Volume 30 Number 1
Barrier film for prevention and treatment of radiation dermatitis: a WHAM evidence summary
Author Adj. Prof. Emily Haesler
For referencing For referencing Haesler E for Wound Healing and Management Unit. Barrier film for prevention and treatment of radiation dermatitis: a WHAM evidence summary. Wound Practice and Research 2022; 30(1):62-64.
DOI https://doi.org/10.33235/wpr.30.1.62-64
![]()
CLINICAL QUESTION
What is the best available evidence for barrier films for preventing and treating radiation dermatitis in people undergoing radiation therapy for cancer?
SUMMARY
Radiation dermatitis (RD) is an acute skin reaction that occurs as a result of radiotherapy used to treat a range of different cancers. Severity of symptoms ranges from erythema to dry desquamation (dry flaky skin with itching) to moist desquamation (serous exudate, oedema and blistering). Level 1 evidence1-3 reporting effectiveness of barrier films for preventing RD. Some studies2-4 showed statistically significant reductions in severity of RD and reduction in patient-reported symptoms.; however, the volume of evidence was small. Largest effect was achieved with a silicone-based barrier film forming gel dressing that was associated with a 41% reduction in risk of grade 2 RD.4 Level 1 and 3 evidence4, 5 failed to demonstrate a benefit in using a barrier film to treat existing RD.
CLINICAL PRACTICE RECOMMENDATIONS
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
A barrier film could be applied as prophylactic protection to reduce the incidence of radiation dermatitis (Grade B).
There is no strong evidence to support the use of a barrier film for healing existing radiation dermatitis.
SOURCES OF EVIDENCE
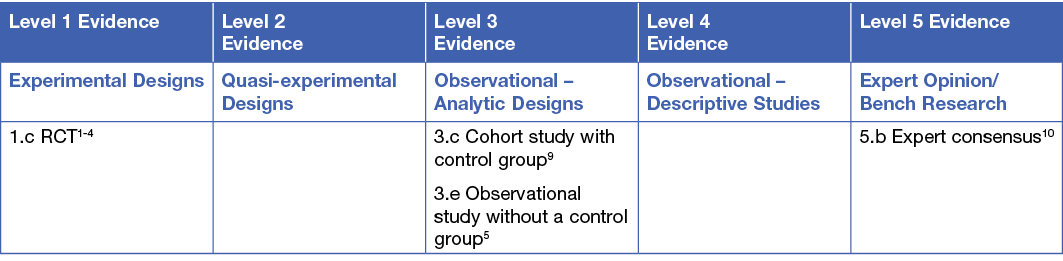
This summary was conducted using methods published by the Joanna Briggs Institute.6-8 The summary is based on a systematic literature search combining search terms related to radiation dermatitis/radiodermatitis and wound dressings and barrier films. Searches were conducted in Embase, Medline, Pubmed, the Cochrane Library and Google Scholar for evidence published up to January 2021 in English. Levels of evidence for intervention studies are reported in the table below.
BACKGROUND
Radiation dermatitis is a common side effect of radiotherapy, which is a type of therapy delivered in the management of cancer. Radiation causes damage to epithelial cells and underlying structures of the skin, usually commencing early during radiotherapy and persisting up to six months following radiotherapy.11, 12 The severity of RD is related to the dose and regimen of radiation and the area of skin over which radiotherapy is administered,11-13 increasing when cell destruction occurs faster than normal cell reproduction. In early stages of RD the skin becomes warmer, itchy and erythema may present. As cumulative exposure to radiation increases, old skin becomes dry and flaky (referred to as dry desquamation). When the rate of new skin cell production cannot replace shedding cells the epidermis breaks down, becomes oedematous and exudate is present (referred to as moist desquamation).12 Pain, skin warmth, pruritus, burning sensations are reported by people experiencing RD.14 Consistent with outcome measures reported in the evidence, when referring to ‘grade’ of RD this evidence summary uses the Radiation Therapy Oncology Group (RTOG) scale for categorising the severity acute of RD.15
Barrier films are applied to the skin in a spray, liquid or gel and dry to form a protective film coating the skin that protect the skin and wound.14
CLINICAL EVIDENCE
Barrier film for preventing radiation dermatitis
Three studies reported on the same no-sting barrier film product† for preventing RD. The barrier film was compared to Sorbolene cream (n = 61, participants acted as own controls) in an RCT conducted with women undergoing radiotherapy for breast cancer. Sorbolene was applied twice daily and the barrier film was applied 2—3 times weekly, with treatments continuing until either moist desquamation occurred or two weeks after radiotherapy completion. The barrier film was statistically significantly superior based on RTOG scores (p = 0.005) and pruritus (p = 0.011). Pain scores were not statistically significantly difference2 (Level 1).
In a multi-arm RCT, participants acted as their own controls to compare the barrier film to corticosteroid (n = 17 in arm) or to no treatment (n = 13 in arm). Compared to topical corticosteroids, people using the barrier film showed no statistically significant difference in time to occurrence of grade 1 RD (32.4 days versus 28.4 days, n = 0.072). However, the barrier film was inferior to corticosteroid for time until occurrence of grade 2 RD (44.5 days versus 53.4 days, p = 0.002). The barrier film was not statistically different to no treatment for time to occurrence of grade 1 RD (32.5 versus 29.4, p = 0.079) or grade 2 RD (44.2 versus 46.6, p = 0.196). There was also no significant difference in either arm for pain outcomes1 (Level 1).
In a third RCT, the barrier film was compared to a regimen of skin hygiene, moisturiser and topical corticosteroid (n = 79 analysed, participants acted as own controls). Skin regions receiving the barrier film were statistically significantly less likely to experience sensitivity (p < 0.001) , pain (p < 0.001), burning sensation (p = 0.005) or oedema (p = 0.005)3 (Level 1).
One RCT (n = 189)4 reported on a silicone-based barrier film forming gel dressing;** for preventing RD. The comparator group received Sorbolene cream. The participants receiving the barrier film forming gel had four weeks free from RD compared to a median of three weeks for Sorbolene, equating to a 41% risk reduction for grade 2 RD4 (Level 1).
Barrier film for treating existing radiation dermatitis
Two studies4, 5 reported on a silicone-based barrier film forming gel4 dressing;†† one of these studies compared the barrier film to Sorbolene cream4 (Level 1) and the second undertook an observational assessment5 (Level 3). The observation study (n = 54) found that there was no statistically significant improvement in dry desquamation (p = 0.40) or moist desquamation (p = 0.589)5 (Level 3). In the larger RCT (n = 189),4 mean RD severity grade over a maximum of four weeks was lower in the barrier film-forming dressing group (mean = 2.4, 95% CI 2.2 to 2.6 versus mean = 2.7, 95% CI 2.5 to 3.0, p = 0.002), and the dressing was associated with slower progression to RD severity of grade 3 (6 weeks versus 5 weeks)4 (Level 1). The observational study5 showed a statistically significant 16.9% improvement in clinical condition (p < 0.05) using the Radiation-Induced Skin Reaction Assessment Scale (RISRAS) score, with improvement noted in erythema, pain, pruritus, burning, inflammation and skin hydration5 (Level 3). However, the RCT4 found no statistically significant difference in scores for pruritus, pain or quality of life4 (Level 1).
The findings from these two studies4, 5 showed weak evidence that this silicone-based barrier film forming gel dressing was associated with statistically significantly faster healing of RD, although the differences may not be clinically significant. A small amount of evidence5 suggested that progression to more severe RD might be delayed, and evidence4, 5 was conflicting on the impact of this product on associated skin signs and symptoms.
CONSIDERATIONS FOR USE
- Selection of a barrier film should be made with consideration to the goals of care, which may differ based on severity of the person’s RD.9
- Silicone-based barrier film forming gel dressing does not need to be removed prior to undergoing radiation therapy.10
- Ability for a dressing to manage exudate is a consideration for people with moist desquamation.9
- The average cost of a no-sting barrier film regimen was comparable to treatment with Sorbolene in Australia in 2004.2
FUNDING
The development of WHAM evidence summaries is supported by a grant from The Western Australian Nurses Memorial Charitable Trust.
CONFLICTS OF INTEREST
The author declares no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
ABOUT WHAM EVIDENCE SUMMARIES
WHAM evidence summaries are consistent with methodology published in Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach, Worldviews Evid Based Nurs. 2015;12(3):131-8. Methods are provided in detail in resources published by the Joanna Briggs Institute as cited in this evidence summary. WHAM evidence summaries undergo peer-review by an international multidisciplinary Expert Reference Group. More information: https://healthsciences.curtin.edu.au/health-sciences-research/research-institutes-centres/wceihp/
WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information.
Copyright © 2022 Wound Healing and Management Unit, Curtin University.
Author(s)
Author Adj. Prof. Emily Haesler, PhD. Wound Healing and Management Unit, Curtin University
Email emily.haesler@curtin.edu.au
References
- Shaw SZ, Nien HH, Wu CJ, Lui LT, Su JF, Lang CH. 3M Cavilon No-Sting Barrier Film or topical corticosteroid (mometasone furoate) for protection against radiation dermatitis: A clinical trial. Journal of the Formosan Medical Association, 2015;114(5):407-14.
- Graham P, Browne L, Capp A, Fox C, Graham J, Hollis J, Nasser E. Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys, 2004;58(1):241-6.
- Møller PK, Olling K, Berg M, Habæk I, Haislund B, Iversen A-M, al. e. Breast cancer patients report reduced sensitivity and pain using a barrier film during radiotherapy: Danish intra-patient randomized multicentre study. Tech Innov Patient Support Radiat Oncol, 2018;7:20e5.
- Chan RJ, Blades R, Jones L, Downer TR, Peet SC, Button E, Wyld D, McPhail S, Doolan M, Yates P. A single-blind, randomised controlled trial of StrataXRT® - A silicone-based film-forming gel dressing for prophylaxis and management of radiation dermatitis in patients with head and neck cancer. Radiother Oncol, 2019;139:72-8.
- Quilis A, Martin J, Rodriguez C, Sanchez P, Ribes JL. Reducing radiation dermatitis during ongoing radiation therapy: an innovative film-forming wound dressing. J Radiat Oncol, 2018;7(3):255-64.
- Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual. https://reviewersmanual.joannabriggs.org/ The Joanna Briggs Institute. 2017.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. New JBI Grades of Recommendation. 2013. Adelaide: Joanna Briggs Institute.
- The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. 2014. www.joannabriggs.org: The Joanna Briggs Institute.
- Bonomo P, Desideri I, Loi M, Ciccone LP, Lo Russo M, Becherini C, Greto D, Simontacchi G, Pimpinelli N, Livi L. Management of severe bio-radiation dermatitis induced by radiotherapy and cetuximab in patients with head and neck cancer: emphasizing the role of calcium alginate dressings. Support Care Cancer, 2019;27(8):2957-67.
- International Society for Nurses in Cancer Care. Evidence-based Guidelines for the Prevention and Management of Radiation Dermatitis. 2021: International Society for Nurses in Cancer Care.
- Berger A, Regueiro C, Hijal T, Pasquier D, De La Fuente C, Le Tinier F, Coche-Dequeant B, Lartigau E, Moyal D, Seite S, Bensadoun RJ. Interest of supportive and barrier protective skin care products in the daily prevention and treatment of cutaneous toxicity during radiotherapy for breast cancer. Breast Cancer, 2018;12(no pagination).
- The Princess Royal Radiotherapy Review Team. Managing Radiotherapy Induced Skin Reactions. 2011. UK: St James’s Institute of Oncology, The Leeds Teaching Hospitals NHS Trust.
- Chen MF, Chen WC, Lai CH, Hung CH, Liu KC, Cheng YH. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer, 2010;10 (no pagination) (508).
- Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer, 2014;14:53.
- Collins A. Assessment and management of radiotherapy-induced skin reactions. Wounds UK, 2018;14(4):64-70.


