Volume 30 Number 2
The cost of venous leg ulcers in a Singapore tertiary hospital: an explorative study
Fazila Aloweni, Thendral Uthaman, Nur L Agus, Tan W Xian, Sivagame Maniya, Ang S Yuh and Chong T Tec
Keywords Cost, venous leg ulcers, compression therapy, healthcare perspective
For referencing Aloweni F et al. The cost of venous leg ulcers in a Singapore tertiary hospital: an explorative study. Wound Practice and Research 2022; 30(2):75-81
DOI
https://doi.org/10.33235/wpr.30.2.75-81
Submitted 16 August 2021
Accepted 6 October 2021
Abstract
Background Venous leg ulcers (VLU) are chronic wounds that cause significant socioeconomic burdens on individuals and healthcare systems, yet data on this in the Singapore setting is sparse. Thus, this study aimed to describe the frequency of outpatient and inpatient visits for patients with VLU and the costs of compression therapy.
Method A retrospective review of patients at Singapore General Hospital (SGH) diagnosed with VLU between 2016 and 2018 (primary and secondary) was conducted.
Results A total of 4214 unique patients’ data were extracted; 238 patients (5.6%) received compression therapy. A patient on compression therapy in Singapore was estimated to spend a median of S$451.20 (US$333.26) for VLU. Patients on compression therapy had more visits to the outpatient clinics (Mean=3.26, SD=2.92) than patients who were not (M=1.57, SD=1.16). At outpatient clinics, patients on compression therapy spent more – M=S$309.88 (US$228.88), SD=S$379.90 (US$280.60) – than patients who were not –M=S$173.31 (US$128.01), SD=S$230.88 (US$170.53).
Conclusion Compression therapy was received by minimal patients. As it is only offered at outpatient clinics, patients on compression therapy were found to spend more than their counterparts.
Background
Venous leg ulcers (VLU) are chronic wounds that occur on the lower extremities as a result of vein valve reflux or venous obstruction1. The prevalence of VLU is estimated to be between 0.05–2% in the United States (US)2,3, which is reportedly similar to the rate of heart failures3. In Europe, the prevalence rate was estimated to be between 3–10% in the acute setting and 1–11% in the community setting4. In a recent study in Australia, it was reported that 400,000 people were treated for VLU in the year 2010 alone5, whereas, in Singapore, it was estimated that 15 per 100,000 people experienced venous-related wound conditions among the general population in 20176. When looking at people aged 50 years and above, this number increases to 38 per 100,000 people6. However, there are no other published VLU incidence rates around south-east Asia for comparison.
Risk factors for VLU
Across studies, old age is a consistent and significant risk factor for VLU7–9. This is due to changes in the structural and mechanical properties in the vascular wall that cause arteries to lose their elasticity and compliance in the older population10. In addition, a person’s skin undergoes a variety of changes with age which affect the healing process of wounds10. Therefore, as the ageing population increases, the prevalence of VLU is also expected to increase11. Other common factors that have a role in the development of VLU include obesity, family history, nephrosis, deep vein reflux, deep vein thrombosis, low physical activity, previous injuries, and hypertension7–9,12,13. Other less common but significant risk factors in the formation of VLU are varicose veins and multiple pregnancies12. However, in terms of pathophysiology, venous hypertension has been identified as the sole factor that causes chronic venous insufficiency and VLU14. Untreated venous hypertension results in oedema of the limb and chronic reperfusion injury14. These changes lead to characteristics of venous insufficiency such as aches, heaviness of limbs, itching, pigmentation and, ultimately, in VLU9,14.
Treating VLU
The goal of VLU treatment is to improve venous function12,15, and compression therapy continues to be the mainstay treatment for VLU2,13,14. It helps to reduce venous reflux and inflammatory cytokines, accelerate the capillary flow, and lower capillary fluid leakage, thereby alleviating limb oedema16. Compression therapy includes compression hosiery and two- or four-layer compression bandages. Among these, four-layer compression bandages are considered the best and ideal treatment options17. However, these bandages are thick, bulky and cause difficulties in mobilising and wearing shoes as well as resulting in discomfort17. Additionally, correct application of this bandage is vital as an incorrect application can compromise the level of compression, thus rendering this form of treatment otherwise ineffective17. Two-layer bandages such as the Coban 2 Lite (3M, St Paul, Minnesota) offer reduced compression for patients with mixed aetiology leg ulcers or patients who are unable to withstand high-strength compressions as a result of painful VLU18. Compared to four-layer compression bandages, two-layer bandages are just as safe and offer a more comfortable option for such patients who cannot withstand high compression pressure18.
Quite often, treating VLU involves a combination of compression therapy and localised wound management such as moist dressings and debridement2. However, many VLU patients are susceptible to contact sensitivity, making localised wound management a challenging option14,19. Depending on the severity of the ulcers, surgery may be recommended. For example, superficial venous surgery is effective in treating patients with superficial venous incompetence14. Surgical treatments have also been used for superficial venous reflex20. Superficial venous surgery could also reduce the VLU recurrence rate from 44% to 26%21. Besides compression therapy, wound management and surgeries, enhanced treatments such as negative pressure therapy and bioengineered cellular technologies have also been used to assist in the healing of VLU2. In summary, a combination of treatment modalities may be needed depending on the cause and severity of the venous insufficiency. These treatment options may also occur simultaneously or in stages and could take months to heal.
Effect of VLU on patients and family
VLU cause a significant impact on a patient’s quality of life due to pain and impaired mobility2,11,15. It affects patients’ mental health as well, in the form of depression, social exclusion and anxiety1,2,13. Patients with VLU often experience poor sleep, consistent pain, and mobility restrictions12. A study in the United Kingdom (UK) found half of the patients with VLU face difficulties in their activities of daily living, such as bathing, because of the need to keep compression bandages dry22. Beyond the direct challenges of caring for the wound, patients also reported an inability to engage in previously enjoyed activities such as swimming, gardening and walking due to negative body image or fear of injury12,22. In addition, odours, secretions and leakages from wounds lead to poor self-esteem, self-loathing, disgust, self-isolation and depression1. VLU patients also experience considerable guilt as they continue to depend on friends or family for physical support22.
Cost of treating VLU
The treatment of VLU is often long and arduous and could last between 6 months to 1 year or even longer for some patients7,13. VLU also has the highest recurrence rate2 – between 18–28% within 12 months17,23. Therefore, treating VLU is expensive for both patients and healthcare systems19. Current literature agrees as much; VLU incurs high costs for patients directly and indirectly24. Direct costs are classified as the costs of medication, dressings, bandages and hospital and care facilities, while indirect costs are classified as loss of productivity and reduced quality of life24. Western countries such as the US, the UK and Germany have reported spending approximately 1% of their healthcare budgets in managing chronic venous insufficiency alone1. On average, a patient with VLU would spend €2585 (US$3023.02) in Sweden, €1994 (US$2331.87) in the UK and €9569 (US$11295.67) in Germany for the direct and indirect costs of VLU per year11. A study in the US estimated that VLU patients under Medicare spent an average of US$18,986 in 12 months2. In Australia, a study found VLU patients who received standard treatments to have weekly costs of A$214.61 (US$156.68)11. VLU patients who received guideline-based prevention and treatment were found to have weekly costs of A$294.72 (US$215.17)11.
Being a chronic condition that impacts both patients and healthcare systems, it is therefore essential to examine the costs and use of resources associated with the treatment of VLU. While studies have been conducted in the US, UK, Australia and other European countries to estimate the costs of VLU, no studies have been done in Singapore or in the Asian setting to estimate the costs of VLU per patient. Furthermore, as Singapore faces an increasingly ageing population, it is crucial to understand the direct costs of VLU for a patient in the local setting, especially when on compression therapy. In Singapore, all compression therapies (two- and four-layer compression bandages) are performed by specialist wound nurses in a hospital outpatient setting, unlike in the West where compression dressing services are offered in the community.
Additionally, although healthcare is subsidised in Singapore, the government relies extensively on patient co-payments, i.e., out of own pocket25. The amount of out of own pocket varies by the patient’s income and/or affordability. Therefore, findings from this study can inform care and policies in Singapore and other Asian countries with similar healthcare financing structures or demographics such as Hong Kong, Taiwan and China.
Aims
The aims of this study were twofold – the first was to describe the frequency of outpatient attendances and inpatient admissions for patients with VLU, and the second was to describe the costs of treatment for VLU patients on compression therapy (two- or four-layer compression bandages, excluding hosieries).
Method
Design
A retrospective medical records review of patients diagnosed with VLU using the International Classification of Diseases (ICD-10) codes, regardless of whether the diagnosis was primary or secondary, was conducted between 2016 and 2018 in Singapore General Hospital (SGH), the oldest and largest teaching hospital in Singapore. The codes used were 1209742014 (statis ulcer), 1781953019 (venous ulcer of leg), 2476696011 (venous ulcer), 2477048019 (statis ulcer of leg), 34824018 (peripheral venous insufficiency), 34827013 (chronic venous insufficiency), 350678016 (venous insufficiency of leg), 69941010 (venous ulcer). Using SingHealth-IHiS Electronic Health Intelligence System (eHINTS), we extracted de-identified data relating to VLU. eHINTS is an enterprise data repository and analytics system. It integrates data from multiple healthcare transactional systems, including administration, clinical and ancillary systems and is refreshed daily26.
Current literature defines “direct costs” as those caused by the treatment and care provided27. In accordance, finance data, namely hospitalisation bills, emergency department bills and outpatient clinic bills related to the treatment of the VLU, were extracted. Specifically, bill statements associated with the treatment of VLU for up to 1 year from the date of the first diagnosis were retrieved. Demographic data such as age, gender and date of death were collected. Additionally, subsequent visits to SGH for VLU within 1 year of the admission date, length of hospitalisations, day surgeries, emergency admissions, treatments, number of visits and treatments offered were gathered to estimate the use of hospital resources for VLU. Indirect costs such as loss of productivity, cost of days lost from work27, other non-medical expenses and future consumption were not included in the study due to the study design and limitation of the database. Therefore, in this study, the cost is analysed and presented from the healthcare perspective28. Compression therapy is defined in this study as patients who received either two- or four-layer compression bandages.
In instances where data was missing, last observations were carried forward. In other words, if cost data on a patient’s subsequent visits was missing, the last gathered data on cost was used for all subsequent visits that were missing. It is more likely that the data was lost due to a system error rather than that a patient did not pay at all during a visit. Hence, observing the last observations carried forward would be the best estimate of the cost of VLU for a patient. Since this is a descriptive study rather than a predictive one where results or effects are measured, the effects of bias in using this method are minimal.
Participants
Records of all patients aged 18 years and above and who were first diagnosed with VLU from January 2016 to December 2018 at SGH were retrieved. Patients aged below 18 years who did not seek treatment from SGH, i.e., no visits or treatment records in SGH, were excluded.
Data analysis
All statistical analysis was done on SPSS v25 for Windows. p values less than 0.05 were considered statistically significant. T-tests, Pearson correlations, ANCOVA tests and crosstabs were done to examine the relation between cost and various factors and healthcare resource utilisation.
Results
Patient characteristics
A total of 4214 unique patients’ data was extracted. The median age of patients was 63 years (M=62, SD=14); 59.1% of patients were female (n=2491). Since the date first diagnosed, 48 participants had passed away during the study period.
Use of resources
The median number of visits a patient made to the individual settings in SGH are described in Table 1. In addition, 23 patients visited the day surgery clinic. All patients who visited the day surgery clinic only visited once; thus, there are no mean or median values for this setting. The median number of days a patient spent admitted as an inpatient for VLU was 3 (Min=0, i.e., the patient did not have to stay overnight, Max=273).
Table 1. No. visits made to the outpatient clinics, inpatient admissions and emergency admissions (n=4214)
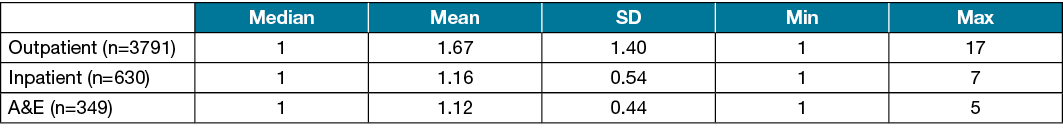
The median number of visits a patient on compression therapy made to the individual settings in SGH is described in Table 2. Again, all patients on compression therapy who visited the day surgery clinic did so only once. The majority of patients on compression therapy were still female (n=133, 55.9%). The average patient on compression therapy was 65 years old (Min=25, Max=93).
Table 2. No. visits made to the outpatient clinics, inpatient admissions and emergency admissions by patients on compression therapy (n=238)
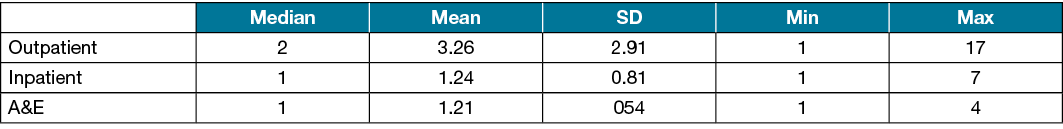
A total of 238 patients (5.6%) received compression therapy (two- or four-layer bandages); 455 patients (10.8%) were offered dressing consumables such as iodosorb powder, 2198 patients (52.2%) had general consultations with doctors or specialty nurses, 10 patients (0.3%) had investigations such as histology, and seven patients (0.2%) had minor surgical procedures. The use of compression therapy was found to be statistically significant in the number of visits a patient made to the outpatient clinics, t (3789)=–18.91, p<0.005. Patients who underwent compression therapy had more visits to the outpatient clinics (M=3.26, SD=2.92) than patients who did not (M=1.57, SD=1.16).
Patients who underwent compression therapy had more emergency admissions (M=1.21, SD=0.54) than patients who did not (M=1.11, SD=0.42), t (347)=–1.44, p=0.01. Patients who underwent compression therapy were also found to have more inpatient admissions (M=1.24, SD=0.81) than patients who did not (M=1.15, SD=0.50), t (628)=–1.20, p=0.03. When adjusting for gender and age, there was a significant difference in the number of accident and emergency (A&E) admissions, F(1,1)=557.69, p=0.02 between patients who received compression therapy and patients who did not. Compared to females, males who received compression therapy (M=1.25, SD=0.78), had more A&E admissions than males who did not (M=1.14, SD=0.48).
Direct costs of VLU for patients on compression therapy
The median total cost of VLU and the median costs at the individual settings for patients on compression therapy in SGH are summarised in Table 3. These sums reflect how much a patient on compression therapy spent in SGH for VLU from the date of diagnosis until completion or discontinuation of treatment or the end of 2018 when the extraction of our medical records ceased.
Table 3. Cost of VLU (in S$) in the outpatient clinics, inpatient settings, emergency admissions and day surgery clinics, and total costs for patients on compression therapy (n=238)
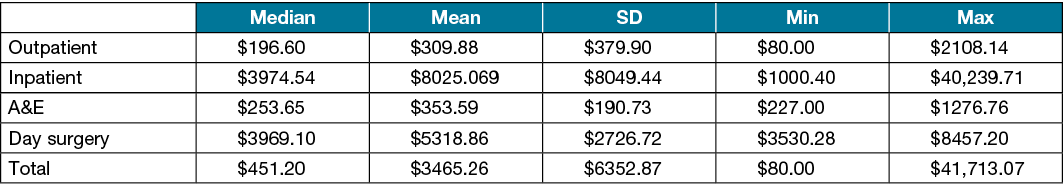
There was a positive, statistically significant correlation between the number of visits to the outpatient clinic and the total spent in the outpatient setting. (r=0.58, n=145, p<0.005). There was also a positive, statistically significant correlation between the number of visits to the outpatient clinic and the total cost spent across all the settings in SGH (r=0.33, n=173, p<0.005).
At outpatient clinics, where compression therapy is offered, patients who underwent compression therapy were found to spend significantly more – M=US$228.50 (S$309.88), SD=US$280.10 (S$379.90) – than patients who did not undergo compression therapy – M=US$127.78 (S$173.31), SD=US$170. 23 (S$230.88), t (1473)=–6.26, p<0.005.
Discussion
To the best of the authors’ knowledge, this study is the first of its kind in Singapore and Asia. While a similar paper on the clinical and economic burden of all types of chronic wounds has been done in Singapore29, it did not study the cost of VLU patients on compression therapy29. In this study, we explored the direct costs of VLU for patients on compression therapy from the healthcare perspective due to the commonality and effectiveness of compression therapy. Although other countries have done similar studies, these estimates may be irreplicable in Singapore due to the differences in healthcare systems and services. The demographic profile of the patients from our study matches the current literature; most VLU patients tend to be female and older5,8,30.
Use of resources
Despite compression therapy being the gold standard for VLU management, it was only received by 238 patients (5.6%) in our study. The low rate of compression therapy in this study is unknown due to the nature of this study design. However, it is possible that these patients needed further investigations before starting compression therapy. Nonetheless, low levels of compression therapy as treatment options have also been reported in previous studies5,13,24. The various reasons posited for the low rate of compression therapy are poor adherence or tolerance to compression therapy or lack of resources to provide the therapy13. Indeed, most available data do not indicate the actual consultations or discussions patients had with doctors and other healthcare professionals. These are the conversations that could explain the low uptake of compression therapy. However, as with most long-term treatments, patients may have refused compression therapy due to reasons such as cost, pain, discomfort or other difficulties in adhering to the treatment.
Further, in Singapore, two- and four-layer compression bandage services are only provided by the wound care nurses in the outpatient clinic within an acute care hospital. This is unlike many other countries such as Australia, Ireland and the UK where compression therapy is provided and managed in the community by nurses15,31,32 or by external nursing agencies in countries like Canada33. This is also supported by our finding that patients who were under compression therapy had more visits to the outpatient clinics than patients who were not. Thus, having to commute to the hospital two to three times a week for treatment might not be convenient and cheap, especially since outpatient treatments require patients to pay out of pocket. Our findings also concurred that patients on compression therapy spent more in the outpatient setting, and thus total cost, than patients who were not.
This study also found patients on compression therapy to have more admissions to the A&E and hospitalisations than patients who were not. The first possibility for this is that patients who are on compression therapy have more visits to the outpatient clinics where compression therapy is exclusively offered. This would facilitate an increase in opportunities to identify infected or deteriorating wounds that would warrant admissions. On the contrary, another possibility is that while our study identified whether a patient was placed on compression therapy or not, it did not examine if they adhered to the therapy until the VLU healed or was better managed. Lack of compliance to compression therapy would mean increased venous reflux and decelerated capillary flow, thus exacerbating the VLU or creating complications that possibly warrant admission to A&E or inpatient wards. A previous study found 62% of hospitalised patients believed compression therapy to be ineffective34.
Previous studies also indicate patients have poor confidence in compression therapy, which will cause a demotivation in treatment adherence and consequently lead to serious events such as hospitalisations34. This study found male patients to have more inpatient admissions than female patients. Literature indicates that women are more likely than men to seek treatment, and men are more likely to care for a condition by themselves35. For a chronic condition such as VLU, specialised treatment by skilled professionals is a necessity. Thus, the more a patient tries to manage a VLU by himself, it’s more likely that he’s delaying administration of the correct treatment which will, in turn, lead to the possibility of increased complications and more inpatient or A&E attendances. Nonetheless, healthcare staff’s skills and knowledge of compression therapy should be assessed to determine the institution’s capability in delivering it as a treatment option for patients with VLU.
Direct cost of VLU for patients on compression therapy
We estimated a median total cost of S$451.20 (US$333.26) for a VLU patient on compression therapy in Singapore. As previously stated, this is the total a patient had spent for VLU from the date of diagnosis until completion of treatment, discontinuation or the cessation of data extraction. Patients who underwent compression therapy were found to spend significantly more than patients who did not. Barnsbee et al.11 had similar findings, where patients who received treatments ideal for their ulcers (including compression therapy) were found to spend more than patients who received general care. Phillips et al.13 also found compression therapy to be a significant factor in increased costs of VLU for a patient. As suggested by Barnsbee et al.11, high costs, despite appropriate or necessary treatment, are a likely result of costs of general consultation, product and the subsequent costs.
Another likely reason for patients spending more on compression therapy is because compression therapy is often offered in the outpatient setting, which requires patients to pay out of pocket rather than relying on insurance11. As mentioned above, compression therapy is only offered in the outpatient setting by wound care nurses; thus, patients who need compression therapy will have to attend the outpatient clinic. This is supported by our finding that the more visits a patient made to the outpatient clinic, the more they would spend.
Limitations
This study has several limitations. Firstly, current literature indicates travel expenses for caregivers to care settings as a direct cost27. This was not calculated in our study. Thus, these estimates are rather conservative and act as an indication of what a typical patient with VLU can expect to spend from the date of diagnosis to completion of treatment or over 2 years. Future studies can include transport costs for a more accurate measure and discuss the implications for both patients (and caregivers if they are accompanied by one) and healthcare organisations. Secondly, it did not examine the reasons compression therapy was not observed by a patient, despite being the gold standard in treatment options for VLU.
Further, as this was a retrospective study, there was no quality of life index to calculate the disability-adjusted life years (DALY). Thirdly, as this is a retrospective study with clearly defined parameters on the data we could and could not access, the costs discussed here cannot be defined with further specificity than that stated in the study. For example, while we have estimated a cost, we are unable to define how much a patient could expect to spend over a month, a year, etc. We are also unable to estimate the costs in cases such as recurrence, which is highly common for VLU. Lastly, this study only offers information on the cost of VLU at SGH. If a patient chose to discontinue their follow-up with SGH or change their healthcare provider, it would not be possible to access their treatment and cost data.
Conclusion
There is no current literature on the frequency of healthcare resource utilisation and the cost of treating VLU for patients on compression therapy in Singapore. Given the long healing time VLU requires, its frequent recurrences and compliance to compression therapy, it is pertinent to understand the costs and burden of disease. The results from this study can help plan, inform and design interventions that could increase the efficacy of the treatment and hospital resources.
Acknowledgements
The authors acknowledge and thank A/Prof Tracy Carol Ayre (group chief nurse, SingHealth), Ms Ng Gaik Nai (chief nurse, SGH) for their continued support, research coordinator Ms Ng Xin Ping and senior staff nurse Chew Suet Mei for their assistance during the data extraction process and Dr Ng Yizhen and Prof Keith Harding for vetting our paper.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study received funding from a grant under the Wound Care Innovation for the Tropics Programme (project number H17/01/a0/0BB9). This was an industry alignment grant under A*STAR and the Skin Research Institute of Singapore of the Biomedical Science Institutes Singapore.
Ethics
This study was approved by the SingHealth Centralised Institutional Review Board in Singapore (CIRB number 2019/2678).
Author(s)
Fazila Aloweni1, Thendral Uthaman*2, Nur L Agus2, Tan W Xian1, Sivagame Maniya3, Ang S Yuh1 and Chong T Tec4
1Division of Nursing, Singapore General Hospital, Singapore
2Division of Nursing Administration, Singapore General Hospital, Singapore
3Division of Nursing, Sengkang General Hospital, Singapore
4Department of Vascular Surgery, Singapore General Hospital, Singapore
*Corresponding author Email Thendral.uthaman@gmail.com
References
- Lal BK. Venous ulcers of the lower extremity: definition, epidemiology, and economic and social burdens. Semin Vasc Surg 2015;28(1):3–5.
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014;17(5):347–56.
- Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, Cartwright D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Hlth 2018;21(1):27–32.
- Meaume S, Gemmen E. Cost-effectiveness of wound management in France: pressure ulcers and venous leg ulcers. J Wound Care 2002;11(6):219–24.
- Weller CD, Bouguettaya A, Britt H, Harrison C. Management of people with venous leg ulcers by Australian general practitioners: an analysis of the national patient-encounter data. Wound Repair Regen 2020;28(4):553–60.
- Goh OQ, Ganesan G, Graves N, Ng YZ, Harding K, Tan KB. Incidence of chronic wounds in Singapore, a multiethnic Asian country, between 2000 and 2017: a retrospective cohort study using a nationwide claims database. BMJ Open 2020;10(9):e039411.
- Meulendijks AM, de Vries FM, van Dooren AA, Schuurmans MJ, Neumann HA. A systematic review on risk factors in developing a first-time venous leg ulcer. J Eur Acad Dermatol Venereol 2019;33(7):1241–8.
- Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: a dual case-control study. J Vasc Surg 1995;22(5):622–8.
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermat 2001;44(3):401–24.
- Karanikolic V, Binic I, Jovanovic D, Golubovic M, Golubovic I, Djindjic N, Petrovic D. The effect of age and compression strength on venous leg ulcer healing. Phlebol 2018;33(9):618–26.
- Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. Int Wound J 2019;16(1):112–21.
- Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 2013.
- Phillips CJ, Humphreys I, Thayer D, Elmessary M, Collins H, Roberts C, Naik G, Harding K. Cost of managing patients with venous leg ulcers. Int Wound J 2020;17(4):1074–82.
- Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. BMJ 2004;328(7452):1358–62.
- Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J 2018;15(1):29–37.
- Nair B. Compression therapy for venous leg ulcers. Indian Dermatol Online J 2014;5(3):378.
- Ashby RL, Gabe R, Ali S, Adderley U, Bland JM, Cullum NA, Dumville JC, Iglesias CP, Kang’ombe AR, Soares MO, Stubbs NC. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet 2014 Mar 8;383(9920):871–9.
- Ivins N, Jones N. Two-layer reduced compression system for lower limb wounds: a non-comparative evaluation. Brit J Commun Nurs 2020;25(Sup4):S10–6.
- Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, Woo K, Romanelli M, Kirsner RS. What’s new: management of venous leg ulcers: treating venous leg ulcers. J Am Acad Dermatol 2016;74(4):643–64.
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, Epstein DM, Nyamekye I, Poskitt KR, Renton S, Warwick J. A randomized trial of early endovenous ablation in venous ulceration. New Eng J Med 2018;378(22):2105–14.
- Barwell JR, Taylor M, Deacon J, Ghauri AS, Wakely C, Phillips LK, Whyman MR, Poskitt KR. Surgical correction of isolated superficial venous reflux reduces long-term recurrence rate in chronic venous leg ulcers. Eur J Vasc Endovasc Surg 2000;20(4):363–8.
- Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, Symonds T. Measuring the impact of venous leg ulcers on quality of life. J Wound Care 2005;14(2):53–7.
- Ragnarson Tennvall G, Hjelmgren J. Original research articles – clinical science: annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair Regen 2005 Jan;13(1):13–8.
- Simka M, Majewski E. The social and economic burden of venous leg ulcers. Am J Clin Dermatol 2003;4(8):573–81.
- Lim MK. Shifting the burden of health care finance: a case study of public–private partnership in Singapore. Health Policy 2004;69(1):83–92.
- Integrated Health Information Systems (IHiS). Project showcase: Electronic Health Intelligence System (eHINTS); 2021 [cited 2020 Jun 2]. Available from: https://www.ihis.com.sg/Project_Showcase/Healthcare_Systems/Pages/eHINTS.aspx
- Nelzen O. Leg ulcers: economic aspects. Phlebol 2000;15(3–4):110–4.
- van Baal P, Meltzer D, Brouwer W. Future costs, fixed healthcare budgets, and the decision rules of cost-effectiveness analysis. Health Econ 2016;25(2):237–48.
- Lo ZJ, Lim X, Eng D, Car J, Hong Q, Yong E, Zhang L, Chandrasekar S, Tan GW, Chan YM, Sim SC. Clinical and economic burden of wound care in the tropics: a 5-year institutional population health review. Int Wound J 2020;17(3):790–803.
- Tennvall GR, Hjelmgren J, Oien R. The cost of treating hard-to-heal venous leg ulcers: results from a Swedish survey. World Wide Wound 2006;Nov:1–7.
- Edwards H, Courtney M, Finlayson K, Lindsay E, Lewis C, Shuter P, Chang A. Chronic venous leg ulcers: effect of a community nursing intervention on pain and healing. Nurs Stand 2005;19(52).
- O’Brien JF, Grace PA, Perry IJ, Hannigan A, Clarke Moloney M, Burke PE. Randomized clinical trial and economic analysis of four-layer compression bandaging for venous ulcers. J Br Surg 2003;90(7):794–8.
- Lorimer K. Happenings – continuity through best practice: design and implementation of a nurse-led community leg-ulcer service. Can J Nurs Res Arch 2004;1:105–13.
- Bainbridge P. Why don’t patients adhere to compression therapy? Br J Commun Nurs 2013;18(Sup12):S35–40.
- Callam MJ. Leg ulcer and chronic venous insufficiency in the community. In: Venous disease. London: Springer;1999. p.15–25.



