Volume 30 Number 2
Length of stay and readmissions for people with diabetes-related foot ulceration admitted to two public tertiary referral hospitals in Australia
Sarah M Manewell, Sarah J Aitken, Vanessa L Nube, Anna M Crawford, Maria I Constantino, Stephen M Twigg, Hylton B Menz, Cathie Sherrington and Serene S Paul
Keywords Hospitalisation, diabetic foot, foot ulcer, length of stay, readmission
For referencing Manewell SM et al. Length of stay and readmissions for people with diabetes-related foot ulceration admitted to two public tertiary referral hospitals in Australia. Wound Practice and Research 2022; 30(2):82-90.
DOI
https://doi.org/10.33235/wpr.30.2.82-90
Submitted 4 September 2021
Accepted Accepted 26 October 2021
Abstract
Aims/hypothesis To identify hospital admissions and length of stay (LOS) and to investigate readmissions, cumulative LOS and associated factors for diabetes-related foot ulceration (DFU).
Methods Routinely-collected hospital admission data were used to identify DFU-related hospital admissions in two public hospitals between 2012–17. Readmission and cumulative LOS were investigated using negative binomial regression.
Results DFU-related admission was required by 749 patients. Median LOS was 8–10 days (stable across 2012–17). Readmission within 28 days was required by 62 patients (8%) and was significantly more likely with increasing comorbidities (incidence rate ratio [IRR] 1.38, 95% confidence intervals [95% CI] 1.02–1.88). Readmission within 1 year was required by 206 patients (28%), and was significantly more likely for males, unplanned admissions and increasing revascularisation requirements (IRR 1.34–1.70), and significantly less likely for those requiring minor and major amputation (IRR 0.33–0.64). The median cumulative LOS was 13 days (IQR 7–29), and was significantly longer for males, older age, unplanned admissions, those requiring dialysis, and those with increasing revascularisation requirements, comorbidities and mental health or behavioural disorders (IRR 1.02–2.30), and significantly shorter for those with more podiatry attendance (IRR 0.96, 95% CI 0.95–0.97).
Conclusions/interpretation Results provide an important benchmark for DFU outcomes. Predictors for patients at risk of readmission and prolonged cumulative LOS were identified which may be used to guide intervention.
Introduction
Up to 25% of individuals with diabetes will develop a diabetes-related foot ulcer (DFU) throughout their lifetime which has a substantial impact to the patient and healthcare systems1. Hospital admission is frequently required for the management of DFU2–5, and often relates to secondary infection2,4 which is considered potentially preventable6. Other reasons for DFU-related hospital admissions include ischaemia2 and orthopaedic intervention7. A European study found that 46% of individuals with DFU required inpatient care8. In Australia, there were approximately 12,000 hospital admissions for the management of DFU over 1 year5. Patients, as well as healthcare providers, have a shared desire to reduce unnecessary hospital admission, length of stay (LOS), and readmissions.
When hospital admission is required for the management of DFU, LOS and readmission are important outcomes for monitoring healthcare performance over time. Findings outside Australia indicate an average LOS for DFU-related admissions ranging from 6–28 days3,4,9–11. One study followed a cohort of individuals with DFU and identified that DFU-related readmissions within 30 days represented 14.6% of all hospital admissions for this cohort3. Importantly, as readmission is often required for DFU-related care3, the number of days spent in hospital over a period of time (i.e. cumulative LOS) helps us to understand the burden for individuals with DFU and associated service requirements.
Predictors of readmission and cumulative LOS for DFU-related admissions are also important for guiding service delivery and informing future intervention studies. Compared with other populations in Australia, higher rates of diabetes-related foot complications are experienced by Aboriginal and Torres Strait Islander people12; subsequently referred to as Aboriginal people throughout this paper13. Related to this, a key priority for Australian public health outcomes is to improve foot health outcomes for Aboriginal people14. Another key priority for public health outcomes, both within Australia15 but also from an international perspective16, is the recommendation for specialist interdisciplinary management in the care of individuals with DFU. In particular, podiatry care is an important component of preventing diabetes-related foot complications17 and the care of this population is a key function of podiatry services within the public health sector and primary care. As such, knowing whether there are differences in outcomes for individuals who are known to local preventive and management services, versus those who are not, will provide insight into the impact of such services. The aims of this study were therefore twofold – to identify hospital admissions and LOS for DFU and, for these patients, to investigate readmissions, cumulative LOS and associated factors.
Materials and methods
Cohort and datasets
This longitudinal retrospective study analysed admission data from two public principal referral hospitals18, the Royal Prince Alfred and Concord Repatriation General Hospitals, within the Sydney Local Health District (SLHD), New South Wales, Australia, where inpatient care was provided for DFU. Hospital admissions with a discharge date from 1 July 2012 until 30 June 2017 were included, with data up to 30 June 2018 used in analysis to enable calculation of readmissions.
The hospital admission dataset included patients aged 18 years or older at the admission date of incident admission with a diagnosis of DFU as determined by ICD-10-AM codes E10.73, E11.73, E13.73 and E14.73. To identify patients who required hospital admission related specifically to the care of DFU, each hospital admission was categorised into one of two groups – related or unrelated to DFU – using the principal and subsequent diagnosis code for each admission (see supplementary material for further information regarding categorisation of admissions). Only patients with hospital admission(s) categorised as related to DFU were included in the analysis.
Clinic appointment data from SLHD’s podiatry department were also obtained and linked to the hospital admission data to identify which patients were known to SLHD’s two high risk foot services (HRFS) and eight podiatry services. The model of care within SLHD aims to provide interdisciplinary HRFS consultation for people with DFU, primarily in the non-admitted setting, in order to optimise care and to avoid unnecessary hospital admission. The two HRFS include podiatrists, endocrinologists, diabetes educators, vascular surgeons, orthopaedic surgeons, orthotists and pedorthists, and are well integrated with primary care and hospital in the home. Additionally, HRFS provide consultation for inpatients admitted with DFU; however, this is dependent on the attending medical team identifying and referring patients for consultation. Patients with healed DFU and patients at risk but with no history of DFU are offered ongoing care from podiatry services as part of a lifelong foot protection program. Podiatry services provide primary and secondary preventive care across eight centres within SLHD.
The geographic boundaries of SLHD encompass a 126km2 metropolitan area19. The population living in SLHD is estimated at 640,000 people (2016), with 12% aged 65 and over, is culturally diverse, with a language other than English spoken while at home by 43% of the population, and socioeconomically diverse, with 11% of households being classified as low income while 32% are classified as high income19.
This project was approved by the following ethics committees with a waiver of consent: Aboriginal Health and Medical Research Council (HREC ref. 1424/18); Concord Repatriation General Hospital (LNR/15/CRGH/249); Royal Prince Alfred Hospital (LNR/15/CRGH/249). All stages of this project were guided by an Aboriginal Reference Group.
Outcome measures and data analysis
All patient characteristics were measured from hospital admission data, with the exception of whether patients were known to local HRFS or podiatry services, which was measured by linking scheduling data to the hospital admission data. Patient characteristics were obtained from each patient’s incident admission. Sex, Aboriginal status, receiving care for a mental health or behavioural disorder, current smoking status and requirement for dialysis were measured as categorical data. Admission type was also measured as a categorical variable and aimed to compare unplanned with planned admissions. However, admission type was recorded as unknown for a large proportion of the cohort (71 patients, 9.5%) and, due to the large patient loss this would represent if excluded from regression analysis, unknown admission type was also included as a category.
Whether patients were known to local HRFS or podiatry services at the time of their incident admission (defined as 4 weeks prior to, during or 4 weeks post-admission) was also measured as categorical data. This timeframe was chosen to represent whether or not patients were known to these services around the time of their incident admission. Minor amputation(s), major amputation(s) and revascularisation procedure(s) were measured as discrete count variables, as was a modified Charlson Comorbidity count. For each patient, a count of conditions from the Charlson Comorbidity Index was calculated20; however, diabetes was excluded as all patients had this particular condition. Age was measured as a continuous variable and presented in years. For patient characteristics and outcomes, categorical and count variables were presented as proportions of the total cohort, while continuous variables which were normally distributed were presented as mean with standard deviation.
Admission LOS was measured in days as a discrete count outcome. Admission LOS across financial years (1 July until 30 June) based on date of discharge were skewed and reported as median with IQR and compared using the Kruskall-Wallis analysis of variance. Patient outcomes of readmissions (within 28 days and 1 year) and cumulative DFU-related LOS were all measured as discrete counts. From the date of discharge for each patient’s incident admission, the number of DFU-related readmissions within 28 days and within 1 year were identified. Patients were deemed to have required DFU-related readmission if the admission date of a subsequent DFU-related admission was 1 or more days following the previous admission’s discharge date. From 1 July 2012 until 30 June 2017, cumulative outcomes were identified for each patient including the total number of DFU-related admissions, days spent in hospital for DFU-related care (i.e. cumulative LOS), minor amputations and major amputations. These cumulative outcomes were identified by adding discrete count outcomes from each DFU-related admission the patient required during 2012–17. From 4 weeks prior to each patient’s incident admission until 30 June 2017, the number of appointments attended with a podiatrist within SLHD, either as part of the HRFS or podiatry services, was also identified. Mortality was reported based on mortality during any DFU-related admission from 2012–17. All patient outcomes were presented as proportions of the total cohort, with the exception of cumulative LOS which was skewed and presented as median with IQR.
Predictors of the number of readmissions (within 28 days and 1 year) and cumulative LOS per patient were identified using negative binomial regression. Possible predictors included outcomes obtained during each patient’s incident admission, with the following additional predictors also included in models examining cumulative LOS – number of amputations during 2012–17, number of HRFS appointments, and number of podiatry service appointments from 4 weeks prior to incident admission until 30 June 2017. Predictors from univariable regression with a p value <0.2 were included in a multivariable regression model. Where there was evidence of collinearity (r≥0.7), only the variable with the strongest association from univariable regression was included in the multivariable regression model. Regression results were reported as incidence rate ratios (IRR) with 95% confidence intervals (95% CI) and p values. A p value of <0.05 was considered statistically significant. All analyses were conducted using SAS Enterprise Guide v7.1 (SAS Institute Inc. North Carolina, USA).
Results
Patient and admission characteristics
During 2012–17, care for DFU was provided for 1,062 patients through 2,102 hospital admissions across the two hospitals in SLHD. Of these, 1,250 (59.5%) admissions were DFU-related; 637 (30.3%) admissions had a principal diagnosis of DFU and an additional 613 (29.2%) admissions were DFU-related. These 1,250 DFU-related admissions, required by 749 (71%) patients (Table 1), were included in the analysis.
Table 1. Patient characteristics at incident admission (n=749)
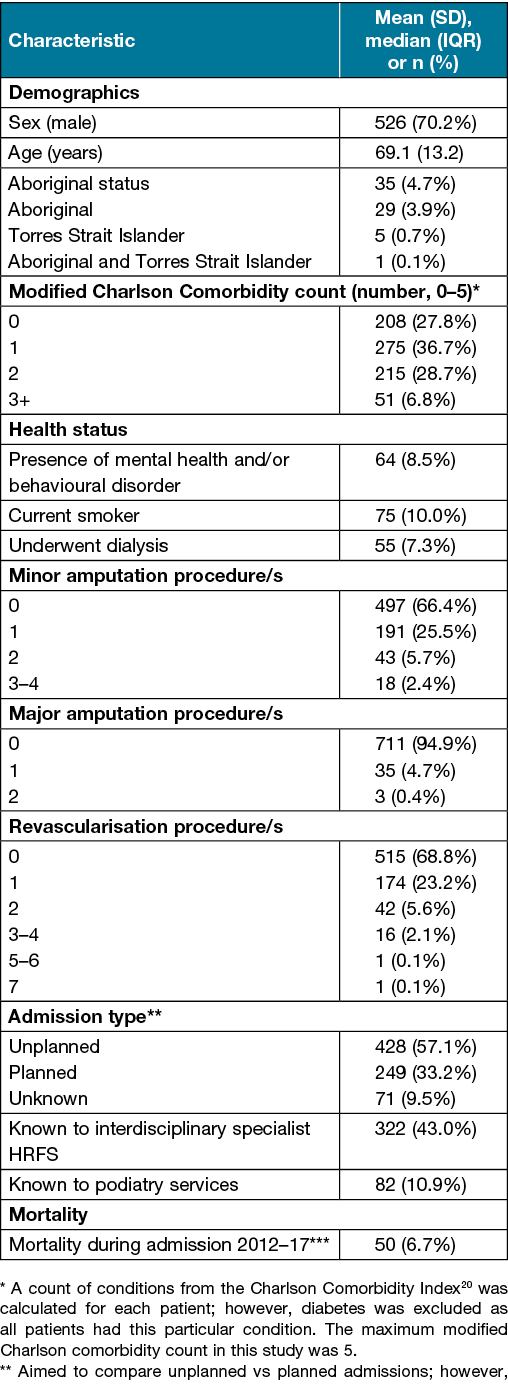
Median LOS (IQR) for DFU-related admissions was 10 days (5–18) during 2012–13, 8 days (4–17) during 2013–14, 9 days (5–16) during 2014–15, 10 days (4–19) during 2015–16 and 8 days (4–16) during 2016–17. Median LOS remained stable during 2012–17 (p=0.15).
Patient outcomes
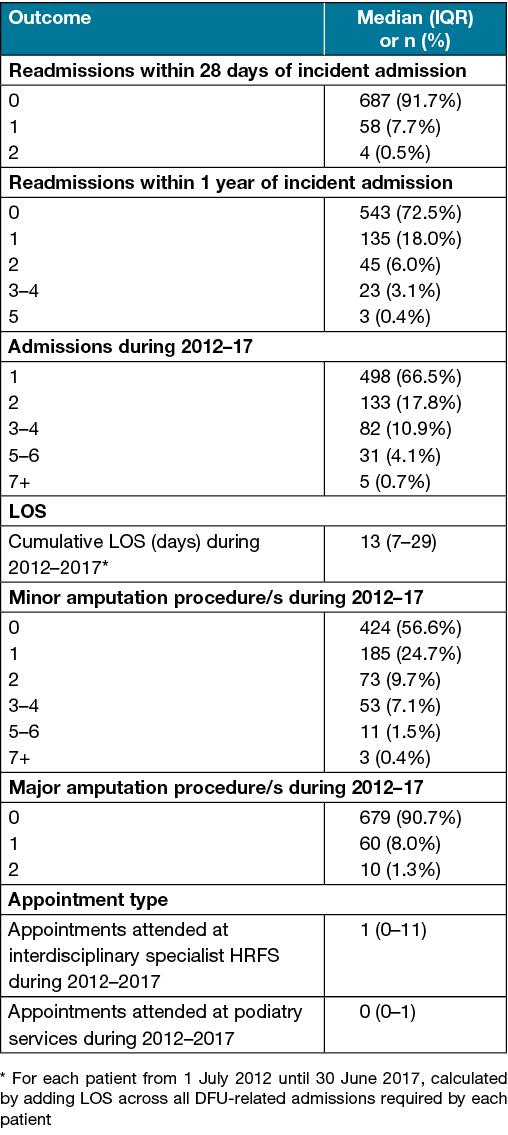
One to two DFU-related readmissions were required within 28 days of the incident admission for 62 patients (8.3%). One to five DFU-related readmissions were required within 1 year of incident admission for 206 patients (27.5%). One to eight DFU-related admissions were required by each patient during 2012–17. The median cumulative LOS for DFU-related care per patient during 2012–17 was 13 days (IQR 7–29; overall range 1–236). One to ten minor amputations were required by 325 patients (43.4%) during 2012–17. One to two major amputations were required by 70 patients (9.3%) during 2012–17 (Table 2).
Table 2. Patient outcomes during 2012–17
Predictors of readmission within 28 days
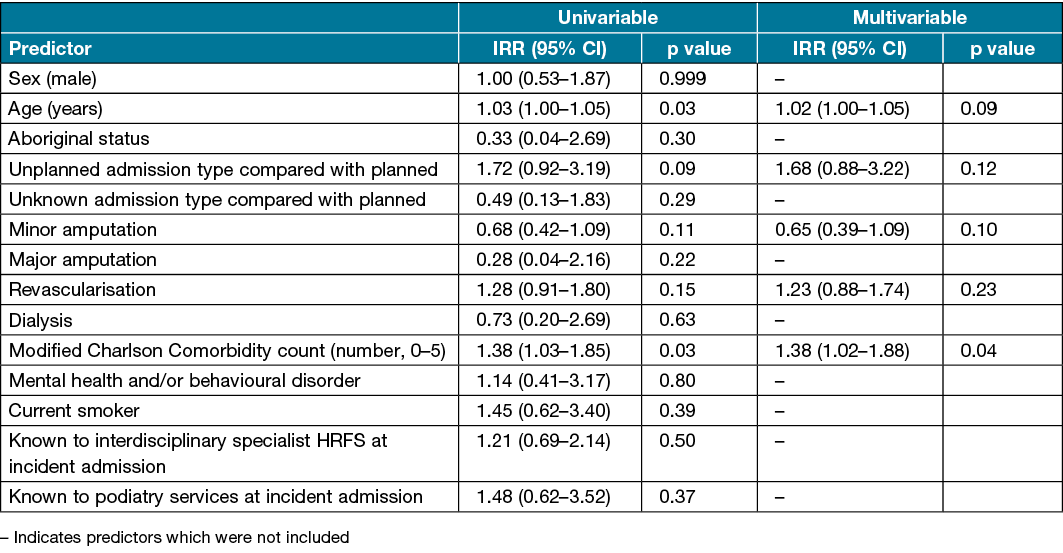
One patient (0.1%) was excluded from the 28 day readmission regression analysis due to missing data. Univariable analysis found that increasing age and higher modified Charlson Comorbidity counts were associated with an increased risk of readmission within 28 days (Table 3). In multivariable analysis, only a higher modified Charlson Comorbidity count (IRR 1.38, 95% CI 1.02–1.88) was independently associated with increased risk of readmission within 28 days (Table 3).
Table 3. Predictors of number of readmissions within 28 days
Predictors of readmission within 1 year
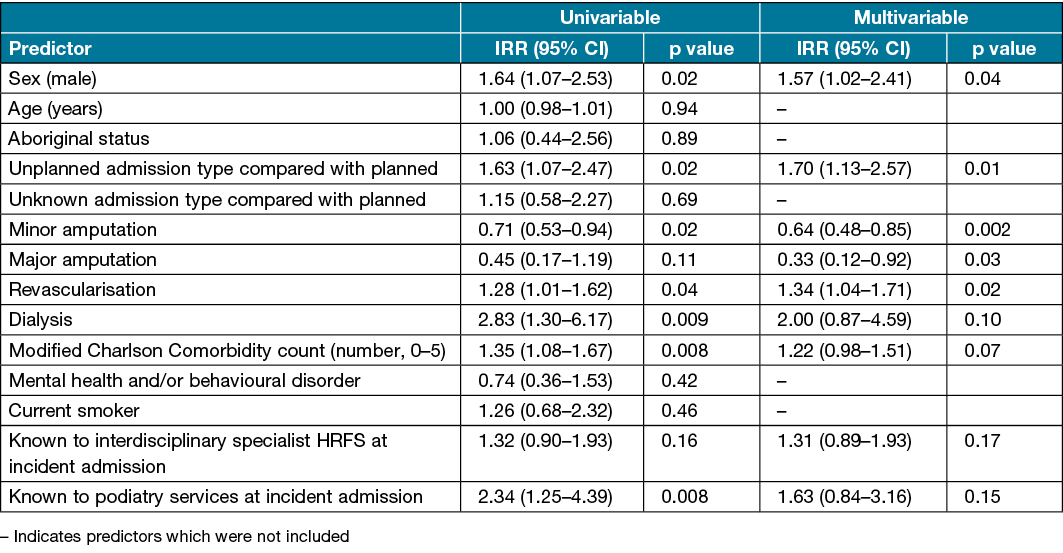
One patient (0.1%) was excluded from the 1 year readmission regression analysis due to missing data. Univariable analysis found that male sex, unplanned admissions, revascularisation, dialysis, a higher modified Charlson Comorbidity count, and being known to podiatry services at incident admission were associated with an increased risk of readmission within 1 year, while minor amputations were associated with reduced risk (Table 4). Multivariable analysis found that male sex (IRR 1.57, 95% CI 1.02–2.41), unplanned admissions (IRR 1.70, 95% CI 1.13–2.57) and requiring revascularisation (IRR 1.34, 95% CI 1.04–1.71) were independently associated with an increased risk of readmission within 1 year. Minor amputations (IRR 0.64, 95% CI 0.48–0.85) and major amputations (IRR 0.33, 95% CI 0.12–0.92) were independently associated with reduced risk (Table 4).
Table 4. Predictors of number of readmissions within 1 year
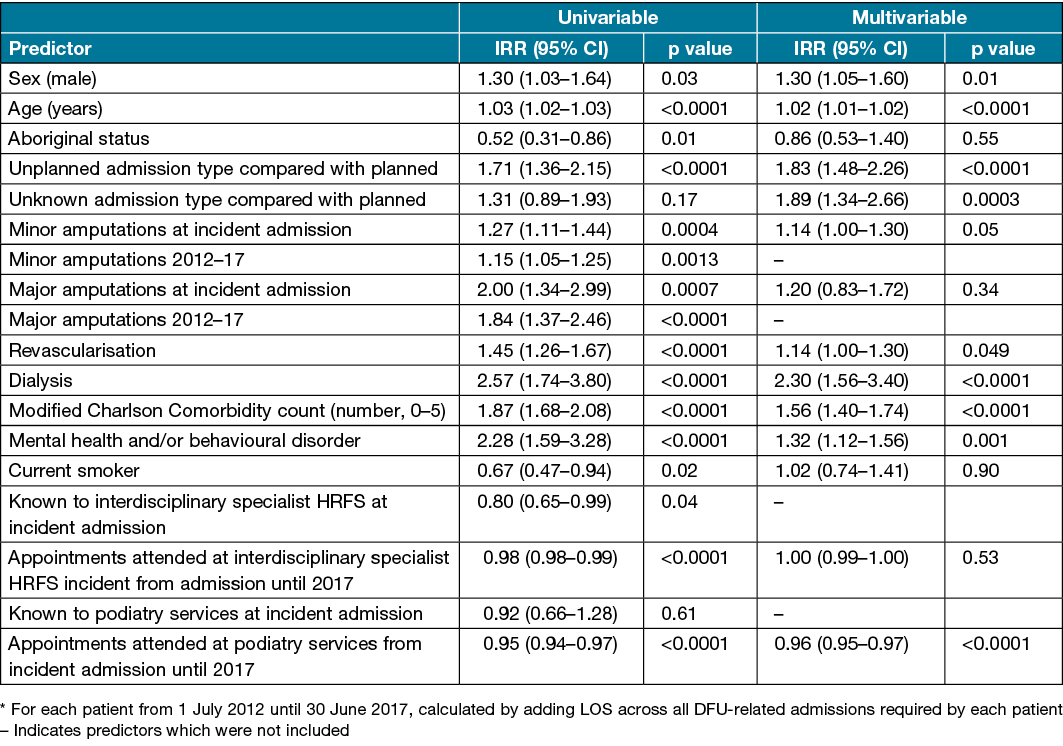
Predictors of cumulative LOS
Three patients (0.4%) were excluded from cumulative LOS regression analysis due to missing data. Univariable analysis found that male sex, increasing age, unplanned admissions, minor and major amputations (at incident admission and throughout 2012–17), revascularisation, dialysis, a higher modified Charlson Comorbidity count and receiving care for a mental health or behavioural disorder were associated with longer cumulative LOS. In contrast, recorded Aboriginal status, being a current smoker, being known to the HRFS at incident admission, and the number of appointments attended at HRFS or podiatry services from incident admission until 2017 were associated with lower cumulative LOS (Table 5). Multivariable analysis found that male sex (IRR 1.30, 95% CI 1.05–1.60), increasing age (IRR 1.02, 95% CI 1.01–1.02), unplanned admissions (IRR 1.83, 95% CI 1.48–2.26), revascularisation (IRR 1.14, 95% CI 1.00–1.30), dialysis (IRR 2.30, 95% CI 1.56–3.40), a higher modified Charlson Comorbidity count (IRR 1.56, 95% CI 1.40–1.74) and receiving care for a mental health or behavioural disorder (IRR 1.32, 95% CI 1.12–1.56) were associated with longer cumulative LOS. Attending more appointments with the podiatry services from incident admission until 2017 (IRR 0.96, 95% CI 0.95–0.97) was associated with shorter cumulative LOS (Table 5).
Table 5. Predictors of cumulative LOS* from 2012–17
Discussion
This study presents a unique, systematic and reproducible approach to quantify outcomes for inpatients who presented to two large tertiary hospitals for DFU-related care. This approach is important as results from the current study indicate that identifying hospital admissions using only a principal diagnosis of DFU misses approximately half of the target cohort. This relates to the complexities for hospital coding in selecting the principal diagnosis for each admission, as the principal diagnosis may reflect the presenting acute condition (e.g. cellulitis) or the underlying chronic condition (i.e. DFU)21,22. Optimal methods for capturing a cohort of patients with DFU using administrative data have been discussed previously23 and results from the current study support the need to consider additional diagnoses to aid improved sensitivity for identifying DFU-related admissions.
In the current study, 749 patients required 1,250 DFU-related hospital admissions from 2012–17, with 325 patients (43.4%) requiring minor amputation and 70 patients (9.3%) requiring major amputation. These findings are difficult to compare with findings from other local health districts due to methodological differences. For example, the current study focused on patients, while other studies focused on associated admissions or procedures24,25. This supports the need for a national approach to track key indicators for patients with DFU such as the recently established Australian Diabetic Foot Ulcer Minimum Dataset26. As a point of reference, 13.8 diabetes-related lower limb amputations (minor and major) were required per 100,000 people in SLHD during 2016–18, with a range between 6.5 and 22.8 amputations per 100,000 people among the state’s local health districts27.
Median LOS was 8–10 days for DFU-related hospital admissions from 2012–17. These results are comparable to a study in Thailand where a median LOS of 8 days for DFU-related hospital admission was reported28. In Australia, one study reported an average LOS of 17 days for diabetes-related foot complications29; however, the sample differed from the current study’s specific focus on DFU-related inpatient care. In the current study, LOS remained stable across financial years, which is comparable with a study in the USA where average LOS was 7.0 days in 2005 and 6.8 days in 20109.
Following their incident admission, 8.3% of patients required DFU-related readmission within 28 days and 27.5% of patients required DFU-related readmission within 1 year. The proportion of patients in the current study who required short-term readmission (within 28 days) is lower than a study in the USA which reported that 30% of patients with DFU returned to hospital within 30 days. However in the USA study, all presentations to emergency departments and hospital admissions were included for care both related and unrelated to DFU management4. Of interest, another study in Germany reported a significant reduction in the proportion of patients who required readmission within 3 months for those with diabetes and neuroischaemic ulceration, from 16.4% to 8.8%, following the implementation of a new system integrating care across inpatient and outpatient settings30.
The cumulative median LOS for patients with DFU over the 5 years from 2012–17 was 13 days. Other studies reporting cumulative LOS for diabetes-related foot complications have only tracked patients for 12–14 months8,31. In Australia, one study reported a median cumulative LOS of 29 days over 14 months; however, this study focused on diabetes-related foot infections31 which differed from the current study’s broader topic of DFU-related inpatient care. Another study in Europe followed patients with DFU for up to 12 months and reported an average LOS of 16 days8. As both other studies reported longer cumulative LOS over shorter follow-up periods (compared with the current study’s 5 year period), this suggests that patients with DFU in SLHD may have better outcomes.
Multivariable regression findings identified several predictors of readmission and cumulative LOS. One interesting finding was that minor and major amputations were associated with fewer presentations to hospital within a medium timeframe (1 year readmission). However, minor and major amputations were not associated with presentations to hospital within a short timeframe (28 day readmission) and were not associated with time spent in hospital over an extended timeframe (cumulative LOS). These findings are relevant as preventing amputation is a priority32; however, when amputation is required, timely decision making to support patient care is indicated. Of interest, findings from the current study were different to a study in the USA where minor amputation was associated with lower chance of readmission within a short timeframe of 30 days4. This difference in outcomes may relate to the USA study including presentations to emergency departments and hospital admissions for any purpose, while the current study specifically targeted admissions related to DFU.
The patient phenotype for those with DFU commonly involves a series of comorbidities, especially in patients with DFU who require hospital admission9. The current study found that a higher modified Charlson Comorbidity count was linked to adverse outcomes, including more readmissions within 28 days and higher cumulative LOS. Additionally, the requirement for revascularisation was linked to more 1 year readmissions and a higher cumulative LOS, which is similar to another study regarding patients with peripheral arterial disease that reported particularly high rates of readmission within 1 year (70%)33. The requirement for dialysis and receiving care for mental health or behavioural disorders were also linked to a higher cumulative LOS. These findings emphasise that patients with this profile are a high-risk group requiring progressive in-hospital care, and with greater associated costs9. Whether intensifying aspects of resource provision in these DFU patient subgroups would lead to fewer readmissions or reduced cumulative LOS and be cost-effective remains to be determined.
Unexpectedly, multivariable regression findings identified two predictors not significantly associated with readmissions and cumulative LOS. Firstly, recorded Aboriginal status was not associated with readmissions or cumulative LOS. Another Australian study also reported no difference in cumulative LOS over 14 months for Aboriginal people versus non-Aboriginal people with diabetes-related foot infections31 which encourages consideration of other predictors which may influence outcomes for Aboriginal people. One predictor may include a higher risk of leaving before medical treatment is complete, which may relate to a wide range of factors34. Also of note, Aboriginal people represented 4.7% of the current study’s cohort, five times higher than the proportion of the population in SLHD (0.9%)35. However, 4.7% was still a small portion of the study cohort, which may contribute to the null findings. This encourages a larger scale, more focused investigation, such as a case-control study, to better understand readmission and LOS outcomes for Aboriginal people, with a goal of ongoing improvement.
Secondly, recorded current smoking status was not associated with readmissions or cumulative LOS. These findings are in contrast with a study in the USA where current smoking was associated with higher odds of readmission within 30 days3. This difference in outcomes may relate to the broader admission intake criteria and the higher proportion of patients who were identified as being current smokers (27%) in the USA study compared with only 10% in the current study.
Another focus of the current study was to consider outcomes for patients who required DFU-related admission based on whether they were known to local HRFS and podiatry services. At the time of their incident admission, 322 patients (43.0%) were known to local HRFS. Given that HRFS aim to optimise care and help avoid unnecessary hospital admissions in this cohort, this proportion is low36. Within SLHD, prompt access to HRFS consultation for people with DFU has been achieved with rapid triage, prioritisation based on clinical need and workforce strategies. Local initiatives targeting primary care with education, promotion of foot assessment and referral pathways have been ongoing; however, findings from the current study suggest scope for improvement in patients being identified for treatment before hospital admission is indicated. We propose that the proportion of admitted patients with DFU who are known to local HRFS and podiatry services is an indicator of access to preventive foot care, which warrants further monitoring, although no current benchmark exists. Multivariable analysis from the current study found that being known to local HRFS was not predictive of readmissions or cumulative LOS. These findings are similar to another study in the UK which compared LOS before and after the implementation of a coordinator for inpatients with diabetes and foot complications, with no significant reduction in LOS37. However, the current study found that attending more appointments with the podiatry services from incident admission until 2017 was associated with lower cumulative LOS. This may be because patients who attend more podiatry appointments are traditionally ulcer-free while they attend, as ulcers are most often managed in HRFS. Patients who attend more podiatry appointments may also be more proactive in preventive care, and/or podiatry services may help to reduce the rate of future DFU and the associated LOS for inpatient care38. These findings are consistent with a study in the USA which identified that individuals with DFU who received care from a podiatrist in the previous year were less likely to require admission to hospital39.
Limitations
The current study has some limitations which must be considered, including its retrospective design. Hospital admission data are acknowledged to under-report numerous outcomes, including inpatients who receive care for DFU40 as well as recorded Aboriginal status41. Furthermore, hospital admission data does not include variables which may potentially influence the outcomes investigated in this study such as ulcer severity. The HRFS and podiatry services included as predictors in the current study primarily see outpatients who live within SLHD’s geographic boundaries; however, the admitted patient population is more likely to include people who reside outside SLHD’s geographic boundaries, including regional and remote areas. As such, results from the current study are not generalisable to other settings or populations. Furthermore, the current study’s dataset did not include consultations with other providers, including podiatrists working in the private sector, general practitioners, vascular surgeons and district nurses.
Overall, the current study provides insight into the health-related journey for patients with DFU who required inpatient management over a 5 year period. The findings from this study can also guide the design for future service improvement projects and intervention studies for patients with DFU, such as increased access to public podiatry ambulatory services and improved support for inpatients who have a higher Charlson Comorbidity count. Previous intervention studies have demonstrated positive outcomes for patients with diabetes-related foot complications, including reduced LOS38 and reduced rate of hospital readmissions30, which supports the importance for ongoing service improvement and monitoring.
Acknowledgements
Many thanks to members of our Aboriginal Reference Group who have overseen this project – C Daylight, L Wright, A Mumbulla, A Crawford and R Lyons. Thank you also to K Schemann from the Sydney Informatics Hub, the University of Sydney, for advice regarding statistical analysis.
Author contributions
Author contributions included: conception and design SMM, VLN, AMC, MIC, SMT, HBM, CS and SSP; acquisition and analysis of data SMM, SJA, VLN, MIC and SSP; interpretation of data SMM, SJA, AMC and SSP; and manuscript drafting SMM and SSP. All authors provided important intellectual content during manuscript revision and approved this final manuscript for publication.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by a SLHD Allied Health Research Small Grant which supported the time commitments for the lead author to analyse and interpret the data. This financial support had no involvement in the study design, collection, analysis or interpretation of data.
Ethics statement
This project was approved by the following ethics committees with a waiver of consent: Aboriginal Health and Medical Research Council (HREC ref. 1424/18); Concord Repatriation General Hospital (LNR/15/CRGH/249); Royal Prince Alfred Hospital (LNR/15/CRGH/249). All stages of this project were guided by an Aboriginal Reference Group
Appendix
Click here to download the appendix
Author(s)
Sarah M Manewell*1,2, Sarah J Aitken3,4, Vanessa L Nube2,5, Anna M Crawford2,5, Maria I Constantino5, Stephen M Twigg5,6, Hylton B Menz7, Cathie Sherrington8 and Serene S Paul1
1School of Health Sciences, Faculty of Medicine and Health, The University of Sydney, NSW, Australia
2Podiatry Department, Sydney Local Health District NSW Health, NSW, Australia
3Vascular Department, Concord Repatriation General Hospital, Sydney Local Health District NSW Health, NSW, Australia
4Concord Clinical School, Faculty of Medicine and Health, The University of Sydney, NSW, Australia
5High Risk Foot Service, Diabetes Centre Royal Prince Alfred Hospital, Sydney Local Health District NSW Health, NSW, Australia
6Central Clinical School, Faculty of Medicine and Health, The University of Sydney, NSW, Australia
7School of Allied Health, Human Services and Sport, La Trobe University, Melbourne, VIC, Australia
8Institute for Musculoskeletal Health, Faculty of Medicine and Health, The University of Sydney/Sydney Local Health District, NSW, Australia
*Corresponding author Email sarah.manewell@health.nsw.gov.au
References
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217–28.
- Pemayun TGD, Naibaho RM. Clinical profile and outcome of diabetic foot ulcer, a view from tertiary care hospital in Semarang, Indonesia. Diabet Foot Ankle 2017;8(1) :1312974.
- Holscher CM, Hicks CW, Canner JK, Sherman RL, Malas MB, Black JH 3rd, et al. Unplanned 30-day readmission in patients with diabetic foot wounds treated in a multidisciplinary setting. J Vasc Surg 2018;67(3):876-86.
- Remington AC, Hernandez-Boussard T, Warstadt NM, Finnegan MA, Shaffer R, Kwong JZ, et al. Analyzing treatment aggressiveness and identifying high-risk patients in diabetic foot ulcer return to care. Wound Repair Regen 2016;24(4):731–6.
- Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Practice Res 2014;22(1):20–33.
- Australian Institute of Health and Welfare. National Healthcare Agreement: PI 18 – Selected potentially preventable hospitalisations; 2017. Available from: https://meteor.aihw.gov.au/content/index.phtml/itemId/630028
- Kılıçoğlu Öİ, Demirel M, Aktaş Ş. New trends in the orthopaedic management of diabetic foot. EFORT Open Rev 2018;3(5):269–77.
- Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, Edmonds M, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia 2008;51(10):182–34.
- Hicks CW, Selvarajah S, Mathioudakis N, Perler BA, Freischlag JA, Black JH 3rd, et al. Trends and determinants of costs associated with the inpatient care of diabetic foot ulcers. J Vasc Surg 2014;60(5):1247–54
- Skrepnek GH, Mills JL, Sr., Armstrong DG. A diabetic emergency one million feet long: disparities and burdens of illness among diabetic foot ulcer cases within emergency departments in the United States, 2006–2010. PLoS One 2015;10(8):e0134914.
- Hartemann-Heurtier A, Ha Van G, Danan JP, Koskas F, Jacqueminet S, Golmard JL, et al. Outcome of severe diabetic foot ulcers after standardised management in a specialised unit. Diabetes Metab 2002;28(6 Pt 1):477–84.
- West M, Chuter V, Munteanu S, Hawke F. Defining the gap: a systematic review of the difference in rates of diabetes-related foot complications in Aboriginal and Torres Strait Islander Australians and non-Indigenous Australians. J Foot Ankle Res 2017;10:48.
- Centre for Aboriginal Health, NSW Health. Communicating positively: a guide to appropriate Aboriginal terminology; 2019. Available from: https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2019_008.pdf
- Department of Health. Increased support for Indigenous diabetics; 2019. Available from: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/increased-support-for-indigenous-diabetics-0
- National Association of Diabetes Centres and The Australian Diabetes Society. NADC Collaborative Interdisciplinary Diabetes High Risk Foot Services (HRFS) Standards; 2018. Available from: https://nadc.net.au/hrfs-standards/
- Schaper NC, Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36(S1):e3266.
- International Working Group on the Diabetic Foot. IWGDF Guidelines on the prevention and management of diabetic foot disease; 2019. Available from: https://iwgdfguidelines.org/guidelines/guidelines/
- Australian Institute of Health and Welfare. Australian hospital peer groups. Canberra: AIHW; 2015. Available from: https://nla.gov.au/nla.obj-749954943/view
- Sydney Local Health District NSW Health. A picture of health Sydney Local Health District Health Profile; 2015. Available from: https://www.slhd.nsw.gov.au/planning/pdf/SLHD_Health_Profile.pdf
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83.
- Czahor A. The principles of principal diagnosis selection at your facility. Brief Coding Compliance Strateg 2017;20(5):1–4.
- Anonymous. Coping with principal diagnosis coding conundrums. Brief Coding Compliance Strateg 2011;14(8):10–2.
- Sohn M-W, Budiman-Mak E, Stuck RM, Siddiqui F, Lee TA. Diagnostic accuracy of existing methods for identifying diabetic foot ulcers from inpatient and outpatient datasets. J Foot Ankle Res 2010;3:27.
- Australian Commission on Safety and Quality in Health Care. Australian Atlas of Healthcare Variation 2015; 2015. Available from: https://www.safetyandquality.gov.au/sites/default/files/migrated/SAQ201_07_Chapter6_v7_FILM_tagged_merged_6-8.pdf
- Australian Institute of Health and Welfare. Burden of lower limb amputations due to diabetes in Australia: Australian Burden of Disease Study 2011; 2017. Available from: https://www.aihw.gov.au/getmedia/9292ab2b-4dbb-44ca-846f-832d02db7220/20681.pdf.aspx?inline=true
- Diabetes Feet Australia. The Australian Diabetic Foot Minimum Dataset; 2021. Available from: https://www.diabetesfeetaustralia.org/for-researchers/
- HealthStats NSW. Amputations due to diabetes; 2020. Available from: http://healthstats.doh.health.nsw.gov.au/Indicator/dia_prochos/dia_prochos_comparison
- Thewjitcharoen Y, Sripatpong J, Krittiyawong S, Porramatikul S, Srikummoon T, Mahaudomporn S, et al. Changing the patterns of hospitalized diabetic foot ulcer (DFU) over a 5-year period in a multi-disciplinary setting in Thailand. BMC Endocr Disord 2020;20(1):89.
- Lawrence SM, Wraight PR, Campbell DA, Colman PG. Assessment and management of inpatients with acute diabetes-related foot complications: room for improvement. Intern Med J 2004;34(5):229–33.
- Rumenapf G, Geiger S, Schneider B, Amendt K, Wilhelm N, Morbach S, et al. Readmissions of patients with diabetes mellitus and foot ulcers after infra-popliteal bypass surgery – attacking the problem by an integrated case management model. Vasa 2013;42(1):56–67.
- Commons RJ, Robinson CH, Gawler D, Davis JS, Price RN. High burden of diabetic foot infections in the top end of Australia: An emerging health crisis (DEFINE study). Diabetes Res Clin Pract 2015;110(2):147–57.
- Diabetes Australia. 4400 reasons to end amputations. Available from: https://www.diabetesaustralia.com.au/4400-reasons/
- Aitken SJ, Randall DA, Noguchi N, Blyth FM, Naganathan V. Multiple peri-operative complications are associated with reduced long term amputation free survival following revascularisation for lower limb peripheral artery disease: A population based linked data study. Eur J Vasc Endovasc Surg 2020;59(3):437–45.
- Australian Commission on Safety and Quality in Health Care. Understanding leave events for Aboriginal and Torres Strait Islander peoples and other Australians from health service organisations: a systematic literature review. Sydney: ACSQHC; 2020. Available from: https://www.safetyandquality.gov.au/sites/default/files/2020-07/understanding_leave_events_for_aboriginal_and_torres_strait_islander_people.pdf
- Sydney Local Health District NSW Health. Aboriginal Health Strategic Plan 2018–2022. Available from: https://www.slhd.nsw.gov.au/pdfs/AboriginalHealthStrategicPlan2018-2022.pdf
- Agency for Clinical Innovation. Standards for High Risk Foot Services (HRFS) in NSW; 2014. Available from: https://aci.health.nsw.gov.au/__data/assets/pdf_file/0007/459709/ACI_Standards_for_High_Risk_Foot_Services.pdf
- Cichero MJ, Bower VM, Walsh TP, Yates BJ. Reducing length of stay for acute diabetic foot episodes: employing an extended scope of practice podiatric high-risk foot coordinator in an acute foundation trust hospital. J Foot Ankle Res 2013;6(1):47.
- Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract 2005;70(1):31–7.
- Gibson TB, Driver VR, Wrobel JS, Christina JR, Bagalman E, DeFrancis R, et al. Podiatrist care and outcomes for patients with diabetes and foot ulcer. Int Wound J 2014;11(6):641–8.
- Wraight PR, Lawrence SM, Campbell DA, Colman PG. Retrospective data for diabetic foot complications: only the tip of the iceberg? Intern Med J 2006;36(3):197–9.
- Australian Institute of Health and Welfare. Indigenous identification in hospital separations data: quality report. Canberra: AIHW; 2013. Available from: https://www.aihw.gov.au/reports/indigenous-australians/indigenous-identification-in-hospital-separations/contents/table-of-contents



