Volume 29 Number 2
Measurement properties of quality of life instruments for adults with active venous leg ulcers: a systematic review protocol
Shiwen Liu, Victoria Team, Yunjing Qiu and Carolina D. Weller
Keywords venous leg ulcers, measurement properties, quality of life (QoL), quality of life measures
For referencing Liu S et al. Measurement properties of quality of life instruments for adults with active venous leg ulcers: a systematic review protocol. Wound Practice and Research 2021; 29(2):104-109.
DOI https://doi.org/10.33235/wpr.29.2.104-109
Abstract
Objectives The primary objective is to identify instruments used to measure quality of life (QoL) in studies of people with active venous leg ulcers (VLUs). The secondary objective is to map the qualities of each instrument to make recommendations for clinical practice and future research.
Introduction VLUs have a negative impact on patients’ QoL. Prolonged healing and frequent recurrence leads to pain, prolonged disability and psychosocial morbidity. Accurate measurement of QoL can optimise the evaluation of VLU treatments and guide clinician and patient decision-making.
Inclusion criteria Studies that reported QoL in patients with active VLUs.
Methods This review will identify studies indexed in CINAHL, Ovid MEDLINE, Ovid Emcare and ProQuest from January 2000 to December 2020. Methodological quality will be assessed using the COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) Risk of Bias checklist. A narrative synthesis of general information, methodology and measurement properties of the QoL instruments will be presented. If possible, a random effects meta-analysis will be conducted where appropriate.
What is already known on the topic:
- VLUs are the most common chronic lower limb wound managed in primary care in Australia and their prevalence is predicted to increase.
- Multicomponent compression therapy is recommended to improve healing and reduce the recurrence of VLUs.
- VLUs have a negative impact on patient QoL.
- There are many generic and condition-specific QoL instruments used in VLU studies.
What this study contributes
- This study will identify which instruments report QoL in people with active VLUs.
- This study will map QoL measurement properties, identify the qualities of each tool and make recommendations for clinical practice and future research.
Introduction
Venous leg ulcers (VLUs) are irregular-shaped wounds located between the knee and ankle joints1 which are caused by venous hypertension, venous valve dysfunction, venous outflow obstruction, and calf muscle pump dysfunction2. Individuals suffering from VLUs are subject to a cycle of prolonged healing and recurrence3. The severity of VLUs is assessed by the CEAP (clinical manifestation, aetiology, anatomy and pathophysiology) classification which is used to guide treatment based on the level of severity and evaluate the outcomes of specific treatments4. The categories are classified from C0 to C6 – C0 indicating no visible or palpable signs of venous disease and C6 showing active venous ulcer4.
Epidemiology of VLUs
In Australia, VLUs are the most common chronic lower limb wound managed in primary care5. Active VLUs occur between 1.5 and 3.0 per 1,000 persons at the age of 65 and the incidence increases to 20 per 1,000 persons for patients older than 80 years6. It is estimated that VLU prevalence in Australia is 0.33% in people aged over 60 years old7. The prevalence of VLUs is predicted to increase as people are living longer and suffer from various chronic illnesses8.
Management of VLUs
Best practice recommends multicomponent compression therapy to reduce hydrostatic pressure in the lower limbs9,10. Other therapies adjuvant to compression – such as debridement, wound dressing, medications, surgery, the use of devices, physiotherapy and psychological interventions – have also been adopted, although there is a lack of high quality studies for reporting effectiveness on healing and recurrence11.
Multi-component compression systems containing an elastic bandage are more effective than single systems containing inelastic bandages12. However, the efficacy of compression treatment can be limited by the patient’s adherence to compression treatment which depends on many factors13, including pain and discomfort related to compression bandages, lack of knowledge and understanding of the treatment, physical limitations, psychosocial issues and cost12,14,15. Due to a lack of awareness of VLU management clinical practice guidelines, primary care clinicians may not recommend compression therapy5. Reported discrepancies between patients’ and clinicians’ understanding of VLU management can lead to suboptimal VLU care and can impact negatively on compression adherence5. Suboptimal compression adherence can delay time to healing16 and extend episodes of pain and impaired mobility10, thereby impacting on quality of life (QoL)5.
Quality of life
QoL is defined as “the functional effects of an illness and its consequent therapy upon a patient, as perceived by the patient”17(p368). QoL is a broad concept which describes the domain of physical and psychological aspects of wellbeing and social functioning18. VLUs have a profound impact on patients’ QoL15. Factors related to physical and psychosocial functioning and treatments impact on the QoL of patients with leg ulceration18.
High rates of ulcer recurrence and prolonged time to healing are associated with negative emotions such as frustration, anxiety and pessimism19. These negative emotions, pain and reduced mobility affect the QoL of VLUs patients19. Healing of VLUs can be influenced by poor adherence to compression treatment20. Compression therapy combined with different adjunctive agents may promote healing and improve QoL11.
QoL can be measured by using different QoL instruments8. QoL instruments can be categorised as either generic and VLU-specific instruments8. Generic instruments can be used with any group of people with any disease, such as 36-Item Short Form Health Survey (SF-36)8 and EuroQol-five dimensions questionnaire (EQ-5D)15. VLU-specific instruments – including VEnous INsufficiency Epidemiological and Economic Study-Quality of Life (VEINES-QOL)8, Sheffield Preference-based Venous Ulcer questionnaire (SPVU-5D)21, Chronic Venous Insufficiency Questionnaire (CIVIQ)8, the Cardiff Wound Impact Schedule (CWIS)8 and VLU-QoL8 – are typically designed for measuring QoL related to VLU patients8.
Significance of the study
Many published studies that report the impact of VLUs on QoL have already mentioned QoL measures in their studies22–25. However, it is not clear which QoL instrument is most appropriate to guide clinical care and research in patient with VLUs. This study will investigate which QoL instrument is the most appropriate for assessing QoL in people with VLUs in clinical practice and future research.
Review questions
- Which instruments are used to assess the impact of VLUs on QoL of adults with active VLUs?
- Which QoL instruments have the best measurement properties?
- Which QoL instruments are optimal for an assessment of the impact of VLUs on QoL of adults with active VLUs in clinical practice?
Methods
Inclusion criteria
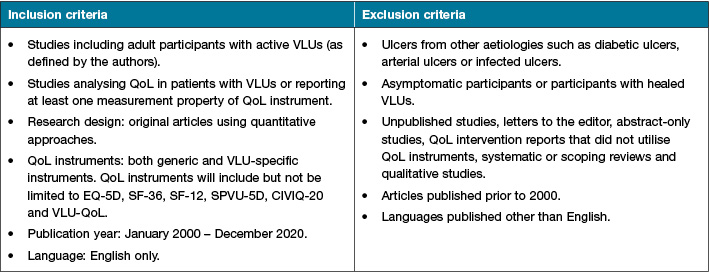
Eligible studies that include adult patients with active VLUs (CEAP: C6) will be included. There are no restrictions on gender, race, educational background or geographical locations. This review will exclude patients with healed VLUs or any leg ulcer that is not VLU, including diabetic ulcers, arterial ulcers and mixed aetiology ulcers.
Instruments
This review will include studies that reported both generic and VLU-specific QoL instruments.
Outcomes
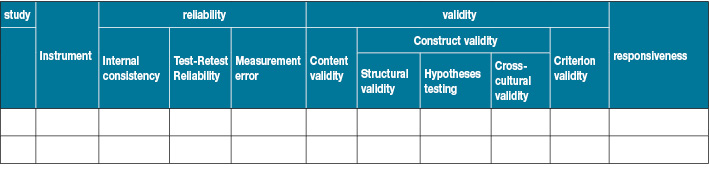
This review will include studies that reported at least one of the following properties of the QoL instruments (Table 1):
- Reliability: Measures of reliability includes internal consistency, test-retest reliability and measurement error.
- Validity: Measures of validity will include content validity, construct validity and criterion validity.
- Responsiveness.
Table 1. Measurement properties26
Types of studies
This review will include published quantitative studies that used QoL instruments to report QoL in adults with active VLUs. This review will exclude letters to the editor, abstract-only studies, QoL intervention reports that did not utilise QoL instruments, systematic or scoping reviews and qualitative studies as these studies cannot provide the measurement of the validity and reliability of QoL instruments. This review will also exclude duplicate publications arising from one project (Table 2).
Table 2. Inclusion and exclusion criteria
This systematic review will be conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines27.
Search strategy
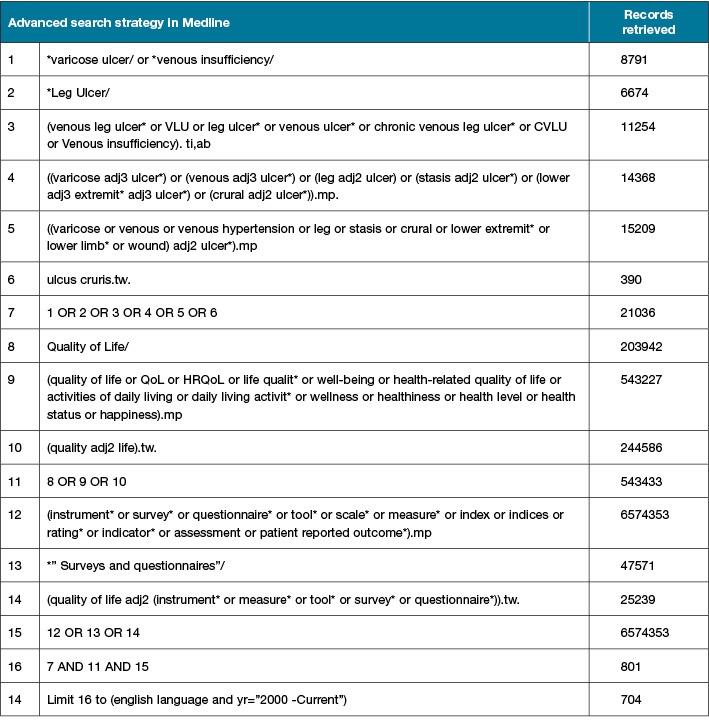
Search terms will combine Medical Subject Headings (MeSH terms) and keywords related to the objective and their words variants (Supplementary Table 1). If additional key words are detected, the search strategy will be modified, and an updated search will be performed to incorporate changes in the systematic review.
Information sources
The following sources will be searched from January 2000 to Dec 2020: CINAHL, OvidMEDLINE, OvidEmcare, ProQuest due to finite resource on this topic since the beginning of VLUs studies.
Study selection
All retrieved articles will be imported to Covidence (https://www.covidence.org/) for study selection. Two review authors (SL and YQ) will independently assess the titles and abstracts of retrieved articles by utilising the pre-set criteria for inclusion. Articles retrieved in the first screening step will be assessed in full text in accordance with inclusion criteria. Two review authors will assess independently to exclude articles that do not meet the inclusion criteria. Any disagreements between the two authors will be resolved by negotiation or with the help of a third review author (VT). Result of the study selection will be presented into a PRISMA flow diagram28.
Assessment of methodological quality
Two review authors (SL and YQ) will independently assess the methodological quality of included studies. In this review, the COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) Risk of Bias checklist will be adopted to assess the methodological quality of retrieved studies29. The COSMIN checklists consist of 10 items – Patient-Reported Outcome Measure (PROM) development and other nine items of measurement properties: content validity, structural validity, internal consistency, cross-cultural validity/measurement invariance, reliability, measurement error, criterion validity, hypotheses testing for construct validity and responsiveness30. Each item requires a “very good”, “adequate”, “doubtful” or “inadequate” response. Overall appraisal rating will be provided by two review authors at the end of the check lists. Any discrepancy will be discussed or resolved by a third review author (VT).
Data extraction
Two review authors will independently extract data to extraction tables that include at minimum general study information, the participant’s information, methodology information and instrument information (Figure 1). Discrepancy will be discussed and resolved by a third review author (VT).

Figure 1. Data extraction information
Data synthesis
The extracted measurement properties will, where possible, be statistically pooled in meta-analysis if multiple studies report the same QoL instruments. Heterogeneity will be assessed statically using I2 tests. For test-retest reliability, weighted mean intraclass correlation coefficients (ICCs) and 95% confidence intervals will be calculated using a random effects model30. For other measurement properties, weighted means and 95% confidence intervals will be calculated. However, there may be limited scope for meta-analysis because of the limited number of studies reporting a limited range of QoL measures. If this is the case, the result will be reported in narrative and tabular synthesis. The extracted data listed above will be presented into tables (Supplementary Table 2). A ‘criteria for good measurement properties’30 will be introduced to this review to evaluate the result of each study on a measurement property30. Each measurement property will be rated as “sufficient”, “insufficient”, or “indeterminate”30. The overall rating will determine the quality of single studies on measurement properties of a QoL instrument30.
Assessing certainty in the findings
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach will be adopted in this review to evaluate the quality of each instrument as a whole31. The evidence of measurement property data is assumed to be in a high quality at the beginning and the quality of data may be subsequently downgraded due to many reasons such as risk of bias, imprecision, indirectness and inconsistence31. The quality of the evidence will be graded as high, moderate, low or very low31. The grade of the quality of evidence will be also presented in the Summary of findings section.
Acknowledgements
Shiwen Liu: conceptualisation, methodology, investigation, writing of the original draft. Victoria Team: conceptualisation, methodology, writing (review and editing). Yunjing Qiu: methodology, investigation. Carolina Weller: conceptualisation, methodology, writing (review and editing), supervision. Ms Cassandra Freeman, Subject Librarian for MNHS (Medicine, Nursing, Health Sciences) at the Monash University for her dedicated assistance in search strategies development.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Shiwen Liu MNPrac, BN(Hons) student
School of Nursing and Midwifery
Monash University, Level 5 Alfred Centre
99 Commercial Road, Melbourne VIC 3004 Australia
Victoria Team DrPH
School of Nursing and Midwifery
Monash University, Level 5 Alfred Centre
99 Commercial Road, Melbourne VIC 3004 Australia
Yunjing Qiu BN(Hons), PhD candidate
School of Nursing and Midwifery
Monash University, Level 5 Alfred Centre
99 Commercial Road, Melbourne VIC 3004 Australia
Carolina D. Weller* PhD
School of Nursing and Midwifery
Monash University, Level 5 Alfred Centre
99 Commercial Road, Melbourne VIC 3004 Australia
Email carolina.weller@monash.edu
*Corresponding author
References
- Chamanga ET. Understanding venous leg ulcers. Br J Community Nurs 2018;23(Sup9):S6–S15.
- Lim CS, Baruah M, Bahia SS. Diagnosis and management of venous leg ulcers. BMJ 2018;362:k3115.
- Stewart A, Edwards H, Finlayson K. Reflection on the cause and avoidance of recurrent venous leg ulcers: an interpretive descriptive approach. J Clin Nurs 2018;27(5–6):e931–e9.
- Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg-Venous L 2020;8(3):342–52.
- Weller C, Richards C, Turnour L, Team V. Venous leg ulcer management in Australian primary care: patient and clinician perspectives. Int J Nurs Stud 2020;103774.
- Weller CD, Bouguettaya A, Team V, Flegg J, Kasza J, Jayathilake C. Associations between patient, treatment, or wound-level factors and venous leg ulcer healing: wound characteristics are the key factors in determining healing outcomes. Wound Repair Regen 2020;28(2):211–8.
- Cheng Q, Gibb M, Graves N, Finlayson K, Pacella RE. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018;18(1):421.
- Franks PJ, Barker J, Collier M, Gethin G, Haesler E, Jawien A, et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care 2016;25(Sup6):S1–S67.
- Weller CD, Team V, Ivory JD, Crawford K, Gethin G. ABPI reporting and compression recommendations in global clinical practice guidelines on venous leg ulcer management: a scoping review. Int Wound J 2019;16(2):406–19.
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012(11).
- Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, et al. What’s new: management of venous leg ulcers: treating venous leg ulcers. J Am Acad Dermatol 2016;74(4):643–64.
- Welsh L. What is the existing evidence supporting the efficacy of compression bandage systems containing both elastic and inelastic components (mixed-component systems)? A systematic review. J Clin Nurs 2017;26(9–10):1189–203.
- Team V, Chandler PG, Weller CD. Adjuvant therapies in venous leg ulcer management: a scoping review. Wound Repair Regen 2019;27(5):562–90.
- Boxall S, Carville K, Leslie G, Jansen S. Compression bandaging: identification of factors contributing to non-concordance. Cambridge Publishing; 2019. p. 6–20.
- Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. Int Wound J 2019;16(1):112–21.
- Walburn J, Weinman J, Norton S, Hankins M, Dawe K, Banjoko B, et al. Stress, illness perceptions, behaviors, and healing in venous leg ulcers: findings from a prospective observational study. Psychosom Med 2017;79(5):585–92.
- Meaume S, Dompmartin A, Lok C, Lazareth I, Sigal M, Truchetet F, et al. Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomised controlled trial. J Wound Care 2017;26(7):368–79.
- Gil-Lacruz M, Gil-Lacruz AI, Gracia-Pérez ML. Health-related quality of life in young people: the importance of education. Health Qual Life Out 2020;18(1):187.
- Isaac A, Watson C. How venous leg ulcers affect quality of life. Primary Health Care 2016;26(3).
- Scotton MF, Miot HA, Abbade LPF. Factors that influence healing of chronic venous leg ulcers: a retrospective cohort. An Bras Dermatol 2014;89:414–22.
- Cheng Q, Kularatna S, Lee XJ, Graves N, Pacella RE. Comparison of EQ-5D-5L and SPVU-5D for measuring quality of life in patients with venous leg ulcers in an Australian setting. Qual Life Res 2019;28(7):1903–11.
- Green J, Jester R, McKinley R, Pooler A. The impact of chronic venous leg ulcers: a systematic review. J Wound Care 2014;23(12):601–12.
- Phillips P, Lumley E, Duncan R, Aber A, Woods HB, Jones GL, et al. A systematic review of qualitative research into people’s experiences of living with venous leg ulcers. J Adv Nurs 2018;74(3):550–63.
- González-Consuegra RV, Verdú J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs (John Wiley & Sons, Inc). 2011;67(5):926–44.
- Palfreyman SJ, Tod AM, Brazier JE, Michaels JA. A systematic review of health-related quality of life instruments used for people with venous ulcers: an assessment of their suitability and psychometric properties. J Clin Nurs 2010;19(19–20):2673–703.
- Mokkink L, Terwee C, Patrick D, Alonso J, Stratford P, Knol D, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epid 2010;63:737–45.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLoS Med 2009;6(7):e1000097.
- Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, Terwee CB. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res 2018;27(5):1171–9.
- Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147-57.
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. Available from: guidelinedevelopment.org/handbook. 2018.
- Stephenson M, Riitano D, Wilson S, Leonardi-Bee J, Mabire C, Cooper K, et al. Systematic reviews of measurement properties. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Adelaide: JBI, 2020 [ cited 30 May 2021]. Available from: https://doi.org/110.46658/JBIMES-20-13.
Appendix I. Search strategy
Appendix II. Data extraction instrument
1. General and methodology information
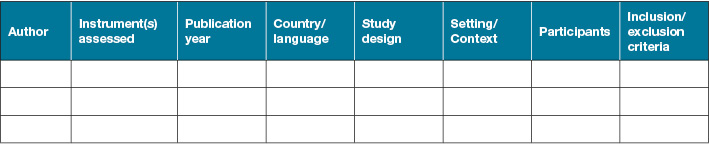
2.Participant’s information
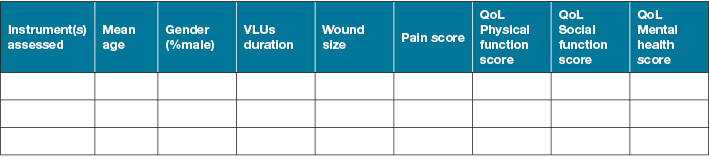
3. Instrument information
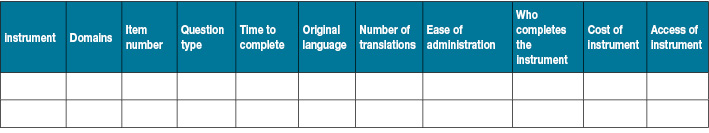
4. Measurement properties32