Volume 30 Number 1
Necrobiosis lipoidica: a review of management and the role of compression
Daniel Zhang and Gabrielle McMullin
Keywords compression therapy, necrobiosis lipoidica, ulceration, skin disease
For referencing Zhang D and McMullin G. Necrobiosis lipoidica: a review of management and the role of compression. Wound Practice and Research 2022; 30(1):11-15.
DOI
https://doi.org/10.33235/wpr.30.1.11-15
Submitted 11 October 2021
Accepted 5 November 2021
Abstract
Necrobiosis lipoidica (NL) is a rare skin disorder that causes significant distress due to its unsightly appearance and propensity to ulcerate. There is a poor understanding of the underlying pathophysiology which ranges from microvascular angiopathy to collagen disruption. Current treatment options, including corticosteroids, immunomodulators and phototherapy, have potentially serious side effects and limited effectiveness. Evidence is sparse and heavily varied, with no well-evidenced guidelines or management approaches that is agreed upon. Properly applied compression therapy has strong evidence in the management of lower limb wounds, and the same physiological effects may have a role in NL therapy. This review seeks to consolidate the current understanding of the underlying pathogenesis and explore current and potential treatment options, including compression therapy, with a focus on the proposed mechanisms of action of therapy for NL.
Introduction
Necrobiosis lipoidica (NL) is a rare, non-infectious granulomatous skin disease of uncertain pathogenesis. Various epidemiological, pathological and therapeutic observations have been made, but with no absolute conclusions regarding precise pathogenesis pathways or correlation between process and presentation. This paper seeks to explore the current understanding and treatment options for NL while also exploring another treatment option, compression therapy. NL management remains a contentious topic, with anti-inflammatory pharmaceutical options generally regarded as the first-line, but with inconclusive evidence and variable effectiveness. Compression therapy has occasionally been used as an adjunct together with pharmaceutical options; however, it has not been used as the sole primary intervention. This review aims to expand the repertoire of treatment modalities with non-pharmacological management for which there are minimal side effects and is generally very well tolerated.
Clinical presentation
NL is characterised by well-demarcated plaques ranging from yellow to red or brown in colour with associated erythema (Figure 1) and a propensity to ulcerate (Figure 2), quoted in up to 30% of cases1. Its distribution is typically seen in the lower limbs bilaterally; however, there have been reports of histologically confirmed NL in other parts of the body including the abdomen, scalp, genitals, face and upper limbs. The lesions tend to begin spontaneously as small, asymptomatic red or violet papules that erode over the course of months to years and may eventually break down into the classical plaque appearance, with or without ulceration. It is predominantly an aesthetic issue but may be associated with pain and pruritus. Ulceration is not uncommon, particularly after a traumatic trigger, which increases the risk of developing an infection. There have also been links with NL and an increased risk of squamous cell carcinoma2, although it is unclear if this is associated with the chronic inflammatory response or a sequelae of NL independently. These lesions persist for years if left untreated but typically remain confined to the region of onset and do not progressively spread.
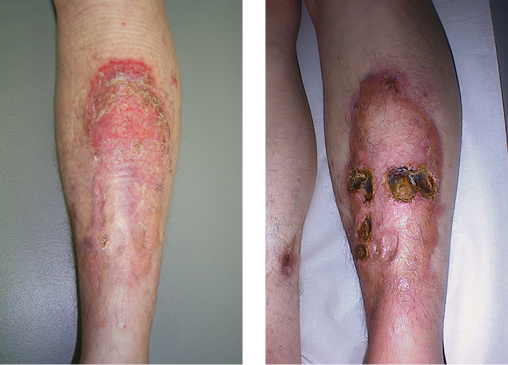
|
Figure 1. An example of NL’s characteristic well-demarcated |
Figure 2. An example of NL’s propensity to ulcerate |
Epidemiology
There is an historical association with diabetes mellitus (DM), but even that is disputed. Studies on patients with NL quote between a 11–62% association with a confirmed diagnosis of DM, and potentially even greater figures when considering patients with impaired glucose tolerance3,4. Indeed, when first identified and named by Oppenheim and Urbach in 1929–1932, it was dubbed necrobiosis lipoidica diabeticorum5. However, epidemiological studies have since identified that there are cases of NL without any indication of DM or impaired glucose tolerance, and absolute figures of rates of NL in DM are low, from 0.3% to up to 2%3,4. It is noted that Type 1 DM has been associated with greater rates of NL; however, glucose control itself does not seem to correlate with disease progression7.
Other epidemiological observations include higher prevalence in females compared to males, with up to a 5:1 distribution7, although previous estimates demonstrate a 3:1 distribution8. The typical age of onset is between 30–40 years of age, but extreme variations such as presence at birth have also been reported. Other risk factor associations that have been identified include obesity, hypertension, dyslipidaemia and smoking9,10.
Pathological processes
NL may resemble multiple other granulomatous skin diseases, including granuloma annulare, erythema nodosum or the even rarer necrobiotic xanthogranuloma4,11. While diagnosis can be made clinically, confirmed diagnosis requires histopathology, often, but not always, demonstrating features of blood vessel wall thickening, collagen and fibrin abnormalities and granulomatous inflammation with multinucleated giant cells.
Vasculitic and inflammatory changes
Whilst there is no widely accepted pathogenesis pathway for NL, it is generally agreed that vascular changes and microangiopathy plays a part4,12. Features of vasculopathy such as inflammation and thickening of vessel walls, as well as immune complex deposition within vessels, have been identified on histological and immunofluorescence examination12,13. Common theories include glycoprotein deposition as seen in many complications associated with DM; indeed, it has been positively identified in patients with NL9, yet this does not account for all cases. The rates of microvascular complications of diabetes such as diabetic nephropathy or retinopathy in patients with NL are not outside the expected range in the general diabetic population7, suggesting that this follows a diabetic pathogenesis pathway. Other features such as immunoglobulins and complement factors in the vessel wall have also been identified, suggesting other aetiology paralleling autoimmune vasculitidies9,13. Indeed, vascular endothelial growth factor (VEGF) has been demonstrated to be involved in the pathogenesis of NL14. VEGF promotes proliferation of endothelial cells, which in excess is an observed phenomenon of microangiopathy14. Thus, while microangiopathy is clearly observed in cases of NL, the precise pathogenesis remains unclear and multiple postulations currently circulate in the literature.
Interestingly, there are conflicting results regarding ischaemia as the primary aetiology, where Boateng et al.15 observed decreased partial pressure of oxygen in the NL lesions, whilst Ngo et al.16 identified increased cutaneous blood flow via laser Doppler flowmetry, suggesting a predominantly inflammatory process. Antibody-mediated vasculitis secondary to aforementioned immunoglobulin deposition may also play a role4. Inflammatory mediators such as tumour necrosis factor 丟 (TNF-丟) have been identified to be raised in NL patients compared to controls, suggesting an aetiological link with inflammation14. TNF-丟 is a potent stimulator of the cell cycle, inducing proliferation and apoptosis, and is an important regulatory factor in the pathogenesis of granulomas4. Nevertheless, there is no conclusive evidence that this is a primary aetiology for NL, although it is interesting to note that TNF-丟 as a therapeutic target has demonstrated promising results4.
Collagen disruption
The other primary aetiological theory for NL stems from collagen abnormalities, in both its synthesis from fibroblasts and the ultrastructural layout within the dermis, subcutaneous tissue and basement membrane4,11. Light and electron microscopy of control-matched biopsies demonstrated collagen degeneration and necrosis surrounded by mononuclear cell infiltrates. Similarly, elastin was necrotic and the collagen-elastin bundles were disorientated or completed lost17; fibroblast function was also diminished17. NL lesions demonstrated disarrayed collagen fibrils and increased collagen cross-linking, likely secondary to elevated levels of lysyl oxidase18. Despite all these positive findings and potential aetiological links, the primary cause or trigger of NL is yet to be confirmed.
Treatment options
There have been numerous treatment options, targeting the identified pathogenesis findings. Treatment rationale has been typically based off successful treatment results of similar pathologies such as granuloma annulare or skin granulomas secondary to sarcoidosis, with links to the identified pathophysiological processes underlying NL. Topical and systemic corticosteroids have been the mainstay of treatment, with a view to reduce inflammation in the lesion4. Phototherapy has been studied, with very variable response rates. Other options included biologic agents such as infliximab, a TNF-丟 inhibitor, and immunomodulators or immunosuppressants such as methotrexate, tacrolimus and fumaric acid esters4,19. A questionnaire of German dermatology experts in 2012 rated topical corticosteroids first-line treatment, with compression, topical calcineurin inhibitors and phototherapy equal second line options10,20. There have been no reports on compression alone in the management of NL lesions.
Corticosteroids
Corticosteroids have been the first-line treatment for NL, with topical and intralesional applications preferred over systemic treatment due to reduced side effect profile. Nevertheless, despite the propensity for systemic steroids to cause hyperglycaemia and hypertension, particularly in the already diabetic population, a short-term course is not contraindicated and may lead to rapid cessation of progressive disease and potentially complete resolution4. These effects can largely be attributed to the up-regulation of anti-inflammatory proteins such as annexin-1 and mitogen-activated protein kinases from the activation of the glucocorticoid receptor21. There is good evidence for resolving active lesions with enlarging borders; however, lesions with features of atrophy or ulceration do not benefit from steroid therapy, in-fact it may worsen the disease4,22. Indeed, in many cases, NL is refractory to steroid treatment, prompting consideration of other options.
Phototherapy
Phototherapy has also been studied for treatment of NL, given its effectiveness in other inflammatory dermatological diseases. The mechanisms underlying the treatment are not well understood, with some demonstrated immunomodulatory effects on various cytokines including TNF-丟, interleukins and granulocyte colony-stimulating factor23. Various methods have been examined, including photodynamic therapy (PDT) with methyl aminolevulinate and psoralen-UV-A (PUVA) therapies. Again, clinical effects see a reduction in active inflamed borders and may resolve superficial lesions; however, it does not have any effect on atrophied areas4. Response rates of PDT have been quoted to be around 40% with some improvement, but in 50% of patients there is no effect19,23. PUVA studies demonstrate up to two-thirds of patients have partial or complete resolution19. One of the main drawbacks to phototherapy is the associated pain with treatment23.
Biological agents
With the identification the role of TNF-丟 in the pathogenesis of NL, drugs such as infliximab, a monoclonal antibody that binds directly to TNF-丟 to inhibit its action, have been used particularly in ulcerating NL, with promising results4,19. It has been shown to be particularly useful in ulcerative disease, where corticosteroids and phototherapy have proven ineffective, with complete resolution in 70% of cases after a course of infliximab19. Infliximab has been given intravenously and intralesionally and is generally well tolerated; however, there is an increased risk of serious infection, including reactivation of latent tuberculosis and infections by opportunistic pathogens24. Infliximab has also been used in non-ulcerated NL with complete resolution at 6 weeks and no significant adverse effects, thus may potentially be a viable first-line option25.
Immunomodulators
Various immunomodulators, including cyclosporine and tacrolimus, as well as topical dermatological treatments such as fumaric acid esters have also been trialled in the management of NL lesions for their anti-inflammatory effects4,10,19. Through inhibiting calcineurin, cyclosporine and tacrolimus inhibit the production of inflammatory cytokines and thus have anti-inflammatory effects which has seen promising results when used both topically and systemically4,19. Various combination therapies of calcineurin inhibitors and other immunomodulatory drugs such as methotrexate have also been used successfully26. Fumaric acid esters have been extensively used in dermatology for inflammatory skin conditions such as psoriasis, with good outcomes due to their inhibition of inflammatory pathways including inhibition of T lymphocyte proliferation, TNF-induced tissue factor messenger ribonucleic acid and TNF-induced binding of inflammatory proteins4,27. It has seen good results in other granulomatous disorders such as granuloma annulare and cutaneous sarcoidosis, and similarly demonstrated promising outcomes in the treatment of NL with less than 10% of patients showing no improvement19. However, these immunomodulating agents are not without their drawbacks, with the calcineurin inhibitors associated with nephrotoxicity, especially relevant given the high incidence of diabetes and potential diabetic nephropathy4. Fumaric acid esters are associated with lymphocytopenia, reported to be as common as in 44% of the test population27.
Compression therapy
Bandaging has been used to treat wounds for millennia, both to protect and to promote healing. There is strong evidence that compression bandaging is beneficial for wound healing, particularly with venous ulcers28. Well recognised clinical effects include reduction in oedema and improved venous and lymphatic outflow; however, less appreciated but just as significant effects include a reduction in inflammatory cytokines, increased local oxygen partial pressure, and reduced lymphocyte adhesion28–30. Local cytokines are reduced after 4 weeks of compression bandaging in lower limb ulcers, including TNF-丟 and various interleukins29. Furthermore, cutaneous microcirculation as measured by laser doppler fluxmetry somewhat counter-intuitively demonstrated improved flow in areas under and around where compression is applied, most pronounced with pressures between 21–30mmHg but still improved with pressures 31–40mmHg30. It is hypothesised that there is an arteriolar vasodilatory response to compression, potentially induced via increased nitric oxide production, resulting in these findings28,30. Thus, there is physiological evidence that compression addresses many of the identified pathogenesis pathways of NL.
However, there is no clear literature on the use of compression in NL. While a survey of German dermatology experts demonstrated that 47% would recommend compression therapy, 13.3% of whom recommend it as first-line, it has not been examined independently10. Some case reports and case series document compression used concurrently with the aforementioned treatment options1,10.
Compression bandaging is a relatively cost-effective treatment option with minimal side effects; indeed, it may improve cardiovascular function by improving venous return30. Unlike pharmacological treatments, however, there is an element of operator skill and requires trained staff to apply the compression bandaging for effective results31. Compression is graded based off interface pressure, with ‘mild’ compression below 20mmHg, ‘medium’ compression between 21–40mmHg and ‘strong’ compression from 41–60mmHg. Various elastic and inelastic bandages are used to apply and maintain this pressure, with the gold standard of triple-layer bandages to ensure static stiffness28,31. The optimal pressure range for clinical benefit and comfort lies between 35–45mmHg, where the benefits of significantly reduced oedema, reduced inflammatory cytokines and improved microcirculation have been observed, yet is not tight to the point of being uncomfortable28–31. However, there are contraindications for compression bandaging, especially at this pressure. Significant peripheral arterial disease with systolic pressures at the ankle less than 50mmHg and severe heart failure may lead to ischaemic limb or worsening heart failure respectively, and thus ‘medium’ to ‘strong’ compression is contraindicated28. There may be a role for a modified compression bandage with reduced pressures in these scenarios.
Conclusion
The pathophysiology of NL is still unclear. There have been pathological processes identified, and individual treatment options for particular pathways have demonstrated some degree of efficacy. The most common and recommended first-line option is corticosteroids; however, the evidence supporting its use is inconclusive, and it may in fact be detrimental for ulcerated disease. Newer treatment options such as tacrolimus have demonstrating positive results for all degrees of disease severity; however, these are associated with significant side effects such as severe immunocompromise. Furthermore, in the subset of patients with multiple other comorbidities, steroids and immunosuppressing agents may be ill-advised due these concerning adverse effects.
Given the strong evidence for compression bandaging in lower limb wounds, the recognised physiological benefits of compression, and the current understanding of some of NL’s mechanisms, compression therapy can be considered for NL lesions. This is particularly relevant for ulcerated NL, where typical, least invasive options of topical steroids or phototherapy have proven ineffective or even detrimental. It has been used together with other treatments with variable results; however, this has not been documented independently. Nevertheless, compression bandaging remains a low-cost, non-invasive treatment option that has minimal side effects and may be considered in the growing arsenal for NL treatment.
Further research needs to be done to better delineate the underlying processes and hopefully identify a clear causative pathway which can then be addressed for direct management. Currently there is no gold-standard to management and treatment should be considered on an individual basis depending on the presenting lesion, the patient’s priorities and the resources available.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author(s)
Daniel Zhang1 and Gabrielle McMullin*2
1Sutherland Hospital, Caringbah, NSW 2229, Australia
2St George Hospital, Kogarah NSW 2217, Australia
*Corresponding author Email gmcmullin@ssvc.us
References
- Franklin C, Stoffels-Weindorf M, Hillen U, Dissemond J. Ulcerated necrobiosis lipoidica as a rare cause for chronic leg ulcers: case report series of ten patients. Int Wound J 2015;12(5):548–554.
- Lim C, Tschuchnigg M, Lim J. Squamous cell carcinoma arising in an area of long-standing necrobiosis lipoidica. J Cutan Pathol 2006;33:581–583.
- O’Toole EA, Kennedy U, Nolan JJ, Young MM, Rogers S, Barnes L. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol 1999;140:283–286.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin 2015;33(3):343–360.
- Oppenheim M. Eigentümlich disseminierte Degeneration des Bindegewebes der Haut bei einem Diabetiker [Degeneration of the connective tissue of the skin in a diabetic patient]. Z Hautkr 1929;32:179.
- Grillo E, Rodriguez-Munoz D, Gonzalez-Garcia A, Jaen P. Necrobiosis lipoidica. Aust Fam Physician 2014;43(3):129–130.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, Nelson CA, Noe MH, Imadojemu S, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermat 2019;155(4):455–459.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol 2013;69:783–791.
- Jockenhöfer F, Kröger K, Klode J, Renner R, Erfurt-Berge C, Dissemond J. Cofactors and comorbidities of necrobiosis lipoidica: analysis of the German DRG data from 2012: cofactors and comorbidities of necrobiosis lipoidica. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2016;14(3):277–284.
- Erfurt-Berge C, Dissemond J, Schwede K, Seitz A-T, Al Ghazal P, Wollina U, et al. Updated results of 100 patients on clinical features and therapeutic options in necrobiosis lipoidica in a retrospective multicentre study. Eur J Dermatol 2015;25(6):595–601.
- Pokharel A, Koirala I. Necrobiotic granuloma: an update. Indian J Dermatopathol Diagn Dermatol 2018;5:27–33.
- Boulton AJ, Cutfield RG, Abouganem D, et al. Necrobiosis lipoidica diabeticorum: a clinicopathologic study. J Am Acad Dermatol 1988;18(3):530–537.
- Quimby SR, Muller SA, Schroeter AL. The cutaneous immunopathology of necrobiosis lipoidica diabeticorum. Arch Dermatol 1988;124:1364–1371.
- Słowik-Kwiatkowska I, Lesiak A, Woźniacka A, Sysa-Jędrzejowska A, Narbutt J. Does inflammation play a role in development of necrobiosis lipoidica? Przegl Dermatol 2014;3:187–91.
- Boateng B, Hiller D, Albrecht HP, Hornstein OP. Cutaneous microcirculation in pretibial necrobiosis lipoidica. Comparative laser Doppler flowmetry and oxygen partial pressure determinations in patients and healthy probands. Hautarzt 1993;44:581–586.
- Ngo B, Wigington G, Hayes K, Huerter C, Hillman B, Adler M, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol 2008;47(4):354–358.
- Oikarinen A, Mörtenhumer M, Kallioninen M, Savolainen ER. Necrobiosis lipoidica: ultrastructural and biochemical demonstration of a collagen defect. J Invest Dermatol 1987;88(2):227–32.
- Lowitt MH, Dover JS. Necrobiosis lipoidica. J Am Acad Dermatol 1991;25(5):14.
- Peckruhn M, Tittelbach J, Elsner P. Update: treatment of necrobiosis lipoidica. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2017;15(2):151–7
- Erfurt-Berge C, Seitz AT, Rehse C, et al. Update on clinical and laboratory features in necrobiosis lipoidica: a retrospective multicentre study of 52 patients. Eur J Dermatol 2012;22:770–5.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med 2005;353(16):1711–23.
- Kota S, Kota S, Modi K, Jammula S, Meher L. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocr Metab 2012;16(4):614.
- Berking C, Hegyi J, Arenberger P, Ruzicka T, Jemec GBE. Photodynamic therapy of necrobiosis lipoidica – a multicenter study of 18 patients. Dermatol 2009;218(2):136–9.
- Basoulis D, Fragiadaki K, Tentolouris N, Sfikakis PP, Kokkinos A. Anti-TNFα treatment for recalcitrant ulcerative necrobiosis lipoidica diabeticorum: a case report and review of the literature. Metabolism 2016;65(4):569–73.
- Conte H, Milpied B, Kaloga M, Lalanne N, Belin E, Jouary T, et al. Treatment of pre-ulcerative necrobiosis lipoidica with infliximab. Acta Derm Venerol 2011;91(5):587–8.
- West EA, Warren RB, King CM. A case of recalcitrant necrobiosis lipoidica responding to combined immunosuppression therapy. J Eur Acad Dermatol Venereol 2007;21:830–1.
- Kreuter A, Knierim C, Stucker M, et al. Fumaric acid esters in necrobiosis lipoidica: results of a prospective noncontrolled study. Br J Dermatol 2005;153:802–7.
- Berszakiewicz A, Sieroń A, Krasiński Z, Cholewka A, Stanek A. Compression therapy in venous diseases: physical assumptions and clinical effects. Postepy Dermatol Alergol 2020;37(6):842–7.
- Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg 2009;49(4):1013–20.
- Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg 2012;55(1):122–8.
- Tidhar D, Keren E, Brandin G, Yogev M, Armer JM. Effectiveness of compression bandaging education for wound care nurses. J Wound Care 2017;26(11):625–31.



