Volume 29 Number 2
Dissemination of microbiota between wounds and the beds of patients with pressure injuries: a cross-sectional study
Mao Kunimitsu, Gojiro Nakagami, Aya Kitamura, Takeo Minematsu, Yuko Mugita, Kazuhiro Ogai, Junko Sugama, Miku Aoki, Chika Takada and Hiromi Sanada
Keywords wound infection, hard-to-heal wounds, hospital linen, microbiome, 16S rRNA gene analysis
For referencing Kunimitsu M et al. Dissemination of microbiota between wounds and the beds of patients with pressure injuries: a cross-sectional study. Wound Practice and Research 2021; 29(2):70-76.
DOI https://doi.org/10.33235/wpr.29.2.70-76
Abstract
Aim Wound infection is a life-threatening complication of pressure injuries (PIs) and is not yet completely preventable. This study aims to explore the dissemination of microbiota between PIs and hospital beds using a culture-independent methodology. This serves as the first step towards developing a new intervention to prevent wound infection.
Methods A cross-sectional study was conducted at a long-term care hospital on patients aged >65 years with PIs. The microbiota of wounds, skin and beds were identified using 16S ribosomal RNA (rRNA) gene sequencing analysis. Zero-radius operational taxonomic units (zOTUs) were used for confirming dissemination which indicates bacteria possessing identical sequences within the V3–V4 region of the 16S rRNA gene.
Results Ten PIs were analysed in this study. All individuals had zOTUs common to samples from their wound, skin and bed (median: 194, interquartile range [IQR]: 121–320). Furthermore, the bed samples were classified into the same clusters as the wound samples from eight sites.
Conclusion Our study is the first to quantitatively show the dissemination of microbiota between PIs and patients’ beds using culture-independent analysis. Preventing the dissemination of bacteria to beds may be an effective therapeutic strategy for the prevention of wound infection.
Introduction
Pressure injuries (PIs) are localised injuries to the skin and/or underlying tissue caused by sustained pressure1, with a reported prevalence of 9–14.5% in the elder care setting2–4. Although their prevalence is declining due to advances in prevention and treatment5, up to 30% of PIs develop infections, which is a life-threatening complication6–8. Therefore, controlling wound infections is a crucial intervention after the development of PIs.
Most patients with PIs are compromised hosts due to old age and malnutrition. Thus, treatment directed at improving the overall condition of the patient (e.g., nutritional support) is provided to treat those with PIs. Additionally, breaking the route of transmission of bacteria by using dressings is recommended1 because the region around the sacrum is susceptible to faecal bacterial contamination. Other bacteria from the peri-wound skin may also pose a potential source of infection9, a bacterial bioburden which is reduced by cleansing these areas. However, these multifaced interventions have not made wound infection completely preventable.
We focused on hospital beds as the microclimate around patients is conducive to bacterial growth10. Furthermore, many patients with PIs are immobile and bed-bound, and PIs are exposed to external bacteria because they are treated as open wounds. Therefore, the bacteria found on sheets and blankets are more likely to colonise the wound. In addition, a previous study of the bed environment of patients with PIs found that the temporal change in the composition of bed microbiota was greater in patients with a PI than in those without11. This suggests that the presence of a PI is likely to affect the patient’s microbiome and the dissemination of microbiota may occur between PIs and patients’ beds. However, no studies have yet quantitatively demonstrated bacterial dissemination of microbiota with the beds of patients with PIs, and no interventions for beds have been implemented.
Traditionally, culture methods have been used to detect bacteria. However, 99% of microbes in the environment, including beds, cannot be cultivated12. Therefore, comprehensive investigation of bacteria has been difficult. Recent innovations in sequencing technology have gradually improved culture-independent bacterial identification methods based on bacterial DNA sequences. This study analysed the microbiota of wounds and patients’ beds by detailed analysis of the 16S ribosomal RNA (rRNA) bacterial gene. This is the first step towards understanding the dissemination of microbiota from PIs and the development of a new intervention for the prevention of wound infection.
Materials and methods
Ethics
The study protocol was approved by the ethics committee of the Graduate School of Medicine, The University of Tokyo (Approval No. 11812) and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants enrolled in this study.
Study design and setting
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. A cross-sectional study was conducted at a long-term care hospital in Ishikawa Prefecture, Japan, between October and November 2018. At this hospital, patients are bathed and sheets are changed once a week, clothes are changed twice a week, and incontinence pads are changed three times a day as part of routine care. Sheets, clothes and diapers may also be changed as needed. Standard wound care is provided according to national guidelines13.
Participants
All the participants were recruited during routine rounds by the PI team. The participants were hospitalised patients aged 65 years or older. The inclusion criterion was the presence of d2, D3, D4, D5 or unstageable PIs determined using the DESIGN-R® tool14. With this tool, PIs are divided into six categories as follows: d1 for persistent redness; d2 for lesions that extend into the dermis; D3 for lesions extending into the subcutaneous tissue; D4 for lesions extending to the muscle, tendon or bone; D5 for lesions extending into the articular or body cavity; and DU for unstageable PIs due to necrotic tissue that completely covers the wound bed. Patients with deteriorating systemic condition were excluded. Wounds too small to collect swab samples or located on the toes were also excluded. In addition, we excluded wounds with biofilm because previous studies indicated that the presence of biofilm affected the microbiota composition15.
Data collection
The presence of biofilm was detected by the wound blotting method16. In this method, polysaccharides in the exudate are collected by attaching a nitrocellulose membrane to the wound surface, and biofilms are visualised by staining with alcian blue. The membrane was obtained and stained as previously described17. The presence of biofilm was evaluated by a researcher who was blinded to wound outcome. Patients’ demographic characteristics (age, gender, body mass index, disease, Braden Scale score18, degree of independence and mattress type), location of PIs, treatment, dressing type and duration of PIs were collected from medical records. The DESIGN-R® was scored by a nurse expert in wound care management to assess the severity of PIs.
Sample collection
The samples were collected from three sites – the wound, bed and skin. All samples were obtained using a flocked swab (Puritan, Guilford, ME) soaked in saline with 0.1% Tween-20. Wound samples were collected by swabbing a 1x1cm square at the centre of the wound bed using the Levine technique before wound cleansing19. The patients’ bed samples, defined as the environment formed by the body of the patient, sheet and blanket in this study, were obtained from sheets around the buttock. Skin samples were taken from the patients’ back skin to examine commensal skin bacterial communities of patients. Samples of the bed and skin were collected from a 4.4×4.4cm square area on the surface by swabbing twice with the Z stroke technique20. Sampling was conducted 3–6 days after changing sheets in order to collect comparable levels of bacterial contamination among patients. The swab samples were stored at –80˚C until DNA extraction.
Next-generation DNA sequencing
Genomic DNA was isolated from swab samples using a QIAmp DNA Mini Kit (Qiagen N.V., Venlo, Netherlands) as previously described21. To determine the copy number of the 16S rRNA gene, real-time polymerase chain reaction (PCR) was conducted using universal primer pairs and a universal probe. All reactions were performed using the AriaMx Real-Time PCR System (Agilent Technologies, Santa Clara, CA).
The 16S rRNA gene from each DNA sample was amplified using first PCR primers (Forward: 5'-ACACTCTTTCCCTACACGACGCTCTTCCGATCT-CCTACGGGNGGCWGCAG-3'; Reverse: 5'-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-ACTACHVGGGTATCTAAKCC-3') with Ex Taq® Hot Start Version (TaKaRa Bio Inc., Shiga, Japan) using a thermal cycler (GeneAtlas G02; Astec Co., Ltd., Fukuoka, Japan). Cycling conditions were: 94˚C for 2 minutes; followed by 30 cycles at 94˚C for 30 seconds, 50˚C for 30 seconds, 72˚C for 60 seconds and 72˚C for 5 minutes. The PCR amplicons were purified using Agencourt AMPure XP (Beckman Coulter, Inc., Brea, CA) according to the manufacturer’s instructions. The second round of PCR was performed with the purified PCR products as template. Cycling conditions were: 94˚C for 2 minutes, followed by 10 cycles at 94˚C for 30 seconds, 55˚C for 30 seconds, 72˚C for 30 seconds and 72˚C for 5 minutes. After the purification of the amplicons and quantification of the DNA concentration with a Qubit® dsDNA HS Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA) using AriaMx Real-Time PCR System, an equimolar mixture of all the amplicons was outsourced (FASMAC Co., Ltd., Kanagawa, Japan) for Illumina Miseq 16S amplicon sequencing.
Microbiome analysis
First, raw pair-end sequences were filtered using Sickle (version 1.3)22 on the basis of the Q score and combined using PANDAseq (version 2.11)23. Second, the chimeric sequences were eliminated by USEARCH (version 8.0.1623_i86linux64)24, based on the chimera-checked operational taxonomic units (OTUs) GreenGene database (version 13.8)25. Finally, the non-chimera sequences were filtered by size (>300 bp accepted) followed by analysis with Qiime (version 1.9.1)26.
The 16S rRNA gene amplicon analysis was performed as follows: sequences were first assigned to OTUs at 97% and 100% similarity using the ‘pick_de_novo_otu.py’ command. Then, the OTUs at 100% similarity were analysed as zero-radius OTUs (zOTUs)27. When the same zOTU was detected in different microbiota samples, we assumed bacterial dissemination between these samples because zOTUs indicate bacteria with identical sequences within the V3–V4 regions. The number of zOTUs common to an individual’s wound, skin and bed samples was calculated. The OTUs clustered using a threshold of 97% sequence identity were used to confirm the composition of the microbiota. Samples with unassigned microbes for over 15% of the microbiome were excluded from further analyses.
The zOTUs were used for the evaluation of alpha and beta diversity. For the alpha diversity, the samples were rarefied with a depth of 14,000 (minimum read number among all samples) followed by calculation of the Shannon diversity index. The rarefaction curves from the Shannon index were used to examine whether the microbiota of the samples was sufficiently characterised. To assess beta diversity, the Bray–Curtis distance for all combinations of different sampling sites was generated. To confirm the validity of zOTU similarity as a test for bacterial dissemination, the unweighted pair group method with arithmetic mean (UPGMA) and principal coordinated analysis (PCoA) plots were performed using the Bray–Curtis distance. The UPGMA is commonly used to construct a phylogenetic tree from a distance matrix and was plotted using R with the ‘vegan package’ in order to classify the bacterial samples. In addition, PCoA plots were used to confirm whether the wound, skin and bed microbiota were classified into the same cluster.
Results
Participants and wound characteristics
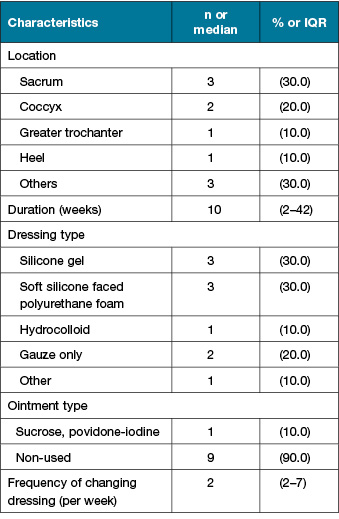
Ten PIs from eight patients were analysed in this study (Figure 1). The characteristics of the study participants are summarised in Table 1. The median age was 85 and the median Braden Scale score was 13 points. Six participants (75.5%) were classified as rank C based on their degree of independence (bed-bound). PI characteristics are shown in Table 2. The median DESIGN-R® total score is 4 points (interquartile range [IQR]: 4–4 points). All PIs were assessed as d2 (superficial), n0 (no necrotic tissue) and i0 (no signs of inflammation). The median of the duration from sheet change to sampling was 4 days (IQR: 3–4 days).
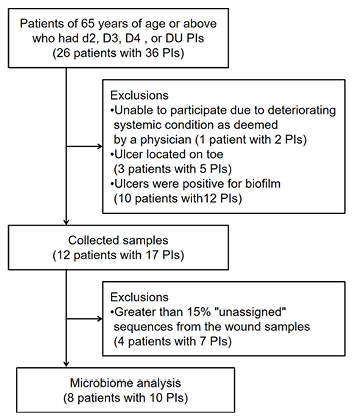
Figure 1. Exclusion criteria for participating in the study
Table 1. Characteristics of participants (n=8)
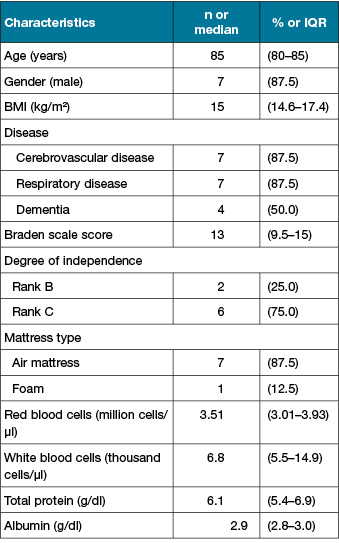
Table 2. PI characteristics (n=10)
Bacterial diversity in bed, wound and skin samples
The relative abundance was calculated from the OTUs cluster using a threshold of 97% sequence identity. The dominant genera in bed samples were Corynebacterium (27.0%), followed by Staphylococcus (26.7%). Staphylococcus (44.6%) and Corynebacterium (35.2%) were dominant in wound samples. Corynebacterium (28.7%) and Staphylococcus (24.5%) were also dominant in skin samples.
A total of 625,472 zOTUs were identified from 26 samples. The median number of zOTUs collected from bed, wound and skin samples was 25,987 (IQR: 22,383–29,932), 22,364 (IQR: 18,898–24,752) and 28,148 (IQR: 27,183–37,414), respectively. The median alpha diversity for the bed, wound and skin microbiome was 11.23 (IQR: 11.01–11.42), 10.26 (IQR: 9.71–10.52) and 11.38 (IQR: 11.16–11.57), respectively. Rarefaction curves of the Shannon index reached a plateau, which indicated that sequencing sufficiently characterised the microbiota (data not shown).
zOTUs common to microbiotas within an individual
All PIs had zOTUs common to the bed and wound (median: 352, IQR: 281–648), the bed and skin (median: 577, IQR: 241–1,398) and wound and skin (median: 1,243, IQR: 1,145–1,406). All wounds had zOTUs common to the bed, wound and skin samples (median: 194, IQR: 121–320) (Figure 2).
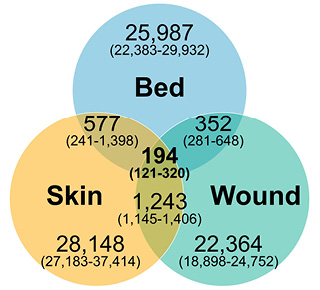
Figure 2. Venn diagram of common zOTUs showing median number of zOTUs (IQR)
Confirmation of the zOTU similarity as a test for bacterial dissemination
Wound and bed samples were classified into the same clusters in eight of the ten study wounds. Furthermore, in three of those eight wounds, the skin samples were also classified into the same clusters. Klebsiella was the dominant genus in Cluster 1; Staphylococcus or Corynebacterium were the dominant genera in Cluster 2; and Cluster 3 was made up of commensal skin bacteria including Staphylococcus, Corynebacterium and Propionibacterium with high diversity (Figure 3). We also assessed the validity of zOTUs for evaluating the dissemination using a PCoA plot. Figure 4 shows that the samples within the same cluster of UPGMA were plotted closely.
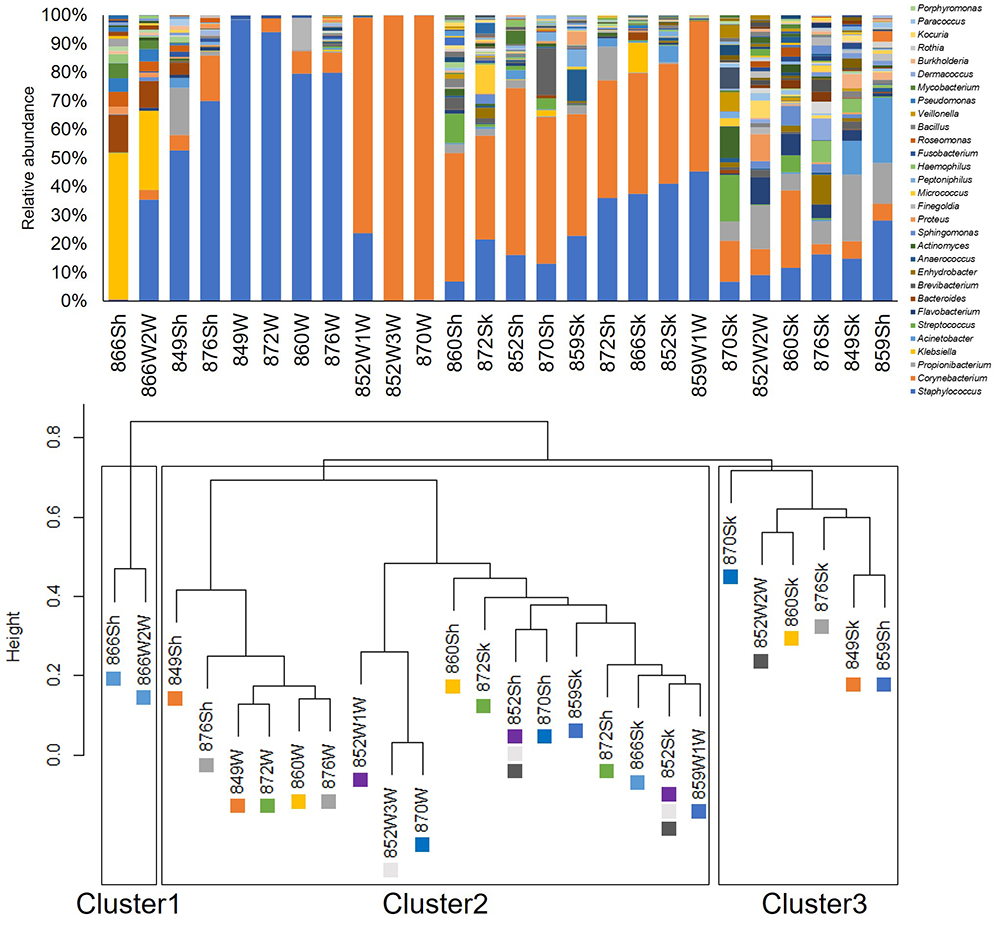
Figure 3. Relative abundance of bacteria classified at the genus level and UPGMA tree
OTUs defined by a 97% identity threshold of the 16S gene sequences, distinguished at the genus level, and used for calculating the relative abundance.
Colour-coded square indicates ID of wounds
W: Wound, Sh: Bed, Sk: Skin
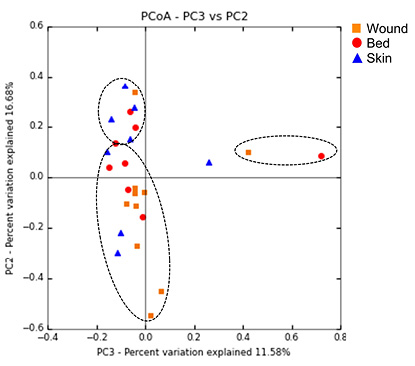
Figure 4. PCoA of microbiome community similarity as measured by Bray–Curtis distance
Ovals indicate the cluster of UPGMA. Red, orange and blue indicate the data collected from the bed, wound and skin, respectively.
Discussion
We explored the microbiota of PIs, back skin and patients’ beds by using 16S rRNA gene analysis. All wounds had several zOTUs common to several microbiota. These microbiota were classified into the same cluster by UPGMA and samples within the same cluster were plotted close to one another for the PCoA. This is, to the best of our knowledge, the first study to quantitatively describe the dissemination of microbiota between wounds and patients’ hospital beds. The result of this study highlights the need for an approach that evaluates the patients’ beds to prevent wound infection.
When the rarefaction curves of the Shannon diversity index reached a plateau it indicates that the sequencing depth of all samples was reasonable, and the sequencing results sufficiently characterised the microbiota, thus confirming the internal validity of sampling and sequencing in this study. In addition, we evaluated the bacterial dissemination using zOTUs defined by a 100% 16S gene sequence identity threshold of the V3–V4 region. The traditional OTU clustering method with a threshold of 97% sequence identity is inadequate for assessing bacterial dissemination. Sequences differing by even one nucleotide (i.e. 99.2% similarity) significantly decreases the degree to which dynamic similarity is observed between two individuals in correlated sequences in comparison to when 100% sequence identity is used28. Several approaches have been proposed to improve the resolution of the 16S data analysis beyond 97% similarity27,29,30. zOTUs were used in this study also to address problems inherent to the standard clustering method. Further, molecular typing has previously been used to evaluate dissemination31; however, this method cannot identify the dissemination of microbiota because it only detects target bacteria. Moreover, the validity of zOTUs for evaluating bacterial dissemination was confirmed in this study by the clustering of microbiota based on the UPGMA tree and PCoA. Thus, the method using zOTUs is suitable for assessing the dissemination of microbiota.
The most abundant genus in patients’ beds was Corynebacterium (27.0%) followed by Staphylococcus (26.7%), which are commonly found on the skin32 and PI33 microbiota. Previous studies of hospital beds focused on pathogens that cause nosocomial infection. Malnick et al. report that Enterococcus faecalis, coagulase-negative Staphylococcus and Bacillus were detected on sheets after they were used overnight10. Moreover, it has been suggested that contaminated hospital linens contribute to Bacillus cereus bacteraemia outbreaks34. Our findings show that not only pathogens but also resident skin, gut and oral bacteria are present in the microbiota of patients’ beds and suggested the need for a comprehensive search of microbiota to understand the dissemination between PIs and patients’ beds.
All PIs had zOTUs common to the bed, wound and skin microbiota, even though the wound location varied. Due to the immobility of many PI patients, patients receive repositioning care by healthcare workers for pressure redistribution1. Therefore, the area of the sheets that is in contact with the wound changes continuously, presenting an opportunity for bacterial dissemination to occur. Additionally, bacteria can be transmitted via healthcare workers35,36, and airborne bacteria significantly increases during dressing changes for burn patients37. Daily wound care by healthcare workers may therefore contribute to bacterial dissemination to patients’ beds. In addition, bacterial attachment between the wound and the bed during wound care might be re-disseminated to the wound as the patient continues to lie on the bed. This indicates that, no matter how well the wound is cleansed, if there are infectious bacteria present in the bed sheet, the bacteria may re-disseminate to the wound and cause infection. Thus, to establish a new intervention to prevent wound infection whilst the patient remains in bed, a longitudinal study in a larger cohort is needed to clarify the direction of this bacterial dissemination. Furthermore, animal studies should be conducted to confirm whether interventions in the external environment can alter the wound microbiota.
Limitations
The present study has several limitations. First, we could not investigate bacterial dissemination of deep wounds due to the presence of biofilm. Therefore, the degree of dissemination for deep wounds is not clear. Second, we could not verify the relationship between bacterial dissemination and wound outcome because no wounds in the study had symptoms of inflammation. A longitudinal study is thus needed to clarify the causal relationship between bacterial dissemination in hospital beds and wound infection. Third, most participants in this study were immobile and spent 24 hours a day in bed. Therefore, further studies are required to generalise our findings for individuals who are not bed-bound.
In conclusion, the present cross-sectional study was performed to examine the dissemination of microbiota between PIs and hospital beds using a culture-independent methodology. Our results show the bacterial dissemination in all wounds, and further studies are required to clarify the impact of bacterial dissemination in order to develop intervention strategies against the dissemination of microbiota and subsequent infections.
Acknowledgements
Alcian blue staining and destaining solutions were provided by Saraya Co., Ltd which had no role in planning and performing the study. The authors would like to thank all patients and clinical staff for their cooperation during this study.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by a grant from the Japanese Society of Wound, Ostomy, and Continence Management and JSPS KAKENHI – Grant Number 18K19669.
Author(s)
Mao Kunimitsu MHS, RN1,2
Gojiro Nakagami PhD, RN1,3
Aya Kitamura PhD, RN1
Takeo Minematsu PhD3,4
Yuko Mugita PhD, RN1
Kazuhiro Ogai PhD5
Junko Sugama PhD, RN6
Miku Aoki PhD, RN7
Chika Takada MSN, RN8
Hiromi Sanada* PhD, RN, CWOCN, FAAN1,3
Faculty of Medicine Building 5-307, 7-3-1, Hongo, Bunkyo-Ku, Tokyo, 113-0033, Japan
Email hsanada@g.ecc.u-tokyo.ac.jp
1 Department of Gerontological Nursing/Wound Care Management, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
2 Japan Society for the Promotion of Science, Tokyo, Japan
3 Global Nursing Research Center, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
4 Department of Skincare Science, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
5 AI Hospital/Macro Signal Dynamics Research and Development Center, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Ishikawa, Japan
6 Research Center for Implementation Nursing Science Initiative, School of Health Sciences, Fujita Health University, Aichi, Japan
7 Division of Nursing, Faculty of Medical Sciences, University of Fukui, Fukui, Japan
8 Department of Nursing, Sengi Hospital, Ishikawa, Japan
* Corresponding author
References
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA: 2019.
- Gunningberg L, Hommel A, Bååth C, Idvall E. The first national pressure ulcer prevalence survey in county council and municipality settings in Sweden. J Eval Clin Pr 2013;19(5):862–7.
- Igarashi A, Yamamoto-Mitani N, Gushiken Y, Takai Y, Tanaka M, Okamoto Y. Prevalence and incidence of pressure ulcers in Japanese long-term-care hospitals. Arch Gerontol Geriatr 2013;56(1):220–6.
- Moore Z, Cowman S. Pressure ulcer prevalence and prevention practices in care of the older person in the Republic of Ireland. J Clin Nurs 2012;21(3–4):362–71.
- VanGilder C, Lachenbruch C, Algrim-Boyle C, Meyer S. The international pressure ulcer prevalence TM survey: 2006–2015: a 10-year pressure injury prevalence and demographic trend analysis by care setting. J Wound Ostomy Cont Nurs 2017;44(1):20–8.
- Braga IA, Pirett CCNS, Ribas RM, Filho PPG, Filho AD. Bacterial colonization of pressure ulcers: assessment of risk for bloodstream infection and impact on patient outcomes. J Hosp Infect 2013;83(4):314–20.
- Braga IA, Brito CS, Filho AD, Filho PPG, Ribas RM. Pressure ulcer as a reservoir of multiresistant Gram-negative bacilli: risk factors for colonization and development of bacteremia. Brazilian J Infect Dis 2017;21(2):171–5.
- Khor HM, Tan J, Saedon NI, et al. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr 2014;59(3):536–41.
- Konya C, Sanada H, Sugama J, et al. Skin debris and micro-organisms on the periwound skin of pressure ulcers and the influence of periwound cleansing on microbial flora. Ostomy Wound Manage 2005;51(1):50–9.
- Malnick S, Bardenstein R, Huszar M, Gabbay J, Borkow G. Pyjamas and sheets as a potential source of nosocomial pathogens. J Hosp Infect 2008;70(1):89–92.
- Kunimitsu M, Nakagami G, Kitamura A, et al. Temporal changes in the diversity and composition of the bed microbiota of patients with pressure ulcers. J Japanese Soc Wound, Ostomy, Cont Manag 2020;24(4):366–78.
- Amann RI, Ludwig W, Schleifer K. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 1995;59(1):143–69.
- Japanese Society of Pressure Ulcers. JSPU guidelines for the prevention and management of pressure ulcers (4th Ed.). Jpn J PU 2016;18(4):455–544.
- Matsui Y, Furue M, Sanada H, et al. Development of the DESIGN-R with an observational study: an absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen 2011;19(3):309–15.
- Nakagami G. Biofilm differentially affects wound healing according to the bacterial community in pressure ulcers. In: Dominique Sigaudo-Roussel, editors. Proceedings of the 21st Annual Meeting of the European Pressure Ulcer Advisory Panel, 2019 Sep 18–20; Lyon, France.
- Nakagami G, Schultz G, Gibson DJ, et al. Biofilm detection by wound blotting can predict slough development in pressure ulcers: a prospective observational study. Wound Repair Regen 2017;25(1):131–8.
- Mori Y, Nakagami G, Kitamura A, et al. Effectiveness of biofilm‐based wound care system on wound healing in chronic wounds. Wound Repair Regen 2019;27(5):540–7.
- Braden BJ, Bergstrom N. Clinical utility of the Braden scale for predicting pressure sore risk. Decubitus 1989;2(3):44–46,50–51.
- Levine NS, Lindberg RB, Mason ADJ, Pruitt BAJ. The quantitative swab culture and smear: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma 1976;16(2):89–94.
- Rushing J. Obtaining a wound culture specimen. Nursing (Lond) 2007;37(11):18.
- Ogai K, Nagase S, Mukai K, et al. A comparison of techniques for collecting skin microbiome samples: swabbing versus tape-stripping. Front Microbiol 2018;9:2362.
- Joshi NA, Fass JN. Sickle: a sliding-window, adaptive, quality-based trimming tool for FastQ files; 2011 [cited May 17 2021]. Available from: URL: https://github.com/najoshi/sickle
- Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: PAired-eND Assembler for Illumina sequences. BMC Bioinformatics 2012;13:31.
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26(19):2460–1.
- DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006;72(7):5069–72.
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7(5):335–6.
- Edgar RC. UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. BioRxiv 2016;1–21.
- Tikhonov M, Leach RW, Wingreen NS. Interpreting 16S metagenomic data without clustering to achieve sub-OTU resolution. ISME J 2015;9(1):68–80.
- Callahan BJ, McMurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J 2017;11(12):2639–43.
- Amir A, Daniel M, Navas-Molina J, et al. Deblur rapidly resolves single-nucleotide community sequence patterns. Am Soc Microbiol 2017;2(2):e00191–16.
- Tan TY, Tan JSM, Tay H, Chua GH, Yong Ng LS, Syahidah N. Multidrug-resistant organisms in a routine ward environment: differential propensity for environmental dissemination and implications for infection control. J Med Microbiol 2013;62(5):766–72.
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol 2018;16(3):143–55.
- Wolcott RD, Hanson JD, Rees EJ, et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen 2016;24(1):163–74.
- Sasahara T, Hayashi S, Morisawa Y, Sakihama T, Yoshimura A, Hirai Y. Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis 2011;30:219–26.
- Jullian-Desayes I, Landelle C, Mallaret M-R, Brun-Buisson C, Barbut F. Clostridium difficile contamination of health care workers’ hands and its potential contribution to the spread of infection: review of the literature. Am J Infect Control 2017;45(1):51–8.
- Mitchell AH, Spencer M, Edmiston CE. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: a review of the literature. J Hosp Infect 2015;90(4):285–92.
- Bache SE, Maclean M, Gettinby G, Anderson JG, Macgregor SJ, Taggart I. Airborne bacterial dispersal during and after dressing and bed changes on burns patients. Burns 2015;41(1):39–48.



