Volume 29 Number 1
Topical corticosteroids for prevention and treatment of radiation dermatitis: a WHAM evidence summary
Emily Haesler
For referencing Haesler E for Wound Healing and Management Centre. Topical corticosteroids for prevention and treatment of radiation dermatitis: a WHAM evidence summary. Wound Practice and Research 2021; 29(1):55-58.
DOI https://doi.org/10.33235/wpr.29.1.55-58
Clinical question
What is the best available evidence for the use of topical corticosteroid preparations for preventing and treating radiation dermatitis in people undergoing radiation therapy for cancer?
Summary
Radiation dermatitis (RD) is an acute skin reaction that occurs as a result of radiotherapy used to treat a range of different cancers. Severity of symptoms ranges from erythema to dry desquamation (dry flaky skin with itching) to moist desquamation (serous exudate, oedema and blistering). Topical corticosteroid preparations are sometimes suggested in regimens to prevent and treat RD. Level 1 evidence from systematic reviews (SRs)1-3 for preventing RD with corticosteroid creams was conflicting, with only some studies showing reduction in incidence of less severe RD. Strong evidence on effectiveness in preventing severe RD was lacking. Level 1 evidence from SRs1, 4 suggested that there might be some benefit in reducing the progression of mild RD if a corticosteroid of moderate potency is used to manage symptoms, particularly pruritis. This decision should be made in the context of the person and the severity of their symptoms.
Clinical practice recommendations
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
Use of a corticosteroid cream to treat symptoms (particularly pruritus) of mild-to-moderate radiation dermatitis could be considered for short durations (Grade B).
Sources of evidence
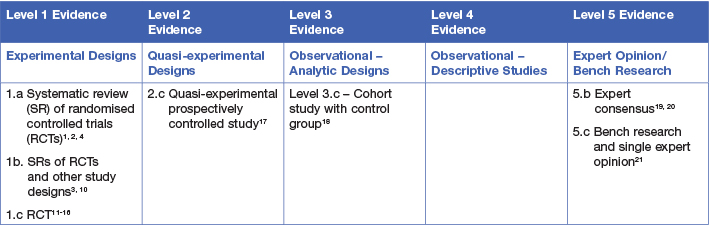
This evidence summary was developed using methods published by the Joanna Briggs Institute.5-9 The summary is based on a systematic literature search combining search terms related to radiation dermatitis/radiodermatitis and topical preparations/creams. Searches were conducted in Embase, Medline, Pubmed, the Cochrane Library and Google Scholar for evidence published up to January 2021 in English. Levels of evidence for intervention studies are reported in the table below.
Background
Radiation dermatitis (also called radiodermatitis) is a common side effect of radiotherapy, which is a type of therapy delivered in the management of cancer. Radiation causes damage to epithelial cells and underlying structures of the skin, usually commencing early during radiotherapy and persisting up to six months following radiotherapy.22, 23 The severity of RD is related to the dose and regimen of radiation and the area of skin over which radiotherapy is administered,22-24 increasing when cell destruction occurs faster than normal cell reproduction. In early stages of RD the skin becomes warmer, itchy and erythema may present. As cumulative exposure to radiation increases, old skin becomes dry and flaky (referred to as dry desquamation). When the rate of new skin cell production cannot replace shedding cells the epidermis breaks down, becomes oedematous and exudate is present (referred to as moist desquamation).23 Pain, skin warmth, pruritus, burning sensations are reported by people experiencing RD.1 Consistent with outcome measures reported in the evidence, when referring to ‘grade’ of RD this evidence summary uses the Radiation Therapy Oncology Group (RTOG) scale for categorising the severity of acute RD.25
Topical corticosteroids are medicated preparations in cream, ointment and gel formulations. After application, active ingredient is absorbed into the skin where it has anti-inflammatory, anti-proliferative and vasoconstrictive effects. Corticosteroid creams are available at different potencies; most preparations reported in the evidence for RD are considered to be moderately potent.21
Clinical evidence
Evidence on corticosteroid topical preparations to prevent radiation dermatitis
One SR1 summarised findings from four RCTs exploring corticosteroid preparations. Two RCTs found no significant effect for topical corticosteroids and a third found no statistically significant differences between two different corticosteroid treatments. One less robust RCT found a small positive effect (odds ratio [OR] = 0.07, 95% CI 0.01 to 0.84, p = 0.04) for topical prednisolone compared to no treatment1 (Level 1).
A 2010 SR3 summarised findings from four RCTs, two of which were reported by Chan et. al. (2014),1 and a 2006 SR2 summarised three small RCTs, only one of which was not reported in the other two reviews. The first of these three additional studies reported a statistically significant result favouring a corticosteroid compared to no product for preventing erythema (p = 0.011)2 and reducing severity of symptoms (p = 0.0033).3 The other two studies found no difference between corticosteroid cream and no product2, 3 (Level 1). A fourth SR10 noted that there was an increased risk of moist desquamation and bacterial infections when using a corticosteroid. Evidence suggested this may be due to the product’s effect in thinning the skin10, 19 (Levels 1 and 5).
Since the publication of the SRs above, additional studies have been published.11-15, 17, 18 A recent RCT15 compared a topical corticosteroid (0.1% betamethasone valerate) to no topical treatment for people undergoing radiotherapy for head and neck cancer. The control group experienced earlier onset of grade 1 RD than people receiving a corticosteroid. A higher incidence of grade 1 RD was noted two weeks after commencing therapy, becoming statistically significant by three weeks (50% versus 28.8%, p = 0.028). By seven weeks, the corticosteroid group had lower incidence of grades 1 and 2 RD, but there was no difference in the incidence of more severe RD (grades 3 or 4).15 Another recent RCT14 compared a topical corticosteroid to a moisturiser, finding lower rates of RD of all grades14 (Level 1). Erridge et. al. (2016)18 reported reductions in redness, pruritus, pain and discomfort when people at high risk of acute RD used a corticosteroid cream in conjunction with a skin care protocol18 (Level 3).
Five of the newer studies11-13, 16, 17 investigated the corticosteroid mometasone furoate. Ho et. al. (2018)12 reported a statistically significant reduction in the incidence of moist desquamation compared with an oil-based product, (43.8% versus 66.7%, p = 0.012) as well as longer time to develop grade 3 RD (46 days versus 35.5 days, p < 0.001).12 Hindley et. al. (2014)11 reported favourable results for a corticosteroid cream compared to a non-medicated product in reducing acuity of skin reaction (p = 0.018) and mean erythema score (p = 0.012), and notably reported a relationship between using a corticosteroid and lower levels of anxiety and depression.11 Liao et. al. (2019)13 found that improvements in incidence of more severe RD associated with a corticosteroid only occurred in people receiving lower doses of radiotherapy; however, pruritus and pain were both statistically significantly improved (p < 0.001 for both) compared to using no products, regardless of radiation dose.13 (Level 1). In one arm of their multi-arm study, Shaw et. al. (2015) demonstrated statistically significantly more days until occurrence of grade 2 RD using mometasone furoate compared to a barrier film (53.4 days versus 44.5 days, p = 0.002). There was no difference in time to developing grade 1 RD, or pain score of 3; and also no difference in any of the three outcomes when comparing to no treatment.16 (Level 1). Finally, in a controlled trial,17 people treated with mometasone furoate experienced no statistically significant difference in time to development of RD, maximum grade of RD or quality of life scores compared to people applying either a hydrogel cream or calendula ointment17 (Level 2).
Overall, this evidence arises from studies that are primarily at moderate risk of bias. The evidence is conflicting, but suggests that using a topical corticosteroid may not reduce the incidence of more severe RD. Some evidence indicates that the volume and homogeneity of radiation dose influences the risk of RD13, 24 and that the effectiveness of corticosteroids might correlate with radiotherapy regimens.12, 13, 24 The available evidence did not adequately explore this potential.
Evidence on topical corticosteroid preparations for treating radiation dermatitis
A 2017 meta-analysis4 included nine studies that compared a topical corticosteroid applied once or twice daily to either a placebo, a non-pharmacological preparation or no treatment. Incidence of moist desquamation was statistically significantly lower when using a topical corticosteroid (OR = 0.29, 95% CI 0.19 to 0.45, p < 0.0001). Pooled results from ten studies exploring the effect of corticosteroid cream on reducing the mean RD severity score also showed a statistically significantly improvement compared to controls (standard mean difference = –0.47, 95% CI –0.61 to –0.33, p < 0.00001). Pruritus and burning were significantly lower in the six studies that reported these outcome measures; however, four studies that reported on pain did not show positive outcomes for corticosteroids compared to control4 (Level 1).
An earlier SR1 summarised findings from two RCTs reporting comparison of topical corticosteroids to placebo for treating existing RD. In one study, the corticosteroid cream had no impact on severity of RD at two weeks following completion of treatment. The second study showed a significant reduction in maximum severity of RD compared to placebo (mean difference = –1.62, 95% CI –2.03 to –1.21, p < 0.001) and in reducing severity of RD two weeks following completion of treatment (MD = –0.55, 95% CI –0.71 to –0.39, p < 0.001). One study comparing two different corticosteroids reported significant results, but this study had a very small sample size.1 All three of these studies were included in the meta-analysis by Haruna et. al. (2017)4 (Level 1).
A recent RCT15 compared a topical corticosteroid (0.1% betamethasone valerate) to no topical treatment for people undergoing radiotherapy for head and neck cancer. Although the study provided evidence that the topical corticosteroid was associated with delayed onset of RD, the rate of healing of grade 2 RD was not different between people using a corticosteroid cream versus no treatment at seven days (46.4% versus 33%) or ten days (25% versus 30%, p = 0.531)15 (Level 1).
One consensus panel20 recommended considering the use of a corticosteroid cream for limited periods for managing grade 2 RD (dry desquamation) or grade 3 RD (moist desquamation).20 Another consensus panel recommended limiting the use of corticosteroid creams in treating pruritus due to its effect of thinning the skin, increasing the risk of bacterial skin infection19 (Level 5).
Considerations for use
If a topical corticosteroid is used to treat symptoms of RD, it should be in conjunction with an individualised skin hygiene and care protocol18-20 (Levels 3 and 5).
Use of corticosteroid cream is suggested for short timeframes (1—2 weeks) and at the lowest necessary potency to reduce the risk of skin thinning and subsequent bacterial infection10, 19, 21 (Level 5).
An ointment preparation is most appropriate for non-hairy, dry skin21 (Level 5).
Conflict of interest
The author declares no conflicts of interest.
Funding
Funded by the Nurses Memorial Charitable Trust
Author(s)
Emily Haesler
for Wound Healing and Management Centre, Curtin University (WHAM@Curtin)
References
- Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer, 2014;14:53.
- Bolderston A, Lloyd NS, Wong RK, Holden L, Robb-Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer, 2006;14(8):802-17.
- Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, Giotis A, Czarnota G, Pang J, de Angelis C. Prophylaxis and management of acute radiation-induced skin reactions: A systematic review of the literature. Curr Oncol, 2010;17(4):94-112.
- Haruna F, Lipsett A, Marignol L. Topical management of acute radiation dermatitis in breast cancer patients: A systematic review and meta-analysis. Anticancer Res, 2017;37(10):5343-53.
- Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. https://synthesismanual.jbi.global: Joanna Briggs Institute, 2021.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. New JBI Grades of Recommendation. 2013. https://jbi.global/sites/default/files/2019-05/JBI-grades-of-recommendation_2014.pdf: Joanna Briggs Institute.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. JBI Levels of Evidence. 2013. https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf: Joanna Briggs Institute.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. 2014. https://jbi.global/sites/default/files/2019-05/JBI%20Levels%20of%20Evidence%20Supporting%20Documents-v2.pdf: Joanna Briggs Institute.
- Munn Z, Lockwood C, S. M. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach. Worldviews Evid Based Nurs, 2015;12(3):131-8.
- Butcher K, Williamson K. Management of erythema and skin preservation; advice for patients receiving radical radiotherapy to the breast: A systematic literature review. J Radiother Pract, 2012;11(1):44-54.
- Hindley A, Zain Z, Wood L, Whitehead A, Sanneh A, Barber D, Hornsby R. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: Results of a randomized trial. Int J Radiat Oncol Biol Phys, 2014;90(4):748-55.
- Ho AY, Olm-Shipman M, Zhang Z, Siu CT, Wilgucki M, Phung A, Arnold BB, Porinchak M, Lacouture M, McCormick B, Powell SN, Gelblum DY. A Randomized Trial of Mometasone Furoate 0.1% to Reduce High-Grade Acute Radiation Dermatitis in Breast Cancer Patients Receiving Postmastectomy Radiation. Int J Radiat Oncol Biol Phys, 2018;101(2):325-33.
- Liao Y, Feng G, Dai T, Long F, Tang J, Pu Y, Zheng X, Cao S, Xu S, Du X. Randomized, self-controlled, prospective assessment of the efficacy of mometasone furoate local application in reducing acute radiation dermatitis in patients with head and neck squamous cell carcinomas. Medicine (Baltimore), 2019;98(52):e18230.
- Uysal B, Gamsiz H, Dincoglan F, Demiral S, Sager O, Dirican B, Beyzadeoglu M. Comparative evaluation of topical corticosteroid and moisturizer in the prevention of radiodermatitis in breast cancer radiotherapy. Indian Journal of Dermatology, 2020;65(4):279-83.
- Sunku R, Kalita AK, Bhattacharyya M, Medhi PP, Bansal S, Borah L, Nayan N, Bora G, Paul M, Saikia S, Kataki AC. Effect of corticosteroid ointment on radiation induced dermatitis in head and neck cancer patients: A prospective study. Indian J Cancer, 2021;(online prior to print).
- Shaw SZ, Nien HH, Wu CJ, Lui LT, Su JF, Lang CH. 3M Cavilon No-Sting Barrier Film or topical corticosteroid (mometasone furoate) for protection against radiation dermatitis: A clinical trial. Journal of the Formosan Medical Association, 2015;114(5):407-14.
- Fenton-Kerimian M, Cartwright F, Peat E, Florentino R, Maisonet O, Budin W, Rolnitzky L, Formenti S. Optimal Topical Agent for Radiation Dermatitis During Breast Radiotherapy: A Pilot Study. Clin J Oncol Nurs, 2015;19(4):451-5.
- Erridge SC, McCabe M, Porter MK, Simpson P, Stillie AL. Prospective audit showing improved patient-assessed skin toxicity with use of betamethasone cream for those at high risk of radiation dermatitis. Radiother Oncol, 2016;121(1):143-7.
- Russi EG, Moretto F, Rampino M, Benasso M, Bacigalupo A, De Sanctis V, Numico G, Bossi P, Buglione M, Lombardo A, Airoldi M, Merlano MC, Licitra L, Denaro N, Pergolizzi S, Pinto C, Bensadoun RJ, Girolomoni G, Langendijk JA. Acute skin toxicity management in head and neck cancer patients treated with radiotherapy and chemotherapy or EGFR inhibitors: Literature review and consensus. Crit Rev Oncol Hematol, 2015;96(1):167-82.
- Gutierrez LC, Khosravi-Shahi P, Alvarez YE. Management of dermatitis in patients with locally advanced squamous cell carcinoma of the head and neck receiving cetuximab and radiotherapy. Oral Oncol, 2012;48(4):293-7.
- Oakley A. 2016. Topical steroid. Available from: https://dermnetnz.org/topics/topical-steroid/. [Accessed January 2021].
- Berger A, Regueiro C, Hijal T, Pasquier D, De La Fuente C, Le Tinier F, Coche-Dequeant B, Lartigau E, Moyal D, Seite S, Bensadoun RJ. Interest of supportive and barrier protective skin care products in the daily prevention and treatment of cutaneous toxicity during radiotherapy for breast cancer. Breast Cancer, 2018;12(no pagination).
- The Princess Royal Radiotherapy Review Team. Managing Radiotherapy Induced Skin Reactions. 2011. UK: St James’s Institute of Oncology, The Leeds Teaching Hospitals NHS Trust.
- Chen MF, Chen WC, Lai CH, Hung CH, Liu KC, Cheng YH. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer, 2010;10 (no pagination)(508).
- Collins A. Assessment and management of radiotherapy-induced skin reactions. Wounds UK, 2018;14(4):64-70.




