Volume 30 Number 1
Pathergy: a review of potential mechanisms and novel therapeutic targets
Anthony Honigman, Johannes S Kern and John W Frew
Keywords Pyoderma gangrenosum, wound healing, pathergy, isomorphic phenomenon, Behçet’s disease
For referencing Honigman A et al. Pathergy: a review of potential mechanisms and novel therapeutic targets. Wound Practice and Research 2022; 30(1):55-61.
DOI
https://doi.org/10.33235/wpr.30.1.55-61
Submitted 5 October 2021
Accepted 23 November 2021
Abstract
Pathergy reaction is the phenomenon of formation of non-healing skin lesions or ulcers following minor injuries. Although conceptually similar to the isomorphic reaction (Koebnerisation), these are two separate phenomena that should be distinguishable to treating clinicians. The underlying pathomechanisms of pathergy are not yet fully understood and subsequently therapy is lacking. Recent advances in the understanding of wound healing through keratinocyte and fibroblast cross-talk and mesenchymal stem cell (MSC) may hopefully foster the development of novel targeted therapies for pathergy-associated wounds and diseases in the near future.
Introduction
Pathergy is a term used to describe hyper-reactivity of the skin that occurs in response to minimal trauma1,2. It is a reaction characterised by non-specific pustules or papules, or enlargement of pre-existing wounds, developing on sites of minor trauma, including blunt trauma. Pathergy is characteristic for pyoderma gangrenosum (PG) and other neutrophilic skin conditions such as Behçet’s disease (BD) and Sweet’s syndrome. Development of a monocytic and neutrophilic cell infiltrate without true vasculitis can be seen on histopathology. It can be elicited via a pathergy test, which leads to the production of an erythematous papule at the site of a skin prick and intradermal injection of saline solution1. The lesions arising from pathergy tend to be non-specific papules or pustules that ultimately may develop in skin ulcers1. This review focuses on the potential underlying mechanisms involved in pathergy and examines novel mesenchymal stem cell (MSC)-based therapy to successfully treat non-healing chronic wounds.
Characteristics
Comparing Koebner phenomenon and pathergy
Koebner phenomenon and pathergy are conceptually similar yet completely separate entities. Koebner phenomenon is the appearance of new skin lesions on previously unaffected skin secondary to trauma, for example in psoriasis. These new lesions are both clinically and histologically identical to the patient’s underlying cutaneous disease3,4. The lesions seen in Koebner phenomenon adopt the same clinical and histological features as the patients’ original skin disease, hence why this condition is also termed the isomorphic response (from Greek: ‘equal shape’). Mechanisms are thought to be related to the presence of T resident memory cells (TRM) in previously affected sites of cutaneous disease.
Disease associations and clinical significance of pathergy
Pathergy is seen in a range of chronic cutaneous diseases across the dermatology specialty. It is therefore imperative for all practising dermatologists, as well as clinicians involved in wound care, to be aware of the pathergy response and understand the proposed underlying pathomechanisms involved. A greater understanding of pathergy and pathergy-associated reactions is of essential importance for initiating treatment and minimising occurrence of painful chronic wounds which may become necrotic or secondarily infected or may scar. Pathergy-associated diseases such as PG have an increased morbidity and mortality rate when compared to the general population and this, coupled with the unpredictability and chronic nature of the disease, may place a burden on the healthcare system5. A comparison of the aetiologies, clinical presentation, proposed pathogenesis and diagnosis of these pathergy-associated diseases can be seen in Table 1.
Table 1. Comparison of pathergy-associated disease
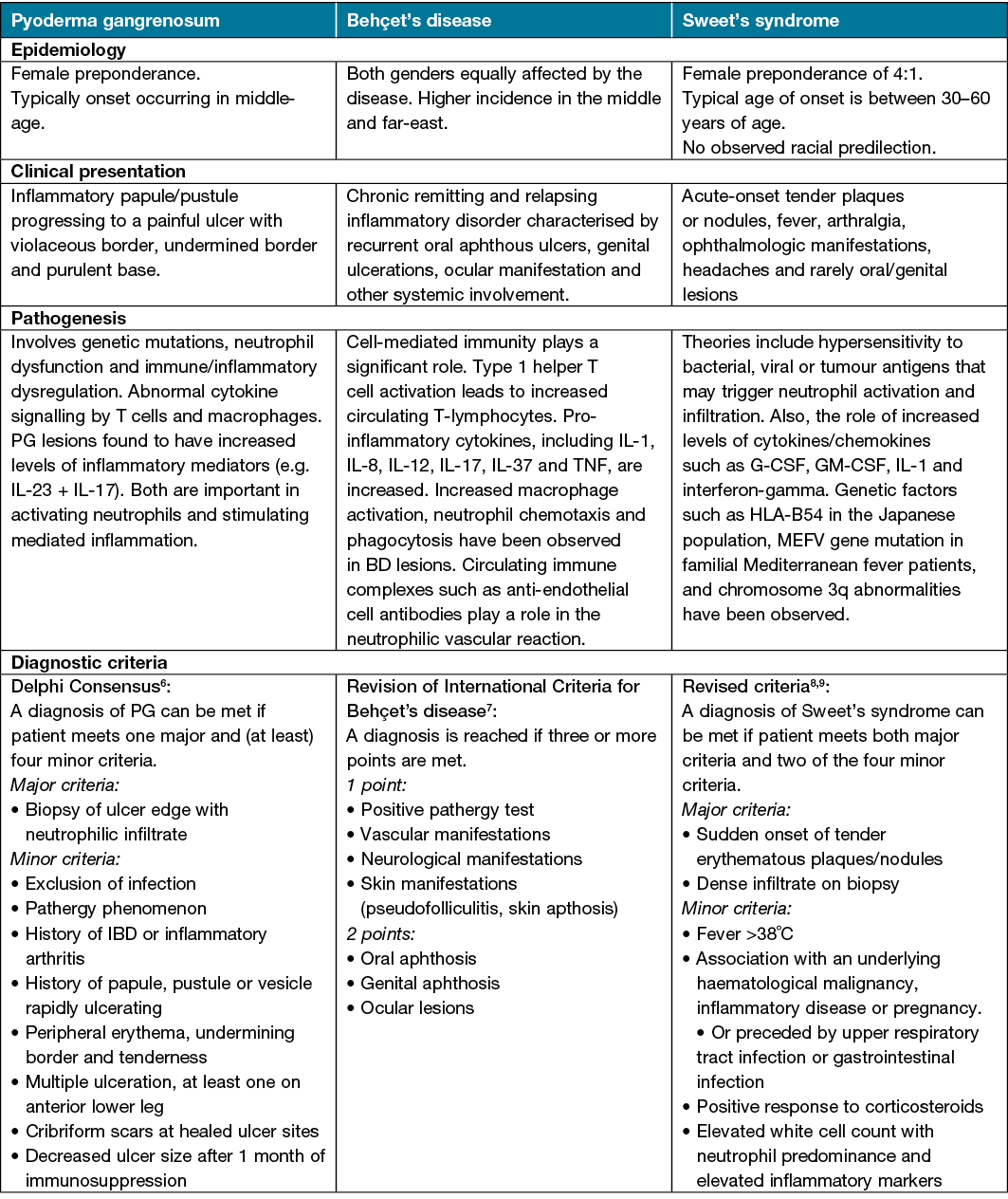
Pyoderma gangrenosum (PG)
PG is a rare, chronic inflammatory skin disease characterised by rapidly progressing painful pustules which progressively break down, forming larger ulcers with violaceous undermined borders. The incidence of disease ranges from 0.3–5.8 per 100,000 individuals, with an increased mortality rate when compared with the general population5,10. Pathergy is seen in 25–50% of patients with PG and is more common in PG associated with systemic disease11. Pathergy may be triggered by incidental or iatrogenic trauma. Examples of pathergy-induced PG include wound infection, surgical procedures that include caesarean section, breast reduction, central line insertion and stoma formation12,13.
Behçet’s disease (BD)
BD is universally recognised as a multisystemic inflammatory disease of unknown aetiology with chronic course and unpredictable exacerbations; its clinical spectrum varies from pure vasculitic manifestations with thrombotic complications to inflammatory involvement of multiple organs and tissues, including orogenital mucosa, skin and eyes14.
Although the pathergy phenomenon is seen in various disease entities, pathergy testing is only indicated in establishing the diagnosis of BD. A positive skin pathergy test (SPT) or skin pathergy reaction (SPR) is a hyper-reactivity response to needle-induced trauma, characterised by papule or pustule formation within 24–48 hours after sterile needle prick. The aim of the SPT is the generation of pathergy lesions in BD patients by administration of a minimal skin puncturing trauma. Positive SPT is the only diagnostic test for BD and is one of the minor criteria for BD diagnosis; it has been derived by the International Study Group of Behçet’s Disease15. Several studies have shown higher positive SPT rates in those with active disease16–18.
Sweet’s syndrome
The development of pathergy lesions has also been reported in other neutrophilic dermatoses, including Sweet’s syndrome. The syndrome is characterised by a constellation of clinical symptoms, physical features and pathologic findings which include fever, neutrophilia, tender erythematous cutaneous lesions and a diffuse infiltrate consisting predominantly of mature neutrophils typically located in the upper dermis19. Cutaneous pathergy at sites of trauma have been reported in the literature and include sites where procedures such as biopsies, intravenous catheters placement, vaccination and venepuncture have been performed, as well as at locations of animal scratches and insect bites20–22.
Pathomechanisms of pathergy
Despite being well-known to dermatologists since its first description in 1937, the mechanisms underlying the pathergy phenomenon and its aetiology is not yet fully understood. It has been suggested that pathergy may be driven by either a non-specific hyperinflammatory response to traumatic insult, an exaggerated response to microbial antigens, or interaction between genetic and environmental factors.
Normal wound healing versus pathergy
Wound healing, as a normal biological process in the human body, is achieved through a number of precisely and highly programmed phases: 1) rapid haemostasis; 2) appropriate inflammation; 3) mesenchymal cell differentiation, proliferation and migration to the wound site; 4) suitable angiogenesis; 5) prompt re-epithelialisation (re-growth of epithelial tissue over the wound surface); and 6) proper synthesis, cross-linking and alignment of collagen to provide strength to the healing tissue23. This process involves a continuous sequence of signals and responses in which platelets, fibroblasts, epithelial, endothelial and immune cells come together outside their usual domains to orchestrate a very complex event that results in tissue repair. These signals, which are mainly growth factors and cytokines, orchestrate the initiation, continuation and termination of wound healing24,25.
After epidermal injury, there is an immediate release of inflammatory mediators from damaged cells and the induction of an acute inflammatory response (Figure 1:1). Degranulating platelets, resident tissue macrophages and mast cells also release mediators into the tissue milieu causing arteriolar dilatation, resulting in increased blood flow to the area1,26.
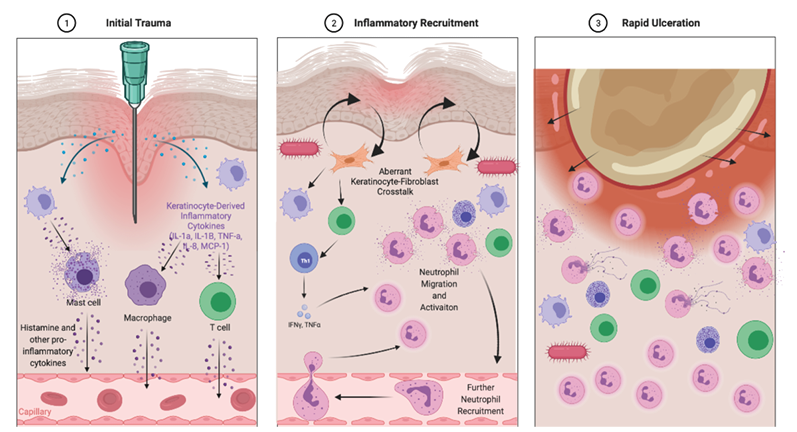
Figure 1. Schematic representation of the proposed pathogenesis of pathergy and deviations from the normal process of wound healing
1=initial trauma results in the release in keratinocyte-derived inflammatory cytokines which lead to stimulation and activation of a variety of resident inflammatory cells. These then signal via other chemokines including histamine to promote inflammation. An increase or aberration in the type of inflammation present is the first potential step which may produce a pathergy-like response
2=inflammatory recruitment from the circulation into the tissue primarily consists of neutrophils which interact with T cells, macrophages and fibroblasts to promote a persistent pro-inflammatory response. The presence of bacteria may also contribute to the ongoing inflammatory response via mediaxtors such as TLR5 leading to a pathergy-type response. Aberrant fibroblast–keratinocyte interactions are proposed to be associated with the resultant ulceration, possibly through promoting breakdown of the basement membrane and again represent another potential cause of pathergy
3=the self-perpetuating inflammatory cascade then results in the characteristic inflammatory and histological findings of pathergy-associated diseases such as BD and PG. All these potential deviations from normal wound healing require further mechanistic investigations and may represent novel therapeutic targets for pathergy-related diseases
In response to specific chemo-attractants, monocytes also infiltrate the wound site and become activated macrophages that release growth factors such as platelet-derived growth factor and vascular endothelial growth factor which initiate the formation of granulation tissue27. These events are the normal prerequisites of the repair of the wound. The heightened or abnormal inflammatory activity occurring in the pathergy reaction can be regarded as a deviation from the normal course of events in the cutaneous wound healing response to the minimal trauma of the SPT provocation. Whilst specific mechanisms which lead directly to pathergy are not well understood, any deviation along this wound healing pathway may hold the potential to induce a pathergy-like response.
Inflammatory cells and mediators
The main histopathological findings in BD-associated pathergy is a mixed dermal inflammatory cell infiltration with lymphocytes, neutrophils and sparse eosinophils, condensed at perivascular sites28. The density and severity of inflammatory cells range from perivascular mononuclear cell infiltration with minimal neutrophil infiltration, to dense perivascular and interstitial mixed cells infiltration with predominantly neutrophils. The difference in histopathological findings can be explained by the diversity of individual immune response to the stimulating agents2. Immunohistochemical examination of pathergy site revealed Human Leukocyte Antigen-DR isotype (HLA-DR) expression of keratinocytes and inflammatory cells, Intracellular Adhesion Molecule (ICAM) and e-selectin expression by endothelial cells28. Inflammatory infiltrate had a dominance of CD3(+), CD4(+), CD45Ro(+) cells and small collections of neutrophil elastase positive cells were detected.
When compared with normal skin, sites of pathergy in BD show significant increases in the messenger RNA expression of interleukin‑8, monocyte chemoattractant protein 1, interferon-g, IL‑12 and IL‑1029 (Figure 1:1). BD patients also have increased numbers of mature dendritic cells, monocytes, lymphocytes, chemokines and cytokines (including IFN-γ, IL‑10, IL‑12 and IL‑15)30.
Non-specific hyperinflammatory response theory
Injured epidermal and dermal cells produce various chemokines, cytokines, growth factors, antimicrobial peptides altogether leading to an inflammatory reaction in response to trauma31. Specifically, TLR and nucleotide-binding oligomerization domain (NOD)-like receptors expressed by keratinocytes activate intracellular pathways and the release of cytokines IL‑6, TNF-α and IL‑1β. These cytokines activate dendritic cells in the dermis which triggers the release of IL‑12, IL‑23 and interferons. Keratinocytes also release chemokines which attract neutrophils, mature dendritic cells and T-lymphocytes to the dermis which leads to the polymorphonuclear cells and mixed inflammatory infiltrate seen in the dermis on histopathology28,30. One study hypothesised that immune processes triggered at the site of nonspecific trauma may provide insights into a dysregulated immune system, triggering a pathergy response in BD patients; it demonstrated an exaggerated Th-1-type immune response in BD patients30. Other studies showed that minimal mechanical skin trauma causes healthy individuals’ uninvolved skin to induce proinflammatory cytokines, including IL‑1β, IL‑6, IL‑8 and IL‑12/2332,33, suggesting that skin damage from trauma activates an innate cutaneous response which may be amplified due to genetic and environmental factors in pathergy-related diseases such as PG and BD.
Genetic implications in pathergy
Genetic factors have also been implicated in the activation of both the innate and adaptive immune systems in both BD and PG, and therefore may play a role in the mechanism of pathergy. HLA-b51 is a genetic marker that has been highly associated with BD in patients from many different ethnic groups, including European, Mediterranean and Asian peoples34.
The reactivity of the ‘pathergy test’ is suggested to be correlated with HLA-B51 in Mediterranean countries35,36. In PG patients, a number of genes including Signal Transducer And Activator Of Transcription 1 (STAT1), IAA-Leu-resistant1 (ILR1), Mitogen-Activated Protein Kinase (MAPK8), interferon regulatory transcription factor 3 and 7 (IRF3, IRF7), Nuclear Factor Kappa B Subunit 1 (NFKB1), MX Dynamin Like GTPase 1 (MX1), Testicular Receptor 4 (TR4), Cluster of differentiation – 40 (CD40), CD40 ligand, Integrin Subunit Alpha M (ITGAM), TLR6 and HLA-A were upregulated in lesions caused by pathergy31. Many of these genes play a role in wound healing. This supports the notion that genetic factors play a role in the pathogenesis of pathergy-related diseases.
Exaggerated response to microbial antigens theory
This hypothesis is founded on the notion that bacterial or microbial elements may induce a pathergy response. Several studies have shown that the proportion of Streptococcus sanguinis (S. sanguinis) was significantly high in the oral bacterial flora of BD patients in comparison with healthy controls37–39. It has been proposed that many BD patients tend to acquire a hypersensitivity against streptococci in their original oral bacterial flora, as demonstrated by a much stronger positive pathergy-type reaction when tested with their own streptococcal antigen compared with those by the ‘pathergy test’35,40. Microbial antigens that have been linked to pathergy-associated conditions include herpes simplex virus, streptococci, staphylococci or Escherichia species37. This theory is supported by reduction in the inflammatory and pathergy response when skin was surgically cleansed with an aseptic epithelial barrier, such as by chlorhexidine or povidone iodide41. It is also supported by reports of a pathergy reaction at injection site in BD patients who had recently received a pneumococcal vaccination42. Another study showed that pathergy-positive BD patients had upregulated TLR5 expression which suggests that microbial or damage-associated signalling may trigger the exaggerated immune response that is characteristic for the pathergy phenomenon43. Similar to the previous theory of a non-specific hyper-inflammatory response, the insertion of an undefined microbial antigen into the skin triggers a cascade of events ending with inflammation in pathergy sites.
Role of keratinocyte–fibroblast interactions in aberrant wound healing
Keratinocytes express numerous growth factors and cytokines which increase wound epithelialisation and ultimately promotes wound healing44. To close the defect in the epidermis, keratinocytes at the wound edge must loosen their adhesion to each other and to the basal lamina and need to develop the flexibility to support migration over the freshly deposited matrix.
Throughout the mid- and late phase of wound healing, cellular interactions become dominated by the interplay of keratinocytes with another critical player involved in wound healing – fibroblasts. The cross-talk between these two cells progressively shifts the microenvironment away from inflammatory to a synthesis-driven granulation tissue45. Mesenchymal–epithelial interactions play a critical role as autocrine/paracrine regulators of fibroblasts and keratinocytes, influencing growth, function and differentiation of these cells and ultimately skin homeostasis24. Apart from paracrine growth factor regulation, the formation of a new basement membrane zone is another example where interaction between keratinocytes and fibroblasts are crucially involved45. Any aberration of these processes may be association with wound breakdown, expansion or the inhibition of re-epithelialisation.
Additionally, scRNA-seq investigations have revealed the dynamic nature of fibroblast identities during wound healing and the powerful wound-induced plasticity of myeloid lineage cells46–49. scRNA-seq analysis infer several pathways fibroblasts follow during wound healing, including contractile and regenerative functions50. Inflammatory cells can directly modulate fibroblast function and contribute directly to pathways involved in wound healing.
Therapies for pathergy-associated diseases
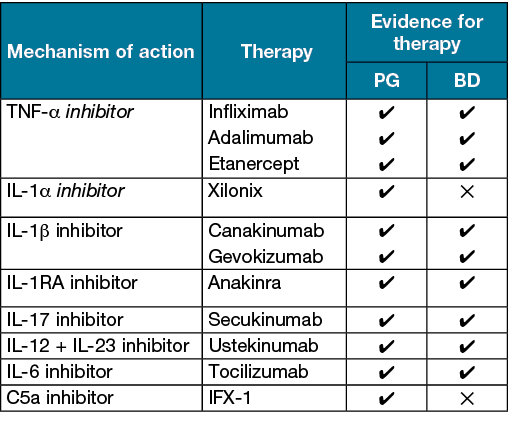
Until recently, therapies for pathergy-associated diseases have focused upon broad immunosuppression with oral steroids or modulation of neutrophil function through therapies such as dapsone and colchicine. In recent years, a number of biological therapies have been used in the treatment of pathergy-associated disease such as PG and BD (Table 2). Through targeting inflammatory mediators such as IL‑1α and IL‑1β, this antagonises inflammation derived both from the keratinocyte ‘alarming’ response as well as inflammation mediated by monocytes, macrophages and neutrophils. Additional targets such as IL‑17 and C5a are involved in the downstream keratinocyte response to neutrophil trafficking and activation. Larger clinical trials will reveal whether these strategies result in clinically significant alterations in vivo.
Table 2. List of biological agents tested for PG and BD in the literature
Mesenchymal stem cells (MSC)
Treating ulcers or wounds caused by pathergy may prove difficult. In PG which has a known pathergy association there is no gold standard of treatment and this can prove challenging for both clinician and patient alike. Most treatment regimens involve topical and systemic immunosuppressants with appropriate wound care and pain management. Recently, the benefits of using human placental tissues in wound regeneration have been documented; one study has shown a 64% wound closure of a PG ulcer after nine weekly applications using this technology27,51.
MSC are key to regenerative wound healing. MSC have spatial memory and respond to local environment. MSC orchestrate wound repair through structural repair via: cellular differentiation; immune-modulation; secretion of growth factors that drive neovascularisation and re-epithelialisation; and mobilisation of resident stem cells52. Viable cryopreserved human placental membranes (vCHPM) is an MSC-based therapy which is a promising strategy in successfully treating non-healing chronic wounds. It contains a combination of growth factors and extracellular matrices as well as viable MSC, fibroblasts and epithelial cells. These components have been shown to decrease inflammation, lower microbial loads and promote tissue regeneration53,54. vCHPM is also a rich source of high molecular weight hyaluronic acid (HC-HA) and pentraxin 3 (PTX3). The HC-HA/PTX3 has a unique ability to promote the death of activated macrophages while downregulating pro-inflammatory cytokines and upregulating anti-inflammatory cytokines55–59. The majority of studies have focused primarily on vCHPM and its role in treating diabetic foot ulcers and venous leg ulcers as opposed to PG or other pathergy-associated diseases.
Conclusion
Pathergy is a result of complex interactions between genetic background, immune-related and environmental factors. Further investigations are needed to understand the pathogenic mechanisms of pathergy to identify novel therapeutic targets for pathergy-associated diseases. Novel monoclonal antibody therapies may provide additional tools to help treat pathergy in the context of diseases such as BD and PG, and the knowledge gained through investigations into the mechanisms of pathergy will have direct relevance to other research in chronic wounds.
Conflict of interest
JWF has conducted advisory work for Janssen, Boehringer-Ingelheim, Pfizer, Kyowa Kirin, LEO Pharms, Regeneron and UCB, participated in trials for UCB, Pfizer and Eli Lilly, and received research support from Ortho Dermatologics.
JSK has received the following grants to institution. For advisory work: Sanofi, Boehringer-Ingelheim; for participation in safety review committee: CSL; for conducting clinical trials: Amryt, CSL, Exopharm, Novartis, AbbVie, Amgen, Boehringer-Ingelheim, BMS, Dermira, Eli Lilly, Pfizer, Principia, Regeneron, Galderma, UCB Biopharma, Astra Zeneca and Argenix.
Ethics statement
No ethical approval was required.
Funding
The authors received no funding for this study.
Author(s)
Anthony Honigman1, Johannes S Kern1,2 and John W Frew†*3–5
†Department of Dermatology, Liverpool Hospital, Suite 7, Level 1, 45–47 Goulburn Street, Liverpool, NSW 2170, Australia
1Dermatology Department, The Royal Melbourne Hospital, Melbourne, VIC, Australia
2The University of Melbourne, Melbourne, VIC, Australia
3Department of Dermatology, Liverpool Hospital, Sydney, NSW, Australia
4Laboratory of Translational Cutaneous Medicine, Ingham Institute of Applied Medical Research, Sydney, NSW, Australia
5University of New South Wales, Sydney, NSW, Australia
*Corresponding author Email john.frew@unsw.edu.au
References
- Varol A, Seifert O, Anderson CD. The skin pathergy test: innately useful? Arch Dermatol Res 2010;302:155–68.
- Srisuttiyakorn C, Aunhachoke K. Pathergy test: the comparison of clinical vs. histopathological evaluation. J Med Assoc Thai 2016 Apr;99(4):412–7.
- Ahad T, Agius E. The Koebner phenomenon. Br J Hosp Med 2015 Nov;76(11):C170–2.
- Zampetti A, Gnarra M, Linder D, Digiuseppe MD, Carrino N, Feliciani C. Psoriatic pseudobalanitis circinata as a post-viral Koebner phenomenon. Case Rep Dermatol 2010;2:183–188.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sex-adjusted population analysis. J Am Acad Dermatol 2019;S0190–9622(19)32494–6.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol 2018;154(4):461–466. doi:10.1001/jamadermatol.2017.5980
- International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014 Mar;28(3):338–47.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986 Mar;37(3):167–74.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol 1994 Oct;31(4):535–56.
- Langan SM, Groves RW, Card TR, et al. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol 2012;132(9):2166–70.
- Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004;43:790–800.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an update review. J Am Acad Dermatol 2015;73(4):691–98.
- Wallace A. Best practice management of peristomal pyoderma gangrenosum. J Comm Nurs 2017;31(1):24–32.
- Caso F, Costa L, Rigante D, Lucherini OM, Caso P, Bascherini V, et al. Biological treatments in Behçet’s disease: beyond anti-TNF therapy. Mediators Inflamm 2014;107421.
- International Study Group for Behçet’s disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990;335:1078–80.
- Karadag AS, Akbay G, Aydin M, Astarci MH, Ek¸sioglu M. Comparison of clinical and histopathologic findings of pathergy test with disposable/sharp and nondisposable/blunt needles in Behçet’s disease. Turk J Med Sci 2009;39:47–51.
- Gilhar A, Winterstein G, Turani H, Landau J, Etzioni A. Skin hyperreactivity response (pathergy) in Behçet’s disease. J Am Acad Dermatol 1989;21(3 Pt 1):547–52.
- Akmaz O, Erel A, Gurel MA. Comparison of histopathological and clinical evaluations of pathergy test in Behçet’s disease. Int J Dermatol 2000;39:121–5.
- Cohen PR. Sweet’s syndrome – a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007;2:34.
- Fett DL, Gibson LE, Su WPD. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc 1995;70:234–240.
- Tan AW, Tan H-H, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol 2006;45:1254–1255.
- Delmonte S, Brusati C, Parodi A, Rebora A. Leukemia-related Sweet’s syndrome elicited by pathergy to arnica. Dermatol 1998;197:195–196.
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4(9):560–582. doi:10.1089/wound.2015.0635
- Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen 2007 Sep-Oct;15 Suppl 1:S46–53. doi:10.1111/j.1524-475X.2007.00225.x. Erratum in: Wound Repair Regen 2008 Jul-Aug;16(4):582.
- Chen L, Shen Z. Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell Mol Immunol 2020;17:64–75.
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746.
- Anselmo DS, McGuire JB, Love E, Vlahovic T. Application of viable cryopreserved human placental membrane grafts in the treatment of wounds of diverse aetiologies: a case series. Wounds 2018;30(3):57–61.
- Ergun T. Pathergy phenomenon. Front Med (Lausanne) 2021 May 25;8:639404.
- Ben Ahmed M, Houman H, Miled M, Dellagi K, Louzir H. Involvement of chemokines and Th1 cytokines in the pathogenesis of mucocutaneous lesions of Behçet’s disease. Arthritis Rheum 2004;50:2291–95.
- Melikoglu M, Uysal S, Krueger JG, Kaplan G, Gogus F, Yazici H, et al. Characterization of the divergent wound-healing responses occurring in the pathergy reaction and normal healthy volunteers. J Immunol 2006;177:6415–21.
- Ji YZ, Liu SR. Koebner phenomenon leading to the formation of new psoriatic lesions: evidences and mechanisms. Biosci Rep 2019;39(12):BSR20193266.
- Sjogren F, Anderson C. Sterile trauma to normal human dermis invariably induces IL1beta, IL6 and IL8 in an innate response to “danger.” Acta Derm Venereol 2009;89:459–65.
- McKenzie F, Arthur M, Ortega-Loayza A. Pyoderma gangrenosum: what do we know now? Curr Dermatol Rep 2018;7:147–157.
- Direskeneli H. Behçet’s disease: infectious aetiology, new autoantigens, and HLA-B51. Annals Rheumatic Dis 2001;60(11):996–1002.
- Kaneko F, Togashi A, Nomura E, Nakamura K. A new diagnostic way for Behçet’s disease: skin prick with self-saliva. Genet Res Int 2014;581468.
- Ohno S, Onguchi M, Hirose S, Matsuda H, Wakisaka A, Aizawa M. Close association of HLA-Bw51 with Behçet’s disease. Arch Ophthalmol 1982;100(9):1455–1458.
- Yokota K, Hayashi S, Araki Y, et al. Characterization of Streptococcus sanguis isolated from patients with Behçet’s disease. Microbiol Immunol 1995;39(9):729–732.
- Isogai E, Ohno S, Takashi K, et al. Close association of Streptococcus sanguis uncommon serotypes with Behçet’s disease. Bifidobacteria Microflora 1990;9:27–41.
- Isogai E, Ohno S, Kotake S, et al. Chemiluminescence of neutrophils from patients with Behçet’s disease and its correlation with an increased proportion of uncommon serotypes of Streptococcus sanguis in the oral flora. Arch Oral Biol 1990;35(1)43–48.
- Kaneko F, Oyama N, Nishibu A. Streptococcal infection in the pathogenesis of Behçet’s disease and clinical effects of minocycline on the disease symptoms. Yonsei Med J 1997;38(6):444–454.
- Van De Ree-Pellikaan C, Kiewiet-Kemper RM, Tchetverikov I, Westerweel PE. Oral ulcerations after placement of orthodontic braces and skin pustules after laser hair removal: novel inducers of pathergy reactions in new onset Behçet’s disease. BMJ Case Rep 2016;7.
- Yurttas B, Taflan SS, Saltoglu N, et al. AB0542 Reactions to Pneumococcal 13-valent vaccine in patients with Behçet syndrome. Annals Rheumatic Dis 2020;79:1567–1568.
- van der Houwen TB, Dik WA, Goeijenbier M, Hayat M, Nagtzaam NMA, van Hagen M, van Laar JAM. Leukocyte toll-like receptor expression in pathergy positive and negative Behçet’s disease patients. Rheumatol 2020;59(12):3971–3979.
- Bolivar-Flores J, Poumian E, Marsch-Moreno M, Montes de OG, Kuri-Harcuch W. Use of cultured human epidermal keratinocytes for allografting burns and conditions for temporary banking of the cultured allografts. Burns 1990;16:3–8.
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 2007 May;127(5):998–1008.
- Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Investig Dermatol 2018;138:811–825.
- Tabib T, Morse C, Wang T, Chen W, Lafyatis R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J Investig Dermatol 2018;138:802–810.
- Januszyk M, Chen K, Henn D, et al. Characterization of diabetic and non-diabetic foot ulcers using single-cell RNA-sequencing. Micromachines (Basel) 2020;11(9):815. doi:10.3390/mi11090815
- Haque A, Engel J, Teichmann SA, et al. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med 2017;9:75.
- Guerrero-Juarez CF, Dedhia PH, Jin S, et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun 2019;10:650.
- Fridman R, Bar-David T, Larsen J, Olson AR. Surgical management of lower extremity pyoderma gangrenosum with viable cryopreserved umbilical tissue: a case series. Wounds 2020 Apr;32(4):101–106.
- Balaji S, Keswani SG, Crombleholme TM. The role of mesenchymal stem cells in the regenerative wound healing phenotype. Adv Wound Care 2012;1(4):159–165. doi:10.1089/wound.2012.0361
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1(2):142–149.
- Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011;6:457–478.
- Cooke M, Tan EK, Mandrycky C, He H, O’Connell J, Tseng SC. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care 2014;23(10):465–474, 476.
- He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem 2009;284(30):20136–20146.
- He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem 2013;288(36):25792–25803.
- He H, Tan Y, Duffort S, Perez VL, Tseng SC. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Invest Ophthalmol Vis Sci 2014;55(3):1647–1656.
- Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J Biol Chem 2014;289(19):13531–13542.



