nutrient29,59. These regimens, however, have been proven to be safely administered in certain populations, and potentially may decrease time to healing and LOS89–92. The inclusion of the omega-3 fatty acids EPA and DHA likely improves the effect of these immune-nutrition regimes as they are metabolised to comparatively less inflammatory and less immunosuppressive metabolites than omega-6 fatty acids10. Dosage of supplementation appears to be more important with Gln than Arg, with ≥0.3g/kg/day via the EN route required to be effective30. Dosage studies have proved that up to 60g/day of Gln appears to be safe to administer. Importantly, the administration of Arg and Gln, for the purpose of improving wound healing, should be provided separately to the overall global protein required. If given as part of the general protein requirements or possibly where insufficient energy intake is consumed, they are utilised via different metabolic pathways negating their beneficial effects30.
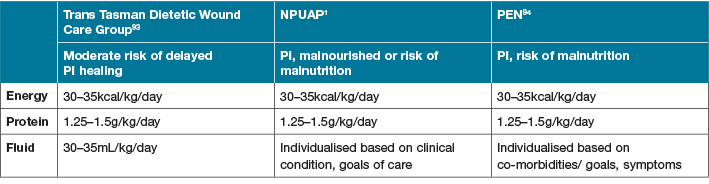
To elucidate the optimal implementation of research into clinical practice, multiple practice guidelines have been developed1,31,93,94. These guidelines all support the use of determining malnutrition risk using appropriate and validated screening tools as the first step of clinical management1,93,94. This should include weight status, weight history, and whether weight loss has occurred. Dietary adequacy of total nutrient intake should also be assessed, with guideline recommendations for macronutrients presented in Table 61,93,94. As part of a comprehensive nutritional assessment, the risk of delayed wound healing due to nutritional compromise should be accounted for; as discussed previously, this is a limiting factor for many studies identified as part of this review. Although all the presented guidelines agree that the current levels of evidence for the use of combined nutritional supplements (containing high protein, Arg +/– Gln, omega-3 fatty acids and micronutrients) remains
low, their use is supported in surgical populations31,88 and for patients with greater than stage II or multiple pressure ulcers where nutritional requirements cannot be met with traditional ON support1,93,94. Findings from this review do not support changes to the current guidelines supporting nutrition and its role in wound healing reported in Table 6 and elsewhere in this discussion. Whilst not an exhaustive list of available guidelines, they are supported by peak nutrition and wound healing groups1,31,87,88,93,94. To ensure optimal wound healing in a clinical setting, following practice guidelines designed for relevant populations is a simple way to translate available evidence into the clinical setting until further large-scale, multi-centre, prospective nutrition intervention studies, including cost effectiveness outcomes, are available.
Table 6. Guideline macronutrient recommendations
Conclusion
Nutrition is an integral component of best practice wound care. Despite the diversity of identified research over the past decade in this area, multiple nutritional strategies for various wound types are evolving. Current available guidelines provide broad consistency regarding macronutrient recommendations; however, evidence for other nutrient recommendations remains lacking, especially relating to optimal timing and dosage. Given the low cost in delivery of nutrition interventions compared to other complementary wound management strategies, such as surgical intervention and specialised wound dressings, this area warrants greater attention. Early nutrition screening and appropriate nutritional intervention for all wound types should be routine clinical practice.
Acknowledgements
Pratyush Misra, brand management, Nestlé facilitated meetings of the authors and encouraged the production of this manuscript.
Conflict of interest
The authors are members of the Nestlé nutrition and wound healing/prevention board and have received an honorarium for this consultancy, but received no direct funding for the production of this manuscript.
Funding
The authors received no funding for this study.
Author(s)
Rochelle Kurmis
BND APD, MClinSci
Allied Health Project Manager, Adult Burns Unit Royal Adelaide Hospital, SA Australia
Michael Woodward*
AM, MB BS MD FRACP, Fellow Wounds Australia
Head, Wound Management Clinic and Director Aged Care Research, Austin Health, Heidelberg
VIC Australia
Email Michael.woodward@austin.org.au
Hayley Ryan
RN, MBA, PGCertWM, AICGG, Cert IV TAA
PhD Candidate
Clinical Nurse Consultant – Wound Management
WoundRescue, Glendale, NSW Australia
Jan Rice
RN, Mast Wound Care, Cert. Plastic & Reconstructive Surgery, Fellow Wounds Australia
Wound Nurse Consultant, VIC Australia
* Corresponding author
References
- European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP) and Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA; 2019.
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian hospitals: a cost-of-illness study. Aust Hlth Rev 2015.
- Clinical Excellence Commission. 2017 NSW pressure injury point prevalence survey report. Sydney: Clinical Excellence Commission; 2018.
- World Union of Wound Healing Societies Concensus Document. Surgical wound dehiscence: improving prevention and outcomes. Wounds Int 2018.
- Thomas DR, Goode PS, Tarquine PH, Allman RM. Hospital-acquired pressure ulcers and risk of death. J Am Geriatric Soc 1996;44(12):1435–40.
- Agarwal E, Ferguson M, Banks M, Bauer J, Capra S, Isenring E. Nutritional status and dietary intake of acute care patients: results from the Nutrition Care Day Survey 2010. Clin Nutr 2012;31(1):41–7.
- Bold J. Supporting evidence-based practice in nutrition and hydration. Wounds UK 2020;16(2):22–8.
- Molnar JA, Vlad LG, Gumus T. Nutrition and chronic wounds: improving clinical outcomes. Plastic Reconst Surg 2016;138(3 Suppl):71s–81s.
- Posthauer ME, Banks M, Dorner B, Schols JM. The role of nutrition for pressure ulcer management: National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, and Pan Pacific Pressure Injury Alliance white paper. Adv Skin Wound Care 2015;28(4):175–88; quiz 89–90.
- Kurmis R, Parker A, Greenwood J. The use of immunonutrition in burn injury care: where are we? J Burn Care Res 2010;31(5):677–91.
- Todd SR, Gonzalez EA, Turner K, Kozar RA. Update on postinjury nutrition. Curr Opin Crit Care 2008;14(6):690–5.
- Caldis-Coutris N, Gawaziuk JP, Logsetty S. Zinc supplementation in burn patients. J Burn Care Res 2012;33(5):678–82.
- Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutr 2010;26(9):862–6.
- Kavalukas SL, Barbul A. Nutrition and wound healing: an update. Plastic Reconstruct Surg 2011;127 Suppl 1:38s–43s.
- Taylor C. Importance of nutrition in preventing and treating pressure ulcers. Nurs Older People 2017;29(6):33–9.
- Little MO. Nutrition and skin ulcers. Curr Op Clin Nutr Metabolic Care 2013;16(1):39–49.
- Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabet/Metabolism Res Rev 2018;34(3).
- Serra R, Grande R, Butrico L, Buffone G, Calio FG, Squillace A, et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J 2016;13(1):88–96.
- Ekinci O, Yanik S, Terzioglu Bebitoglu B, Yilmaz Akyuz E, Dokuyucu A, Erdem S. Effect of calcium beta-Hydroxy-beta-Methylbutyrate (CaHMB), vitamin D, and protein supplementation on postoperative immobilization in malnourished older adult patients with hip fracture: a randomized controlled study. Nutr Clin Prac 2016;31(6):829–35.
- Burkiewicz CJ, Guadagnin FA, Skare TL, do Nascimento MM, Servin SC, de Souza GD. Vitamin D and skin repair: a prospective, double-blind and placebo controlled study in the healing of leg ulcers. Revista do Colegio Brasileiro de Cirurgioes 2012;39(5):401–7.
- Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg 2015;39(11):2776–83.
- de Franciscis S, De Sarro G, Longo P, Buffone G, Molinari V, Stillitano DM, et al. Hyperhomocysteinaemia and chronic venous ulcers. Int Wound J 2015;12(1):22–6.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed) 2009;339:b2535.
- Debats IB, Koeneman MM, Booi DI, Bekers O, van der Hulst RR. Intravenous arginine and human skin graft donor site healing: a randomized controlled trial. Burns 2011;37(3):420–6.
- De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Ventosa M. A randomized double-blind clinical trial with two different doses of arginine enhanced enteral nutrition in postsurgical cancer patients. Europ Rev Med Pharmacol Sci 2010;14(11):941–5.
- De Luis DA, Izaola O, Terroba MC, Cuellar L, Ventosa M, Martin T. Effect of three different doses of arginine enhanced enteral nutrition on nutritional status and outcomes in well nourished postsurgical cancer patients: a randomized single blinded prospective trial. Europ Rev Med Pharmacol Sci 2015;19(6):950–5.
- Marti-Carvajal A, Knght-Madden J, Martinez-Zapata MJ. Interventions for treating leg ulcers in people with sickle cell disease. Cochrane Database Syst Rev 2014(12).
- Perez-Barcena J, Marse P, Zabalegui-Perez A, Corral E, Herran-Monge R, Gero-Escapa M, et al. A randomized trial of intravenous glutamine supplementation in trauma ICU patients. Intensive Care Med 2014;40(4):539–47.
- Blass SC, Goost H, Tolba RH, Stoffel-Wagner B, Kabir K, Burger C, et al. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clinical Nutr 2012;31(4):469–75.
- Tan H, Danilla S, Murray A, Serra R, El Dib R, Henderson T, et al. Immunonutrition as an adjuvant therapy for burns. Cochrane Database Syst Rev 2014(12).
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN 2016;40(2):159–211.
- Theilla M, Schwartz B, Cohen J, Shapiro H, Anbar R, Singer P. Impact of a nutritional formula enriched in fish oil and micronutrients on pressure ulcers in critical care patients. Am J Crit Care 2012;21(4):e102–9.
- Theilla M, Schwartz B, Zimra Y, Shapiro H, Anbar R, Rabizadeh E, et al. Enteral n-3 fatty acids and micronutrients enhance percentage of positive neutrophil and lymphocyte adhesion molecules: a potential mediator of pressure ulcer healing in critically ill patients. Br J Nutr 2012;107(7):1056–61.
- Tihista S, Echavarria E. Effect of omega 3 polyunsaturated fatty acids derived from fish oil in major burn patients: a prospective randomized controlled pilot trial. Clin Nutr 2018;37(1):107–12.
- Soleimani Z, Hashemdokht F, Bahmani F, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabet Complication 2017;31(9):1394–400.
- Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen 2017;25(3):512–20.
- Sakae K, Agata T, Kamide R, Yanagisawa H. Effects of L-carnosine and its zinc complex (Polaprezinc) on pressure ulcer healing. Nutr Clin Pract 2013;28(5):609–16.
- Sakae K, Yanagisawa H. Oral treatment of pressure ulcers with polaprezinc (zinc L-carnosine complex): 8-week open-label trial. Biolog Trace Element Res 2014;158(3):280–8.
- Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev 2014(6):Cd003216.
- Wilkinson E. Oral zinc for arterial and venous leg ulcers. Cochrane Database Syst Rev 2014(9).
- Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabet Complication 2017;31(4):766–72.
- Gottschlich MM, Mayes T, Khoury J, Kagan RJ. Clinical trial of vitamin D2 vs D3 supplementation in critically ill pediatric burn patients. JPEN 2017;41(3):412–21.
- Li X, Tang L, Lin YF, Xie GF. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin Implant Dent Related Res 2018;20(5):793–8.
- Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z. Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biolog Trace Element Res 2018;181(2):207–15.
- Afzali H, Jafari Kashi AH, Momen-Heravi M, Razzaghi R, Amirani E, Bahmani F, et al. The effects of magnesium and vitamin E co-supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen 2019;27(3):277–84.
- Leigh B, Desneves K, Rafferty J, Pearce L, King S, Woodward MC, et al. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care 2012;21(3):150–6.
- Cereda E, Klersy C, Serioli M, Crespi A, D’Andrea F. A nutritional formula enriched with arginine, zinc, and antioxidants for the healing of pressure ulcers: a randomized trial. Annal Int Med 2015;162(3):167–74.
- van Anholt RD, Sobotka L, Meijer EP, Heyman H, Groen HW, Topinkova E, et al. Specific nutritional support accelerates pressure ulcer healing and reduces wound care intensity in non-malnourished patients. Nutr 2010;26(9):867–72.
- Wong A, Chew A, Wang CM, Ong L, Zhang SH, Young S. The use of a specialised amino acid mixture for pressure ulcers: a placebo-controlled trial. J Wound Care 2014;23(5):259–60, 62–4, 66–9.
- Banks MD, Ross LJ, Webster J, Mudge A, Stankiewicz M, Dwyer K, et al. Pressure ulcer healing with an intensive nutrition intervention in an acute setting: a pilot randomised controlled trial. J Wound Care 2016;25(7):384–92.
- Armstrong DG, Hanft JR, Driver VR, Smith AP, Lazaro-Martinez JL, Reyzelman AM, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabetic Med 2014;31(9):1069–77.
- Bauer JD, Isenring E, Waterhouse M. The effectiveness of a specialised oral nutrition supplement on outcomes in patients with chronic wounds: a pragmatic randomised study. J Human Nutr Dietetic 2013;26(5):452–8.
- Kaya SO, Akcam TI, Ceylan KC, Samancilar O, Ozturk O, Usluer O. Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J Cardiothoracic Surg 2016;11:14.
- Moya P, Miranda E, Soriano-Irigaray L, Arroyo A, Aguilar MD, Bellon M, et al. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endoscop 2016;30(11):4946–53.
- Moya P, Soriano-Irigaray L, Ramirez JM, Garcea A, Blasco O, Blanco FJ, et al. Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol: a multicenter randomized clinical trial (SONVI Study). Medicine 2016;95(21):e3704.
- Falewee MN, Schilf A, Boufflers E, Cartier C, Bachmann P, Pressoir M, et al. Reduced infections with perioperative immunonutrition in head and neck cancer: exploratory results of a multicenter, prospective, randomized, double-blind study. Clin Nutr 2014;33(5):776–84.
- Felekis D, Eleftheriadou A, Papadakos G, Bosinakou I, Ferekidou E, Kandiloros D, et al. Effect of perioperative immuno-enhanced enteral nutrition on inflammatory response, nutritional status, and outcomes in head and neck cancer patients undergoing major surgery. Nutr Cancer 2010;62(8):1105–12.
- Fujitani K, Tsujinaka T, Fujita J, Miyashiro I, Imamura H, Kimura Y, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg 2012;99(5):621–9.
- Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, et al. The immunomodulating enteral nutrition in malnourished surgical patients – a prospective, randomized, double-blind clinical trial. Clin Nutr 2011;30(3):282–8.
- Hamilton-Reeves JM, Bechtel MD, Hand LK, Schleper A, Yankee TM, Chalise P, et al. Effects of immunonutrition for cystectomy on immune response and infection rates: a pilot randomized controlled clinical trial. Eur Urol 2016;69(3):389–92.
- Brewer S, Desneves K, Pearce L, Mills K, Dunn L, Brown D, et al. Effect of an arginine-containing nutritional supplement on pressure ulcer healing in community spinal patients. J Wound Care 2010;19(7):311–6.
- Cereda E, Klersy C, Andreola M, Pisati R, Schols JM, Caccialanza R, et al. Cost-effectiveness of a disease-specific oral nutritional support for pressure ulcer healing. Clin Nutr 2017;36(1):246–52.
- Ohura T, Nakajo T, Okada S, Omura K, Adachi K. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial). Wound Repair Regen 2011;19(3):330–6.
- Mayes T, Gottschlich MM, James LE, Allgeier C, Weitz J, Kagan RJ. Clinical safety and efficacy of probiotic administration following burn injury. J Burn Care Res 2015;36(1):92–9.
- Raposio E, Grieco MP, Caleffi E. Evaluation of plasma oxidative stress, with or without antioxidant supplementation, in superficial partial thickness burn patients: a pilot study. J Plastic Surg Hand Surg 2017;51(6):393–8.
- Bell JJ, Bauer JD, Capra S, Pulle RC. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients – results of a pragmatic intervention. Clin Nutr 2014;33(6):1101–7.
- Najmi M, Vahdat Shariatpanahi Z, Tolouei M, Amiri Z. Effect of oral olive oil on healing of 10–20% total body surface area burn wounds in hospitalized patients. Burns 2015;41(3):493–6.
- Fujita T, Okada N, Kanamori J, Sato T, Mayanagi S, Torigoe K, et al. Thermogenesis induced by amino acid administration prevents intraoperative hypothermia and reduces postoperative infectious complications after thoracoscopic esophagectomy. Dis Esophagus 2017;30(1):1–7.
- Babajafari S, Akhlaghi M, Mazloomi SM, Ayaz M, Noorafshan A, Jafari P, et al. The effect of isolated soy protein adjunctive with flaxseed oil on markers of inflammation, oxidative stress, acute phase proteins, and wound healing of burn patients; a randomized clinical trial. Burns 2018;44(1):140–9.
- Vicic VK, Radman M, Kovacic V. Early initiation of enteral nutrition improves outcomes in burn disease. Asia Pacific J Clin Nutr 2013;22(4):543–7.
- Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr 2011;30(5):560–6.
- Khorasani EN, Mansouri F. Effect of early enteral nutrition on morbidity and mortality in children with burns. Burns 2010;36(7):1067–71.
- Li CH, Chen DP, Yang J. Enteral nutritional support in patients with head injuries after craniocerebral surgery. Turkish Neurosurg 2015;25(6):873–6.
- Kim JM, Joh JW, Kim HJ, Kim SH, Rha M, Sinn DH, et al. Early enteral feeding after living donor liver transplantation prevents infectious complications: a prospective pilot study. Medicine 2015;94(44):e1771.
- Yadav PS, Choudhury SR, Grover JK, Gupta A, Chadha R, Sigalet DL. Early feeding in pediatric patients following stoma closure in a resource limited environment. J Pediatr Surg 2013;48(5):977–82.
- Pragatheeswarane M, Muthukumarassamy R, Kadambari D, Kate V. Early oral feeding vs. traditional feeding in patients undergoing elective open bowel surgery – a randomized controlled trial. J Gastrointest Surg 2014;18(5):1017–23.
- Amanollahi O, Azizi B. The comparative study of the outcomes of early and late oral feeding in intestinal anastomosis surgeries in children. Af J Paediatr Surg 2013;10(2):74–7.
- Botella-Carretero JI, Iglesias B, Balsa JA, Arrieta F, Zamarron I, Vazquez C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: a randomized clinical trial. Clin Nutr 2010;29(5):574–9.
- Fabian E, Gerstorfer I, Thaler HW, Stundner H, Biswas P, Elmadfa I. Nutritional supplementation affects postoperative oxidative stress and duration of hospitalization in patients with hip fracture. Wiener klinische Wochenschrift 2011;123(3–4):88–93.
- Anbar R, Beloosesky Y, Cohen J, Madar Z, Weiss A, Theilla M, et al. Tight calorie control in geriatric patients following hip fracture decreases complications: a randomized, controlled study. Clin Nutr 2014;33(1):23–8.
- Roth B, Birkhauser FD, Zehnder P, Thalmann GN, Huwyler M, Burkhard FC, et al. Parenteral nutrition does not improve postoperative recovery from radical cystectomy: results of a prospective randomised trial. Europ Urol 2013;63(3):475–82.
- Gao S, Zheng Y, Liu X, Tian Z, Zhao Y. Effect of early fasting and total parenteral nutrition support on the healing of incision and nutritional status in patients after sacrectomy. Orthopaedic Traumatol Surg Res 2018;104(4):539–44.
- Chen ZH, Lin SY, Dai QB, Hua J, Chen SQ. The effects of pre-operative enteral nutrition from nasal feeding tubes on gastric outlet obstruction. Nutrient 2017;9(4).
- Masters B, Aarabi S, Sidhwa F, Wood F. High-carbohydrate, high-protein, low-fat versus low-carbohydrate, high-protein, high-fat enteral feeds for burns. Cochrane Database Syst Rev 2012(1).
- Marin MC, Osimani NE, Rey GE, de Alaniz MJ. n-3 Fatty acid supplementation in burned paediatric patients. Acta Paediatrica 2009;98(12):1982–7.
- Australian Government, National Health and Medical Research Council. Australian guide to healthy eating [updated 01/05/2017]. Available from: https://www.eatforhealth.gov.au/guidelines/australian-guide-healthy-eating.
- Rousseau AF, Losser MR, Ichai C, Berger MM. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr (Edinburgh, Scotland) 2013;32(4):497–502.
- The Canadian Critical Care Society. The 2015 clinical practice guidelines on critical care nutrition; 2015. Available from: https://www.criticalcarenutrition.com/resources/cpgs/past-guidelines/2015.
- Liu P, Shen WQ, Chen HL. Efficacy of arginine-enriched enteral formulas for the healing of pressure ulcers: a systematic review. J Wound Care 2017;26(6):319–23.
- Alexander JW, Supp DM. Role of arginine and omega-3 fatty acids in wound healing and infection. Adv Wound Care 2014;3(11):682–90.
- Ellinger S. Micronutrients, arginine, and glutamine: does supplementation provide an efficient tool for prevention and treatment of different kinds of wounds? Adv Wound Care 2014;3(11):691–707.
- Chapman BR, Mills K, Pearce LM, Crowe T. Use of an arginine-enriched oral nutrition supplement in the healing of pressure ulcers in patients with spinal cord injuries: an observational study. Nutr Dietetic 2011;68:208–13.
- Trans Tasman Dietetic Wound Care Group. Evidence based practice guidelines for the dietetic management of adults with pressure injuries. Dietitians Association of Australia and Dietitians New Zealand; 2011.
- PEN. Knowledge pathway: wound care and pressure injuries. Category: Health condition/Disease. Dietitians of Canada; 2019.



