Volume 29 Number 3
Wound dressings for treating radiation dermatitis: a WHAM evidence summary
Emily Haesler
For referencing Haesler E for Wound Healing and Management Unit. Wound dressings for treating radiation dermatitis: a WHAM evidence summary. Wound Practice and Research 2021; 29(3):176-179.
DOI https://doi.org/10.33235/wpr.29.3.176-179
CLINICAL QUESTION
What is the best available evidence for wound dressings and barrier films for treating radiation dermatitis in people undergoing radiation therapy for cancer?
SUMMARY
Radiation dermatitis (RD) is an acute skin reaction that occurs as a result of radiotherapy used to treat a range of different cancers. Severity of symptoms ranges from erythema to dry desquamation (dry flaky skin with itching) to moist desquamation (serous exudate, oedema and blistering). There is minimal evidence on the effectiveness of wound dressings for treating RD, and the available evidence for most dressing types is minimal, conflicting and/or at high risk of bias. Level 1 evidence1, 2 suggested using a soft silicone foam dressing to treat moist desquamation was not associated with faster healing but might reduce some signs and symptoms of RD, including pain. Level 3 evidence3 provided support for using a standardised protocol to manage RD, with wound dressings selected based on the severity of RD.
CLINICAL PRACTICE RECOMMENDATIONS
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
A soft silicone foam dressing could be used on existing radiation dermatitis to reduce the impact of some signs and symptoms of more severe radiation dermatitis (Grade B).
There is no strong evidence to support the use of any specific wound dressing for healing existing radiation dermatitis.
SOURCES OF EVIDENCE
This summary was conducted using methods published by the Joanna Briggs Institute.4-6 The summary is based on a systematic literature search combining search terms related to radiation dermatitis/radiodermatitis and wound dressings and barrier films. Searches were conducted in Embase, Medline, Pubmed, the Cochrane Library and Google Scholar for evidence published up to January 2021 in English. Levels of evidence for intervention studies are reported in the table below.
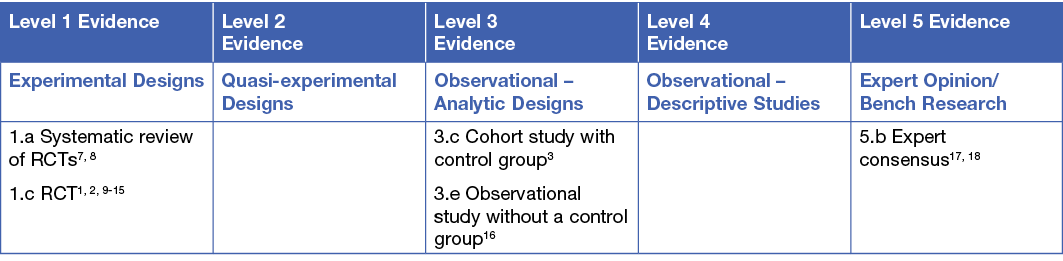
BACKGROUND
Radiation dermatitis is a common side effect of radiotherapy, which is a type of therapy delivered in the management of cancer. Radiation causes damage to epithelial cells and underlying structures of the skin, usually commencing early during radiotherapy and persisting up to six months following radiotherapy.19, 20 The severity of RD is related to the dose and regimen of radiation and the area of skin over which radiotherapy is administered,19-21 increasing when cell destruction occurs faster than normal cell reproduction. In early stages of RD the skin becomes warmer, itchy and erythema may present. As cumulative exposure to radiation increases, old skin becomes dry and flaky (referred to as dry desquamation). When the rate of new skin cell production cannot replace shedding cells the epidermis breaks down, becomes oedematous and exudate is present (referred to as moist desquamation).20 Pain, skin warmth, pruritus, burning sensations are reported by people experiencing RD.7 Consistent with outcome measures reported in the evidence, when referring to ‘grade’ of RD this evidence summary uses the Radiation Therapy Oncology Group (RTOG) scale for categorising the severity acute of RD.22 Wound dressings are used to protect the skin and wound, actively manage signs and symptoms while promoting rapid healing of damaged skin.7
CLINICAL EVIDENCE
Evidence for wound dressings in treating RD reported on soft silicone foam dressing, transparent soft silicone film dressings, silver nylon dressings, polymeric membrane dressings and hydrogel dressings. Two systematic reviews (SRs)7, 8 reported summaries of studies reported in this evidence summary, with both reviews concluding there is insufficient evidence to recommend any specific dressings for promoting healing of RD (Level 1). Consensus panels17, 18 also concluded that the evidence on dressings for treating RD is insufficient, but note that use of a dressing could continue to prophylactically prevent progression to more severe RD17 (Level 5). The evidence addresses a range of outcome measures beyond healing specifically, and several studies reported improvements in signs and symptoms that might be considered significant to people experiencing RD.
In recognition of the changing treatment requirements for skin based on clinical assessment, a retrospectively controlled cohort study investigated use of a standardised skin protocol based on severity of RD. This study noted that a protocol of topical treatment and dressing selection that was standardised based on clinical presentation was associated with statistically significantly (p < 0.0001) fewer interruptions to radiotherapy due to skin complications, but wound healing outcomes were not reported.3 (Level 3).
Soft silicone foam dressing
Two RCTs,1, 2 plus the pilot study14 for one of the RCTs provide evidence on soft silicone foam dressing† for treating RD. In the first RCT,1 foam dressing was compared to standard care (wound cleansing with saline)1 in people undergoing radiotherapy for head and neck cancer who had moist desquamation (n = 88). Time to healing was statistically significantly faster in the foam dressing group (median 16 days, 95% CI 12 to 19 days versus median 23 days, 95% CI 19 to 27, p = 0.009).1 The second RCT,2 which compared the foam dressing to aqueous cream in women undergoing radiotherapy for breast cancer who experienced erythema (n = 80) found no statistically significant difference in average time to healing (11 ± 2 days for dressing versus 13 ± 2 days for aqueous cream, p = 0.49). Progression to moist desquamation was also not statistically significantly different (15% for foam versus 19% for aqueous cream, p > 0.05). However, total time with moist desquamation was lower for those treated with the soft silicone foam dressing (18 weeks versus 25 weeks)2 (both Level 1).
Both studies reported favourable outcomes for the foam dressing with respect to other outcome measures. In one study,1 the average increase in RISRAS scores for RD severity were statistically significantly lower in the foam dressing group (p = 0.009), as were score for wound pain (p = 0.02) and sleep (p = 0.005). In the second study,2 the RISRAS score favoured use of the foam dressing for managing moist desquamation (0.18 versus 0.37, p = 0.043).2 In the smaller pilot study (n = 24), similar statistically significant findings were noted for total RISRAS score (p < 0.001) and erythema score (p°<°0.001).14 (all Level 1).
Zhong et. al. (2013)1 noted no significant difference in neck mobility (p = 0.56) or appearance (p = 0.12) scores associated with using a foam dressing, and Diggelmann et. al. (2010)14 confirmed that the foam dressing was not associated with either build-up or bolus radiation dose (both Level 1). This evidence suggests there may be some benefit to using a foam dressing for managing some signs and symptoms of RD, but this evidence is insufficient and did not show any benefit for improving healing.
Hydrogel dressing
Two RCTs9, 10 investigated a hydrogel dressing for treating moist desquamation, the first compared hydrogel** to a gentian violet dressing in people with the breast, neck or head (n = 30)9 and the second compared hydrogel†† to a simple dry dressing for people with RD in the head, neck, breast and anorectal regions (n = 100).10 The results from the two studies were conflicting. In the largest study, 10 time to healing was significantly slower in those receiving hydrogel (p = 0.03) versus a dry dressing. In contrast, the smaller study9 reported that time to healing was statistically significantly faster when using the hydrogel dressing (hazard ratio = 7.95, 95% CI 2.20 to 28.68, p = 0.002). This study also noted that the hydrogel was associated with a difference in mean healing time of over two weeks, which is clinically significant9 (both Level 1).
Overall, the evidence was insufficient to make any recommendations, particularly considering the risk of bias. One study was very small and likely to be underpowered, 9 and design aspects of the second study may have contributed to the less-than-optimal performance of the hydrogel dressing.10
Transparent soft silicone film dressing
In one RCT, conducted in people with head and neck cancer who acted as their own controls, a transparent soft silicone film dressing‡ was compared to a topical preparation. Treatment commenced prophylactically when radiotherapy started, and for one cohort (n = 11) was continued as a treatment for dry desquamation. The transparent soft silicone film dressing was associated with statistically significantly greater improvement in severity of RD assessed using the RISRAS score (29% improvement, 3.23 ± 0.41 versus 4.32 ± 0.48, p < 0.005). Participants reported the transparent soft silicone film dressing was associated with decreased pain and burning sensations11 (Level 1).
Silver nylon dressing
A small (n = 12) study13 compared silverleaf nylon dressing^ to silver sulfadiazine for treating grade 2 RD in people undergoing radiation therapy for head and neck cancer. The two groups showed no statistically significant differences in severity of RD13 (Level 1). A larger RCT15 (n = 194) compared silverleaf nylon dressing to standard care for treating grade 2 RD in people undergoing radiation therapy for breast cancer. As with the smaller trial,13 there was no statistically significant difference in severity of RD15 (Level 1). There is no current evidence suggesting there is a specific clinical benefit to using a silverleaf nylon dressing to treat RD.
Polymeric membrane dressing
One observational study16 (n = 20) reported participant experiences of pain and sleep while using a polymeric membrane dressing*** for moderate-to-severe RD over a four-week period. In the first 14 days, mean pain scores on a numerical scale reduced from around 6/10 to approximately 1.8/10. Participants self-reported increased sleep. No objective data on healing rates was reported16 (Level 3). This evidence was insufficient to support a specific recommendation to use a polymeric membrane dressing to treat RD.
Nonadherent, non-absorbent dressing
One RCT11 (n = 146) reported on the effectiveness for treating RD of a non-adherent, absorbent dressing††† compared to a gentian violet dressing for treating RD in people receiving radiotherapy for nasopharyngeal cancer. For the primary outcome, wound healing time (defined as absence of moist desquamation and burning), there was no statistically significant difference between the nonadherent dressing and the gentian violet dressing (median = 14 days, 95% CI 12 to 14 versus 14 days, 95% CI 12 to 16, p = 0.09). There was a trend for the nonadherent dressing to be associated with less severe pain (p = 0.07). Sleep, neck mobility, and psychosocial outcomes were not statistically significantly different12 (Level 1).
CONSIDERATIONS FOR USE
- Selection of a wound dressing should be made with consideration to the goals of care, which may differ based on severity of the person’s RD.3 Consider:
- Ability of the dressing to manage exudate is a consideration for moist desquamation3, 11
- Debridement properties of the dressing should be considered when wound debridement is required but the patient cannot tolerate mechanical debridement techniques.3
- Transparent soft silicone film dressings and soft silicone foam dressings can remain in situ during radiotherapy.3, 14
FUNDING
The development of WHAM evidence summaries is supported by a grant from The Western Australian Nurses Memorial Charitable Trust.
CONFLICTS OF INTEREST
The author declares no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
ABOUT WHAM EVIDENCE SUMMARIES
WHAM evidence summaries are consistent with methodology published in Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach, Worldviews Evid Based Nurs. 2015;12(3):131-8. Methods are provided in detail in resources published by the Joanna Briggs Institute as cited in this evidence summary. WHAM evidence summaries undergo peer-review by an international multidisciplinary Expert Reference Group. More information: https://healthsciences.curtin.edu.au/health-sciences-research/research-institutes-centres/wceihp/
WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information.
Copyright © 2021 Wound Healing and Management Unit, Curtin University.
†Mepilex® Lite (Mölnlycke, Gothenberg, Sweden)
**2nd Skin® Aquaheal® (Spenco®, USA)
††Intrasite™ Gel (Smith & Nephew, USA)
‡Mepitel® Film (Mölnlycke, Gothenberg, Sweden)
^Silverleaf nylon dressing (Vomaris [formerly Silverleaf Medical Products], Tempe, AZ)
***PolyMem® (PolyMem, Texas, USA)
†††Product not named
Author(s)
Author Adj. Prof. Emily Haesler PhD
Wound Healing and Management Unit, Curtin University
Email emily.haesler@curtin.edu.au
References
- Zhong WH, Tang QF, Hu LY, Feng HX. Mepilex Lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: a systematic controlled clinical trial. Med Oncol, 2013. Dec;30(4):761.
- Paterson P, Poonam P, Bennett NC, Peszynski RI, Beekhuizen MV, Jasperse M, editors. Randomized intra-patient controlled trial of Mepilex Lite dressings versus aqueous cream in managing radiation-induced skin reactions; 2012.
- Bonomo P, Desideri I, Loi M, Ciccone LP, Lo Russo M, Becherini C, Greto D, Simontacchi G, Pimpinelli N, Livi L. Management of severe bio-radiation dermatitis induced by radiotherapy and cetuximab in patients with head and neck cancer: emphasizing the role of calcium alginate dressings. Support Care Cancer, 2019; 27(8):2957-67.
- Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual. https://reviewersmanual.joannabriggs.org/ The Joanna Briggs Institute; 2017.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. New JBI Grades of Recommendation. Adelaide: Joanna Briggs Institute; 2013.
- The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. www.joannabriggs.org: The Joanna Briggs Institute; 2014.
- Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer, 2014;14:53.
- Fernández-Castro M, Martín-Gil B, Peña-García I, López-Vallecillo M, García-Puig ME. Effectiveness of semi-permeable dressings to treat radiation-induced skin reactions. A systematic review. Eur J Cancer Care (Engl), 2017;26(6).
- Gollins S, Gaffney C, Slade S, Swindell R. RCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamation. J Wound Care, 2008;17(6):268-70, 72, 74-75.
- Macmillan MS, Wells M, MacBride S, Raab GM, Munro A, MacDougall H. Randomized comparison of dry dressings versus hydrogel in management of radiation-induced moist desquamation. Int J Radiat Oncol Biol Phys, 2007;68(3):864-72.
- Wooding H, Yan J, Yuan L, Chyou TY, Gao S, Ward I, al. e. The effect of mepitel film on acute radiation-induced skin reactions in head and neck cancer patients: a feasibility study. Br J Radiol, 2018;91:bjr.20170298.
- Mak SS, Zee CY, Molassiotis A, Chan SJ, Leung SF, Mo KF, al. e. A comparison of wound treatments in nasopharyngeal cancer patients receiving radiation therapy. Cancer Nurs, 2005;28(6):436–45.
- Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngol Head Neck Surg, 2008;37(1):124-9.
- Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol, 2010; 83(995):971-8.
- Aquino-Parsons C, Lomas S, Smith K, Hayes J, Lew S, Bates AT, MacDonald AG. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Radiat Sci, 2010. December;41(4):215-21.
- Scott A. Polymeric membrane dressings for radiotherapy-induced skin damage. Br J Nurs, 2014;23(10):S24, s6-31.
- Russi EG, Moretto F, Rampino M, Benasso M, Bacigalupo A, De Sanctis V, Numico G, Bossi P, Buglione M, Lombardo A, Airoldi M, Merlano MC, Licitra L, Denaro N, Pergolizzi S, Pinto C, Bensadoun RJ, Girolomoni G, Langendijk JA. Acute skin toxicity management in head and neck cancer patients treated with radiotherapy and chemotherapy or EGFR inhibitors: Literature review and consensus. Crit Rev Oncol Hematol, 2015;96(1):167-82.
- Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, Eaby-Sandy B, Lacouture ME. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer, 2013;21(10):2933-48.
- Berger A, Regueiro C, Hijal T, Pasquier D, De La Fuente C, Le Tinier F, Coche-Dequeant B, Lartigau E, Moyal D, Seite S, Bensadoun RJ. Interest of supportive and barrier protective skin care products in the daily prevention and treatment of cutaneous toxicity during radiotherapy for breast cancer. Breast Cancer, 2018;12 (no pagination).
- The Princess Royal Radiotherapy Review Team. Managing Radiotherapy Induced Skin Reactions. UK: St James’s Institute of Oncology, The Leeds Teaching Hospitals NHS Trust; 2011.
- Chen MF, Chen WC, Lai CH, Hung CH, Liu KC, Cheng YH. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer, 2010; 10: 508.
- Collins A. Assessment and management of radiotherapy-induced skin reactions. Wounds UK, 2018;14(4):64-70.



