Volume 29 Number 2
Physical activity, sleep and wound healing in adults with venous leg ulcers: a prospective observational cohort pilot study protocol
Yunjing Qiu, Victoria Team, Christian R Osadnik, Jane O’Brien, Louise Turnour, Ayoub Bouguettaya, Rosemary A McGinnes and Carolina D Weller
Keywords quality of life, physical activity, venous leg ulcers, Pain, sleep
For referencing Qiu Y et al. Physical activity, sleep and wound healing in adults with venous leg ulcers: a prospective observational cohort pilot study protocol. Wound Practice and Research 2021; 29(2):98-103.
DOI https://doi.org/10.33235/wpr.29.2.98-103
Abstract
Background Adults with venous leg ulcers (VLUs) are less likely to be physically active and show greater sleep disturbances than the general population. Limited evidence suggests these issues contribute to VLU healing delays.
Objectives The primary objective is to determine if physical activity (PA) and sleep levels are associated with VLU healing. The secondary objectives are to: 1) evaluate the feasibility and acceptability of a wrist-worn accelerometer device, wActiSleep-BT device wear (ActiGraph); 2) evaluate the utility of self-reported PA instruments to measure PA for people with VLU; and 3) determine whether PA and sleep levels are associated with i) delayed healing, ii) self-reported quality of life (QoL) and/or iii) self-reported VLU pain.
Design and method This prospective observational cohort pilot study aims to recruit 30 adults with VLUs from three hospital-based outpatient wound clinics in Melbourne, Australia. PA and sleep levels will be measured using the wActiSleep-BT device. VLU healing data will be collected from medical records. Patients’ self-reported outcomes will be collected using questionnaires.
Discussion Findings will provide insight into the relationship between PA and sleep with healing, QoL and pain, and determine the feasibility and acceptability of the wActiSleep-BT device. Findings will also inform the potential utility of self-reported instruments in estimating PA level in people with VLUs.
Contribution of the paper statements
What is already known about the topic?
- VLUs are a common and costly problem.
- Adults with VLUs are more likely to be inactive.
- Sleep disturbances are more common in adults with VLUs than the general population.
- Increased PA may improve wound healing outcomes for adults with VLUs.
What this paper adds
- An objective evaluation of the level of PA and sleep by adults with VLUs.
- The relationship between PA, sleep, VLU healing, as well as QoL and pain.
- The validation of self-reported instruments for assessing PA level.
- A feasibility assessment of the wActiSleep-BT device to examine levels of PA and sleep in adults with VLUs.
- An acceptability assessment of wearing the wActiSleep-BT device by adults with VLUs.
Introduction
Background and rationale
Venous leg ulcers (VLUs) are a common and costly public health problem of global significance1,2. The cost of treating VLUs is estimated at greater than US$2.5 billion per year in the United States3,4 and up to £941 million in the United Kingdom5. In 2010 an estimated 400,000 Australians were treated for VLUs, which translated into costs of more than A$3 billion per year6–8. Known risk factors for delayed healing and recurrence of VLUs include chronic venous insufficiency (CVI), older age, female gender, diabetes and hypertension, previous VLU with larger ulcer size, and longer ulcer duration9,10. The natural history of VLUs is a cycle of healing and recurrence11 which has considerable impact on individuals’ health, quality of life (QoL) and socioeconomic costs12. With an ageing population and the growing epidemic of diabetes and obesity13,14, the burden of VLU is expected to rise; coupled with CVI, this burden will continue to reduce patient QoL and increase healthcare costs15. Therefore, it is critical to understand the factors that impede, or aid, VLU healing in order to combat this public health issue.
In addition to compression therapy, lack of physical activity (PA) and sedentary behaviour have been identified as possible factors that impact on VLU healing16. PA is defined by the World Health Organization as any bodily movement produced by skeletal muscles that requires energy expenditure, such as working, playing, carrying out household chores, travelling, and engaging in recreational pursuits, including exercise17. Exercise is a goal-directed, structured, repetitive form of PA, aiming to improve one or more components of physical fitness17. However, the focus of this proposed study is general PA. PA can be graded by intensity according to corresponding metabolic equivalent (MET) values and is classified as light, moderate, vigorous or very vigorous. It is recommended that Australian adults over the age of 65 participate in at least 30 minutes of moderate intensity PA on most, if not all, days of the week18. Distinct from PA, sedentary behaviour refers to time spent physically inactive whilst awake19. However, it is possible to be both sedentary (e.g., sitting for prolonged periods of time) and physically active (e.g., meeting recommended guidelines).
It has been suggested that lack of mobility may be associated with delayed healing time from VLUs20. Subjective and objective PA measures have been reported21,22. Self-reported measures of sedentary time correlate poorly with objective measures, and over-estimation of self-reported PA is well recognised21. Accelerometry offers an objective measurement of PA and sedentary behaviour which may be useful in clinical research22.
Adults with VLU are more likely to be both sedentary and physically inactive than age-matched controls; these adults are also characterised by decreased general mobility with greater dependence on walking aids23–25. Several studies included in two systematic reviews16,26 have investigated the influence of PA on VLU healing27–34. These systematic reviews reported the effects of exercise on VLU wound characteristics such as time to heal, size, and pain, QoL, adverse events and economic outcomes16,26. In the Smith review16 of six studies, four studies reported a non-significant effect regarding the effect of exercise on wound healing, highlighting the importance of conducting future high quality prospective studies incorporating outcomes that capture the significance of the effect of PA and/or exercise for this patient group. The Jull review26, which included two more recent trials32,33, reports clinicians may consider recommending simple progressive resistance and aerobic activity to suitable patients with VLU until further research is produced.
Sleep is the natural, periodic and reversible state of rest for the mind and body wherein consciousness is completely or partially lost and there is decrease in bodily movement and responsiveness to external stimuli35. Cell division, protein synthesis and growth hormone release are increased during sleep36 and decreased sleep may impair wound healing37,38. In healthy volunteers, relatively modest sleep disruption of 72 hours of sleep restriction with 2 hours of sleep per night in a laboratory setting was reported to delay wound healing, possibly due to impairment of the immune response39. Even mild sleep disruption is known to impair immunity by disrupting macrophage and lymphocyte production and activity38.
Sleep disturbances are more common in adults with VLU than the general population; for example, these disturbances have been reported by (217/247) 88% of patients in a longitudinal, observational study40. In addition to this evidence, over (237/763) 30% of patients with VLU in cross sectional data from a Swedish registry showed sleep disturbances41. Patients with VLU report poor sleep quality42 and disrupted sleep due to pain, which may further contribute to delayed VLU healing43. This was highlighted as a potential confounder in the Swedish registry study41, as it is unknown whether the sleep disturbances in VLU patients are the result of pain, or exist without pain. Therefore, questions remain regarding how pain and sleep disturbances affect VLU healing, independent of one another.
As discussed in previous systematic reviews16,26 and cohort research43, previous studies investigating the role of PA and sleep in VLU have been characterised by methodological design weaknesses, bias and performance issues16. There is therefore a need for high quality research to generate transparent evidence with minimal bias to inform treatment and prevention outlines.
This study aims to determine if PA and sleep are associated with VLU healing. We will examine the feasibility and acceptability of wActiSleep-BT device wear, and examine: if PA and sleep levels impact on delayed healing after 12 weeks but up to 24 weeks; adherence to compression therapy; QoL; and VLU pain. This study seeks to address these gaps. We have used the TIDieR checklist to promote full and accurate description of intervention to facilitate replicability and implementation in future research in this area44. TIDieR-PHP is intended to complement and be used as an extension to the appropriate reporting guideline for the study design being used (such as CONSORT, SPIRIT, STROBE, or TREND).
Aims
The primary aim of this study is to determine whether PA and sleep levels are associated with improved ulcer healing (e.g., time to healing, proportion of ulcer healed, rates of changes in ulcer area) at 12 weeks from baseline assessment. The secondary aims are to: evaluate the feasibility and acceptability of a wrist-worn accelerometer device; evaluate the utility of self-reported instruments to measure PA for people with VLU; and determine whether PA and sleep levels are associated with delayed VLU healing after 12 weeks and up to 24 weeks, improved self-reported EQ-5D-5L QoL, and improved self-reported VLU pain.
Design and methods
Study design and setting
This prospective observational cohort study will comprise: the measurement of PA and sleep among adults with VLUs; and the collection of data on factors related to VLU healing. This proof of concept study will also test the use of the wActiSleep-BT device (ActiGraph, Pensacola, Florida) in the VLU population by assessing recruitment feasibility and participant device acceptability.
Research ethics approval
This protocol and informed consent have been approved by Alfred Hospital Health Human Research Ethics Committee (HREC/14/Alfred/2, Project 146/4). This study will be conducted in accordance with the Declaration of Helsinki 1964 and the National Health and Medical Research Council National Statement on Ethical Conduct in Human Research.
Study setting and participants
Participants with VLUs will be invited from three Australian (Melbourne) public hospitals by a research nurse trained in wound care. Patients will be eligible if they have an active VLU/s, and are over 18 years of age. If the participant has more than one VLU, the largest VLU will be selected for measurement. There will be no exclusion criteria, and participants will not be compensated for participating in this research.
Informed consent
A research nurse will provide a verbal explanation of the research study, and provide patients with a written information and consent document (Supplementary Material/Appendix). Written informed consent will be obtained from each patient prior to study commencement for the following items: medical record access; completion of a baseline questionnaire and a 12- and 24-week follow-up questionnaire; wear time of the wActiSleep-BT device; PA log; International Physical Activity Questionnaires (IPAQ) and Rapid Assessment of Physical Activity (RAPA); and wActiSleep-BT device patient acceptability.
Outcome assessment
The research nurse will collect baseline and follow-up assessments. Baseline data to be collected will include age, gender, smoking status, ethnicity, ankle mobility, target ulcer assessment including size (cm2) and duration (weeks), history of diabetes, employment status, medication use, body mass index (BMI), past medical and surgical history.
The primary outcome measure will be healing of the target ulcer (including time to healing, proportion of ulcers healed, and rates of changes in ulcer area) at 12 weeks post baseline assessment. Healing will be defined as 100% epithelialisation with no exudate or scab. Ulcer healing will be measured in days from date of recruitment. Date of healing will be documented in the patient’s medical history. Ulcer size will be documented at baseline, 12 weeks and 24 weeks, and healing date recorded in the data collection form.
Secondary outcomes will comprise:
- Feasibility and acceptability of patient device wear, measured by patient satisfaction, and measured at the end of the 1-week device wear period. Responses to ease of wear, device placement, comfort during sleep, and length of time worn45 will be documented on a five-point Likert scale ranging from ‘Strongly agree’ to ‘Strongly disagree’.
- Delayed healing of target ulcer, defined as healing of the target ulcer after 12 weeks and up to 24 weeks, will be assessed at 12 and 24 weeks.
- Adherence to compression treatment, collected from both the patient’s medical history and via self-report. While compression treatment is part of routine clinical care, adherence is important to measure due to its potential confounding influence on the primary outcome of wound healing. Self-reported adherence to compression treatment will be assessed at baseline, 12 and 24 weeks and measured on a categorical scale of ‘wear everyday’, ‘wear >3 days /week’, ‘wear <2 days per week’ ‘do not wear’. Compression non-adherence will be documented in the data collection form.
- Measurement of self-reported health-related QoL, assessed at baseline, 12 and 24 weeks via the EQ-5D-5L. This instrument is a widely used generic preference-based measure of health outcomes46 suitable for economic appraisal of mobility, self-care, usual activities, pain/discomfort and anxiety/depression and self-rated health (it provides a basis for calculating preference-based health-related QoL) and calculating QALY and conducting cost-utility analysis. This instrument consists of a descriptive system together with a visual analogue 0–100 scale (0 means the worst health imaginable and 100 means the best health), with participants asked to rate five dimensions of health – mobility, self-care, usual activities, pain and anxiety/depression. Scores will be derived to indicate a status of ‘no problems’, ‘slight problems’, ‘moderate problems’, ‘severe problems’ or ‘extreme problems’. The EQ-5D-5L is valid and responsive in detecting change of VLU healing status in an Australian population47.
- Measurement of self-reported VLU pain, assessed at baseline, 12 and 24 weeks via a self-reported 11 point (0–10) numerical rating scale where scores of 0 represent ‘no pain’ and 10 represents ‘maximum pain imaginable’.
- Measurement of self-reported PA, assessed at baseline 12 and 24 weeks. Self-reported PA will be assessed using the RAPA, a nine-item questionnaire designed to assess current levels of PA. It is a valid measure of PA for use in clinical practice with older adults48. Patients will be asked to select one of seven statements to describe light, moderate or vigorous activity levels. Self-reported PA will also be assessed using the IPAQ. The IPAQ, administered at baseline, will be used to assess the types of intensity of PA and sitting time that people do as part of their daily lives which are considered to estimate total PA in MET-min/week and time spent sitting49. As there is no validated self-reported PA instrument specifically for people with VLU, we will use two approaches, the IPAQ and RAPA, to collect subjective PA measurements, thereby allowing for comparison of accuracy and consistency.
- Measurement of objectively quantified PA and sleep via wrist-worn tri-axial accelerometers (ActiGraph). ActiGraph has been reported to provide reliable, objective measurements across a range of clinical and research settings22,50 and the wrist position has been established as an accurate location for activity measurement and may be associated with improved wear-time compliance22,51,52.
Data collection procedure
The study timeline is outlined in Figure 1. The medical records for all patients will be accessed by a research nurse at baseline, 12 weeks and 24 weeks to collect ulcer healing and compression therapy adherence data. These data will be transcribed into a paper-based data collection form developed for this study. Ulcer healing will be recorded as documented in the patient’s medical history.
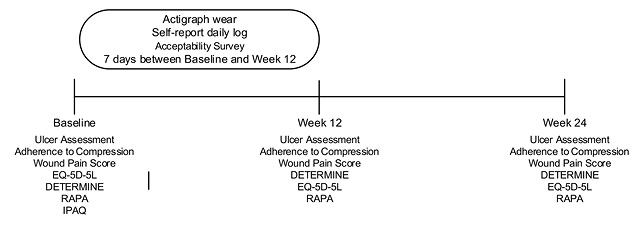
Figure 1. Study timeline. Note DETERMINE: is a questionnaire that can be used to assess individual’s nutritional risks.
All patients will complete baseline, 12- and 24-week questionnaires about compression adherence, QoL, VLU pain and PA levels. Where available, information about adherence to compression treatment will be collected from both the patient’s medical history and via the self-report questionnaire.
Participants will be asked to wear the ActiGraph device for 7 consecutive days and nights during the period between baseline and the week 12 visit (except for water-based activities). Participants will complete a daily log to record sleep and wake times and periods of device removal for longer than 15 minutes. Feasibility and acceptability of using the device data will be recorded.
Sample size
This proof of concept study does not require a sample size calculation53. We plan to recruit a convenience sample of 30 adults with diagnosed VLUs.
Data management
Data collection will be carried out using paper-based data collection instruments (Supplementary Material/Appendix). Participants will complete questionnaires; and data collectors will access participant medical records to collect data. A unique study number will be allocated to each participant and data will be entered centrally into electronic forms created in Qualtrics (Qualtrics, Provo, UT). Data will be downloaded from Qualtrics into Excel and data hygiene will be performed using predetermined coding information outlined in the data dictionary.
PA and sleep data will be imported from wActiSleep-BT devices into ActiLifeTM software54 upon return of equipment after the 7-day monitoring period. Data will first be converted from raw form (units of gravity, collected at 30Hz) into 60-second epochs to allow for efficient data analysis54. To be eligible for analysis, wear time will be validated using default Troiano (2007) algorithms, ensuring a minimum of 8 hours wear data per day on at least 4 days. Non-wear times will be excluded from analysis. Energy expenditure will be calculated using the Freedson VM3 Combination (2011) algorithm and METs defined according to the Freedson Adult (1998) algorithm. To maximise data accuracy from wrist-worn devices, an adapted set of PA cut-off thresholds will be created based on vector magnitude counts. This will be used as follows: sedentary behaviours 0–199; light activity 200–2689; moderate activity 2690–6160; vigorous activity 6161–9642; and very vigorous activity 9643 or greater.
Primary outcomes of interest from PA data include mean step count per day and minutes spent undertaking moderate-vigorous PA. Sleep will be scored using participant log diaries to denote in-bed and out-bed times. Where diary data are missing, we will use the default adult sleep detection algorithm of Cole-Kripke55 to detect sleep, with manual checks for accuracy. Primary outcomes of interest for sleep will be total sleep time, number of awakenings and sleep efficiency (%), where >85% is considered normal.
Data collection forms will be stored securely in a locked room only accessible to the research team and databases will be secured with password protected access.
Statistical methods
Parametric and non-parametric tests will be used to characterise the study sample for key demographic characteristics. The relationship between both PA (e.g., mean daily step count, time spent in moderate-vigorous PA) and sleep (e.g., total sleep time, sleep efficiency) and outcomes of ulcer healing (e.g., time to healing, proportion of ulcer healed, change in ulcer area), pain scores and QoL will be explored using Pearson’s R correlation coefficient (parametric test) or Spearman correlation (non-parametric test).
The accuracy and consistency of two self-reported PA instruments will be examined by comparing responses between the IPAQ and RAPA. Data from each instrument will be categorised as ‘sedentary-light PA and ‘moderate-vigorous PA, and the similarity of responses evaluated via a Chi-squared test.
The association between mean daily step counts and VLU healing will be examined via a combination of regression and survival analyses. Univariate linear regression will first be used to examine the strength of relationship between measures of PA (e.g., mean daily step counts, time spent physically active; exposure variable) and ulcer healing (e.g., change in wound size; dependent variable) in all participants, measured at 12 and 24 weeks. We will consider the coefficient of determination (R2) as the principal measure of interest and will interpret R2 values greater or equal to 0.5 as satisfactory explanatory power to predict ulcer healing. Survival analyses (Cox proportional hazards model) will also be conducted to evaluate the relationship between mean daily step count and time to wound healing. Similar exploratory analyses will be conducted using key sleep variables (e.g., total sleep time, sleep efficiency) as exposure variables. Adjusted multivariate models will be conducted to evaluate the potential influence of age, gender, smoking status, diabetes history, BMI and compression treatment adherence on dependent variables. Statistical significance will be denoted by p<0.05 for all analyses.
Discussion
This protocol outlines the design of a prospective observational cohort study to investigate the association between PA and sleep with ulcer healing, QoL and VLU pain in adults diagnosed with VLUs. We anticipate the results of this study will inform the feasibility of recruitment and determine sample size for a future large-scale randomised controlled trial.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study was supported by funding from Monash Nursing & Midwifery Strategic Grants Scheme 2017.
Author(s)
Yunjing Qiu BN(Hons)1
Victoria Team DrPH1
Christian R Osadnik PhD2
Jane O’Brien PhD3
Louise Turnour1
Ayoub Bouguettaya PhD1
Rosemary A McGinnes DHSc1
Carolina D Weller* PhD
Monash Nursing and Midwifery, Level 5, 99 Commercial Road, Melbourne VIC 3004 Australia
Email Carolina.Weller@monash.edu
1 School of Nursing and Midwifery, Monash University, Level 5 Alfred Centre, 99 Commercial Road, Melbourne VIC 3004 Australia
2 Department of Physiotherapy, Monash University, 47-49 Moorooduc Highway, Frankston VIC 3199 Australia
3 School of Nursing, College of Health and Medicine, University of Tasmania, Launceston TAS 7250 Australia
* Corresponding author
References
- Lal BK. Venous ulcers of the lower extremity: definition, epidemiology, and economic and social burdens. Semin Vasc Surg 2015;28(1):3–5.
- Weller C, Ademi Z, Makarounas-Kirchmann K, Stoelwinder J. Economic evaluation of compression therapy in venous leg ulcer randomised controlled trials: a systematic review. Wound Pract Res 2012;20(1):21–34.
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014;17(5):347–56.
- Ma H, Rosen NA, Iafrati MD, O’Donnell TF. The real costs of treating venous ulcers in a contemporary vascular practice. J Vasc Surg Venous Lymphat Disord 2013;1(1):105.
- Guest JF, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care 2017;26(6):292–303.
- Cheng Q, Gibb M, Graves N, Finlayson K, Pacella RE. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018;18(1):421.
- Australian Wound Management Association. An economic evaluation of compression therapy for venous leg ulcers. Canberra: Australian Wound Management Association; 2013.
- Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Pract Res 2014;22(1):20–33.
- Nelson EA, Adderley U. Venous leg ulcers. Am Fam Physician 2017;95(10):662–3.
- Finlayson K, Wu ML, Edwards HE. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: a longitudinal study. Int J Nurs Stud 2015;52(6):1042–51.
- Finlayson KJ, Parker CN, Miller C, et al. Predicting the likelihood of venous leg ulcer recurrence: the diagnostic accuracy of a newly developed risk assessment tool. Int Wound J 2018;15(5):686–94.
- Kapp S, Santamaria N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J 2017;14(6):1108–19.
- Bray G, Kim K, Wilding J, Federation WO. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obesity Reviews 2017;18(7):715–23.
- Jaacks LM, Siegel KR, Gujral UP, Narayan KV. Type 2 diabetes: a 21st century epidemic. Best Pract Res Clinical Endocrinol & Metabol 2016;30(3):331–43.
- Weller C, Evans S. Venous leg ulcer management in general practice nurses and evidence based guidelines. Aust Fam Physician 2012;41(5):331–.
- Smith D, Lane R, McGinnes R, et al. What is the effect of exercise on wound healing in patients with venous leg ulcers? A systematic review. Int Wound J 2018;15(3):441–453.
- World Health Organisation. Physical Activity. Fact Sheet 385 Geneva 2015. Available from: http://www.who.int/mediacentre/factsheets/fs385/en/.
- Australian Government Department of Health. Recommendations on physical activity for health for older Australians: Department of Health; 2013. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines#chba.
- van der Ploeg HP, Hillsdon M. Is sedentary behaviour just physical inactivity by another name? Int J Behav Nutr Phys Activity 2017;14(1):142.
- Parker CN, Finlayson KJ, Shuter P, Edwards H. Risk factors for delayed healing in venous leg ulcers: a review of the literature. Int J Clin Pract 2015;69(9):967–77.
- Chastin SFM, Dontje ML, Skelton DA, et al. Systematic comparative validation of self-report measures of sedentary time against an objective measure of postural sitting (activPAL). Int J Behav Nutr Phys Activity 2018;15(1):21.
- Gorman E, Hanson HM, Yang PH, Khan KM, Liu-Ambrose T, Ashe MC. Accelerometry analysis of physical activity and sedentary behavior in older adults: a systematic review and data analysis. Eur Rev Aging Phys Activity 2014;11:35–49.
- O’Brien J, Finlayson K, Kerr G, Edwards H. The perspectives of adults with venous leg ulcers on exercise: an exploratory study. J Wound Care 2014;23(10):496–8, 500–9.
- Heinen MM, van der Vleuten C, de Rooij MJM, Uden CJT, Evers AWM, van Achterberg T. Physical activity and adherence to compression therapy in patients with venous leg ulcers. Arch Dermatol 2007;143(10):1283–8.
- Roaldsen KS, Rollman O, Torebjörk E, Olsson E, Stanghelle JK. Functional ability in female leg ulcer patients – a challenge for physiotherapy. Physiother Res Int 2006;11(4):191–203.
- Jull A, Slark J, Parsons J. Prescribed exercise with compression vs compression alone in treating patients with venous leg ulcers: a systematic review and meta-analysis. JAMA Dermatol 2018;154(11):1304–11.
- Heinen M, Borm G, van der Vleuten C, Evers A, Oostendorp R, van Achterberg T. The Lively Legs self-management programme increased physical activity and reduced wound days in leg ulcer patients: results from a randomized controlled trial. Int J Nurs Stud 2012;49(2):151–61.
- Jull A, Parag V, Walker N, Maddison R, Kerse N, Johns T. The prepare pilot RCT of home-based progressive resistance exercises for venous leg ulcers. J Wound Care 2009;18(12):497–503.
- Meagher H, Ryan D, Clarke-Moloney M, O’Laighin G, Grace PA. An experimental study of prescribed walking in the management of venous leg ulcers. J Wound Care 2012;21(9):421–430.
- O’Brien J, Edwards H, Stewart I, Gibbs H. A home-based progressive resistance exercise programme for patients with venous leg ulcers: a feasibility study. Int Wound J 2013;10(4):389–96.
- O’Brien J, Finlayson K, Kerr G, Edwards H. Evaluating the effectiveness of a self-management exercise intervention on wound healing, functional ability and health-related quality of life outcomes in adults with venous leg ulcers: a randomised controlled trial. Int Wound J 2017;14(1):130–7.
- Klonizakis M, Tew GA, Gumber A, et al. Supervised exercise training as an adjunct therapy for venous leg ulcers: a randomised controlled feasibility trial. Br J Dermatol 2018;178(5):1072–1082.
- Mutlak O, Aslam M, Standfield NJ. An investigation of skin perfusion in venous leg ulcer after exercise. Perfusion 2018;33(1):25–9.
- Szewczyk MT, Jawien A, Cwajda-Bialasik J, Cierzniakowska K, Moscicka P, Hancke E. Randomized study assessing the influence of supervised exercises on ankle joint mobility in patients with venous leg ulcerations. Arch Med Sci 2010;6(6):956–63.
- Chokroverty S. Overview of normal sleep. Sleep Disord Med, Springer 2017;5–27.
- Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 2015;3(1):52–62.
- Guo S, DiPietro LA. Factors affecting wound healing. J Dental Res 2010;89(3):219–29.
- House SL. Psychological distress and its impact on wound healing. J Wound Ostomy Continence Nurs 2015;42(1):38–41.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol 2018;124(1):190–200.
- Finlayson K, Miaskowski C, Alexander K, et al. Distinct wound healing and quality-of-life outcomes in subgroups of patients with venous leg ulcers with different symptom cluster experiences. J Pain Symptom Manage 2017;53(5):871–879.
- Hellstrom A, Nilsson C, Nilsson A, Fagerstrom C. Leg ulcers in older people: a national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatric 2016;16(1):25–25.
- Salome GM, de Souza Pellegrino DM, Vieira TF, Blanes L, Ferreira LM. Sleep quality among patients with venous ulcers: a cross-sectional study in a health care setting in Sao Paulo, Brazil. Wounds: Compendium Clinical Res Pract 2012;24(5):124–31.
- Upton D, Andrews A. Sleep disruption in patients with chronic leg ulcers. J Wound Care 2013;22(8):389–90, 92, 94.
- Campbell M, Katikireddi SV, Hoffmann T, Armstrong R, Waters E, Craig P. TIDieR-PHP: a reporting guideline for population health and policy interventions. BMJ 2018;361:k1079.
- Huberty J, Ehlers DK, Kurka J, Ainsworth B, Buman M. Feasibility of three wearable sensors for 24 hour monitoring in middle-aged women. BMC Women Hlth 2015;15:55.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20(10):1727–36.
- Cheng Q, Kularatna S, Lee XJ, Graves N, Pacella RE. Comparison of EQ-5D-5L and SPVU-5D for measuring quality of life in patients with venous leg ulcers in an Australian setting. Qual Life Res 2019;28(7):1903–1911.
- Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prevent Chron Dis 2006;3(4):A118.
- IPAQ. International Physical Activity Questionnaire 2017. Available from: https://sites.google.com/site/theipaq/.
- LaMonte MJ, Buchner DM, Rillamas-Sun E, et al. Accelerometer-measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc 2018;66(5):886–894.
- Rosenberger ME, Buman MP, Haskell WL, McConnell MV, Carstensen LL. Twenty-four hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sports Exerc 2016;48(3):457–65.
- Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sport Med 2017;47(9):1821–1845.
- Larkin L, Gallagher S, Fraser A, Kennedy N. Community-based intervention to promote physical activity in rheumatoid arthritis (CIPPA-RA): a study protocol for a pilot randomised control trial. Int J Rheum Dis 2017;37(12):2095–103.
- ActiGraph Software Department. ActiLife 6 users’ manual. Pensacola, Florida: Actigraph; 2012. Available from: https://actigraphcorp.com/support/manuals/actilife-6-manual/.
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep 1992;15(5):461–9.



