Volume 30 Number 2
The effect of tea tree oil on wound healing in diabetic rats
Yeliz Sürme, Gülsüm Nihal Çürük, Ayça Lekesizcan and Saim Özdamar
Keywords Diabetes, nursing, wound healing, tea tree oil, rats
For referencing Sürme Y et al. The effect of tea tree oil on wound healing in diabetic rats. Wound Practice and Research 2022; 30(2):91-98.
DOI
https://doi.org/10.33235/wpr.30.2.91-98
Submitted 8 August 2021
Accepted 16 November 2021
Abstract
Aim This study was conducted as a randomised controlled study to determine the effect of tea tree oil on acute wound healing.
Methods Rats were divided randomly into two groups, non‑diabetic and ‘diabetic’; rats in the diabetic group were made diabetic by intraperitoneal streptozotocin induction at 50 mg/kg. Each group was then subdivided into sunflower oil, tea tree oil and saline (0.9% NaCl) groups. After incisional wound formation, rats were wound-dressed according to their treatment group every day for 15 days. On day 3, 7 and 15 following the wound formation, 0.5cmx0.5cm full thickness tissue samples were taken and examined histopathologically.
Results On day 3, the epithelisation and inflammatory cell density of the non‑diabetic tea tree oil group was found to be statistically significantly higher than the diabetic saline group. There was a statistical difference in favour of the non‑diabetic tea tree oil group in terms of procollagen and mature collagen density. In addition, the non‑diabetic tea tree oil group had a statistically higher angiogenesis amount than the diabetic and non‑diabetic saline and the diabetic sunflower oil groups on day 15 (p<0.05).
Conclusions It has been determined that tea tree oil has an accelerating effect on wound healing and is an alternative method that can be used in wound dressing.
Introduction
In patients with diabetes mellitus (DM), decreased cellular infiltration, angiogenesis, granulation tissue and collagen delay wound healing1,2. This situation leads to increased prolonged hospital stay, and therefore health spending3. In the meta-analysis conducted by Zhang et al.4 there is a significant relationship between DM and the risk of infection in the surgical wound. In another study, the rate of surgical site infection (6.1%) was higher in patients with DM compared to patients without DM (4.3%), and their duration of hospital stay was significantly longer5.
Selecting the appropriate dressing material for the wound helps prevent wound healing complications6,7. Various problems, such as the emergence of resistant microorganisms in recent years and the lack of effective, new generation antibiotic production, directed healthcare professionals interested in wound care to focus on dressing materials. Thus, the use of herbal products has increased in wound care8,9.
One type of herbal product used in wound treatment is tea tree oil. Tea tree oil, which has antifungal, antibacterial and antiviral effects, has long been used for various purposes, including as an antiseptic10,11. Tea tree oil, which has a long history in integrated medicine, is accepted as a suitable disinfectant for topical use due to its easy application to the skin and is considered as an effective topical antimicrobial product11. In the study conducted by Falci et al.12, tea tree oil was found to be effective on Staphylococcus aureus resistant bacteria which had been isolated from lower extremity wounds. In a study conducted by Chin and Cordell13, it was reported that tea tree oil accelerates the healing of wounds contaminated with S. aureus and abscess complications.
In addition to its antibacterial effect, there are some studies reporting its contribution to wound healing. In the study by González-Palomares et al.14, it was reported that tea tree oil has a significant beneficial effect on wound healing and especially on cell proliferation and muscle function in rats with diabetic ulcers. In another study, an excisional wound was created in rats and a chitosan-based dressing was applied with a mixture of rosemary oil and tea tree oil, and it was found that the applied mixture increased the percentage of wound contraction and reduced oxidative stress in the wound area15.
The nurse is an active member of the surgical team, and plays an important role in the planning and maintenance of preventive, therapeutic and rehabilitative care16. The nurse decides the most appropriate product after assessing the wound, wound care products and wound care practices17,18. The nurse should not only have extensive wound care knowledge, but also be able to extend this knowledge by producing new, evidence- and science-based solutions17,19.
In the literature, no studies have examined the effect of tea tree oil on wound healing acute wounds in DM. In this study, it was aimed to accelerate wound healing with a more economical, easier and more effective care in individuals with DM and contribute to the wound care literature. The study was conducted to determine the effect of tea tree oil on wound healing in diabetic rats.
Materials and methods
Samples
Total of 48 male 2-month-old Sprague-Dawley® rats were used in the study. The adequacy of the sample was decided based on a post-hoc power analysis. Power was found to be 99% for n=47, alpha=0.05 and effect size=0.8. The environment of rats was maintained at an average of 20˚C and humidity between 40–50%. It was ensured that circadian rhythms were regular (12 hours day and 12 hours night). The animals were kept in separate cages after the surgical procedure to prevent them from harming each other. All rats were provided with standard rat feed and tap water during the study. Before starting the study, written approval (Certificate of approval no.16/122) was obtained from Erciyes University’s local ethics committee of animal experiments. All procedures performed on the animals were in compliance with the Helsinki Declaration and the Guide for Care and Use of Laboratory Animals. All efforts were made to minimise animal suffering.
The rats were divided into two groups, a non‑diabetic group (n=24) and a diabetic group (n=24), by a simple randomisation method (n=48); a single dose of 50mg/kg intraperitoneal streptozotocin (STZ) (Sigma Aldrich, Germany) was administered to the latter group to induce hyperglycaemia (n=24). These two groups were then further divided into three subgroups, sunflower oil (n=8), tea tree oil (n=8) and saline (0.9% NaCl) (n=8), also by a simple randomisation method. On day 3 after drug induction, the rats with blood glucose >250mg/dL measured from the tail vein were considered diabetic20. One rat in the non‑diabetic saline group was not able to tolerate anaesthesia and was lost. The study was completed with 47 rats (Figure 1).
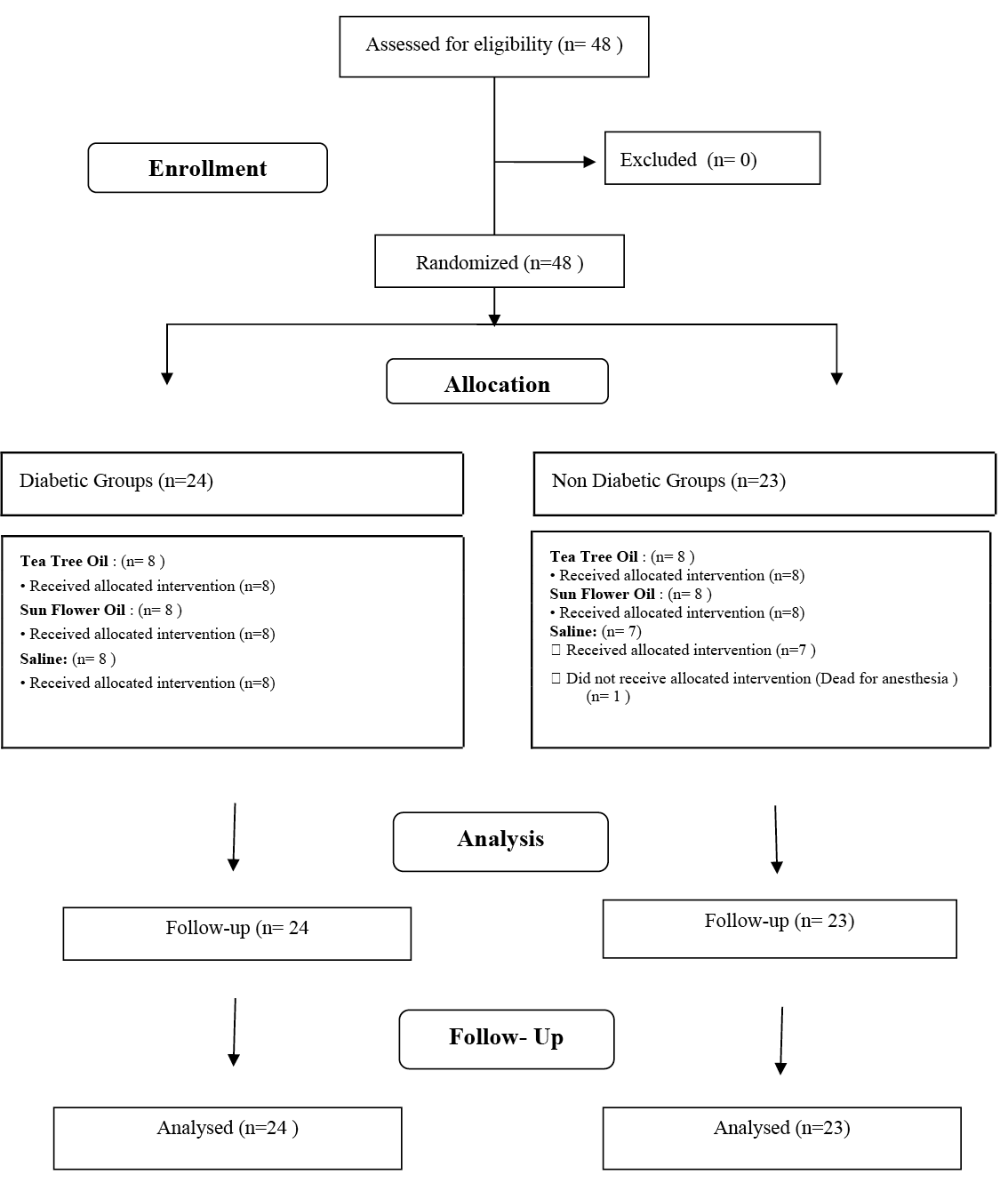
Figure 1. Flow chart of the study
Materials
The tea tree oil and sunflower oil used in the study were obtained from NU-KA Import Export Marketing Industry Limited Company (catalogue number: 9211; density: 0.895 at 10˚C). It has been reported that the minimum amount of terpineol-4 of tea tree oil should be 30%, and the amount of 1,8-cineole should be a maximum of 15%21. The tea tree oil used contains 40.79% terpineol-4 and 2.70% 1,8-cineole. Tea tree oil was diluted in sunflower oil at a rate of 5%. Sunflower oil is one of the essential oils and thought to have no effect on wound healing. 1 ml of the prepared solution was applied to each rat in the diabetic and non-diabetic tea tree oil groups with dressing. This ratio was preferred because no skin irritation was observed when using 5% diluted tea tree oil in the literature22.
Wound formation and follow-up
Rats were anaesthetised with 50mg/kg Ketamine Hydrochloride+10mg/kg Xsilazine intraperitoneally. A 5cm long full-thickness skin incision was made to the cephalic region of the rats’ backs23,24 using a number 15 scalpel and the procedure was completed by suturing with 3/0 silk (Figure 2). In accordance with the defined group, the incisional wound was dressed with either 1ml 5% diluted tea tree oil, sunflower oil or saline solution once daily for 15 days (Figures 3 & 4).
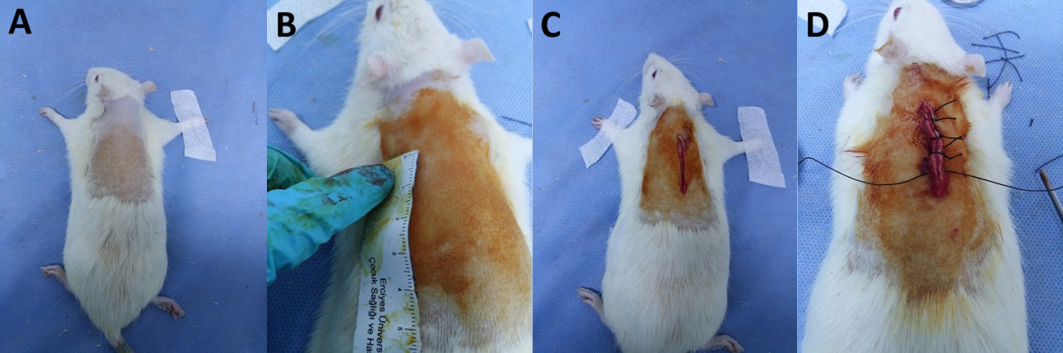
Figure 2. Wound formation process
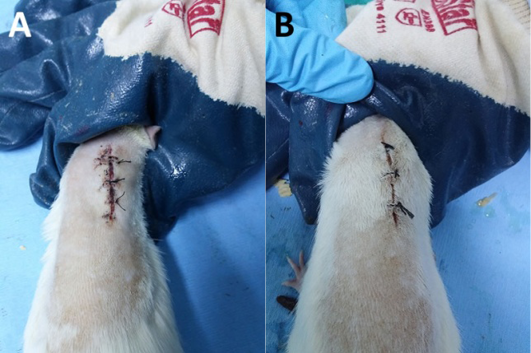
Figure 3. General view of the wound site in the diabetic group on day 3:
A=saline group; B=tea tree oil group
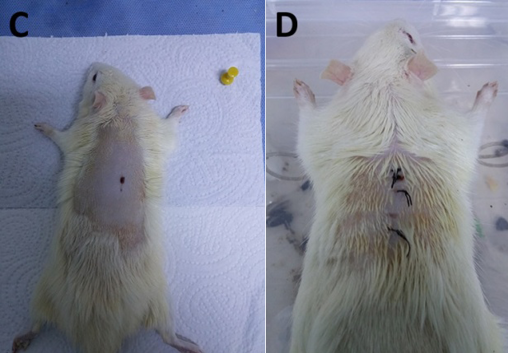
Figure 4. General view of the wound site in the non‑diabetic group on day 15:
C=tea tree oil group; D=sunflower oil group
On days 3, 7 and 15 following wound formation, 0.5cmx0.5cm full thickness tissue samples were taken from the rats under anaesthesia with a biopsy punch, leaving a distance of 1cm between biopsies. Tissue samples were fixed with 4% formaldehyde. Tissues waiting 48 hours in the fixation solution were left in running tap water overnight, then passed through an increasing series of alcohol, then purified with water and transparented with xylene, embedded in paraffin and blocked for light microscopy examination. For histopathological analysis, 5–6伊m sections were examined by Hematoxylin-Eosin (H+E) staining and Masson’s triple staining (trichrome) technique. Histopathological examination was performed by two histologists who were blinded to groups and tissue samples. Histopathological examination was based on inflammatory cell density, procollagen content, mature collagen content, vascularisation, epithelisation and fibroblast density.
Evaluation of tissue samples
In our study, tissue samples were evaluated semi-quantitatively based on the literature25,26. On days 3, 7 and 15, the inflammatory cell density, fibroblast density, amount of procollagen/mature collagen and epithelisation level were evaluated and the data were scored between 1–5 points after H+E and Masson’s triple staining under a light microscope and classified accordingly (Figure 5). During the repair process of a wound, active fibroblasts display a more basophilic cytoplasm which can be easily observed under a light microscope; in this way, fibroblast cells were distinguished from stromal cells27. Therefore, angiogenesis was evaluated by counting vessels one by one under the microscope, the level of angiogenesis is not included in the scoring. At the end of the study, rats were sacrificed by an intraperitoneal high dose (100mg/kg) pentobarbutal injection.

Figure 5. Histopathological scoring
Statistical analysis
Data were analysed with SPSS 22.0 software. Shapiro-Wilk test and Q-Q graphs were used to determine whether the numerical data were suitable for normal distribution. Data with normal distribution were evaluated with Kruskal-Wallis and post-hoc Dunn’s test. The results were reported as mean ± standard deviation (mean±SD). In all results, p<0.05 was considered statistically significant.
Results
Results were based on analysis of inflammatory cell density, fibroblast density, angiogenesis, amount of procollagen/mature collagen and level of epithelisation.
Inflammatory cell density
There was a significant difference between the groups in terms of inflammatory cell density. This difference is due to the results from the non‑diabetic tea tree oil group and the diabetic saline group; the inflammatory cell density of the former was found to be statistically significantly higher than the latter (p<0.05) (Table 1 & Figure 6). The non‑diabetic and diabetic tea tree oil groups did not make a significant difference compared to the diabetic and non‑diabetic sunflower oil groups. Additionally, the diabetic and non‑diabetic sunflower oil groups did not make a significant difference compared to the saline groups.
Table 1. Wound healing parameters on day 3 according to the different groups
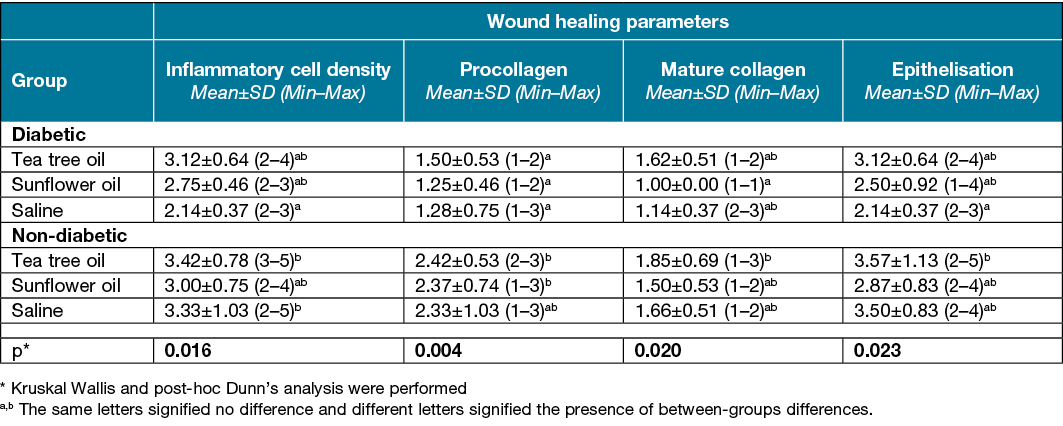
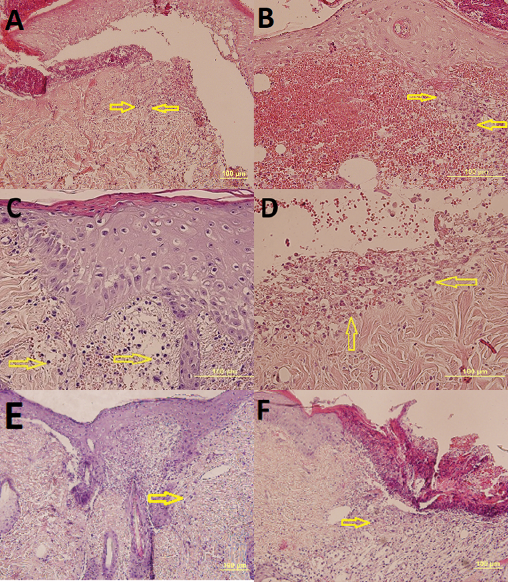
Figure 6. View of day 3 (A, E, F x20 HE) in the following groups; yellow arrows indicate inflammatory cells. Scores are also listed below:
A=diabetic tea tree oil. Score: insufficient (2)
B=diabetic sunflower oil. Score: insufficient (2)
C=non‑diabetic (B, C, Dx40 HE) tea tree oil.
Score: good (enough) (4)
D=non‑diabetic sunflower oil. Score: moderate (3)
E=diabetic saline. Score: insufficient (2)
F=non‑diabetic saline. Score: good (enough) (4)
Fibroblast density and amount of procollagen/mature collagen
Fibroblast density was higher in the non‑diabetic tea tree oil group than the diabetic sunflower oil group. There was a statistical difference in favour of the non‑diabetic tea tree oil group in terms of procollagen and mature collagen density on days 3, 7 and 15 of the study (p <0.05) (Tables 1–3). In addition, it was determined that the procollagen density of the non‑diabetic tea tree oil and non‑diabetic sunflower oil groups were higher on days 3 and 15 than the diabetic tea tree oil and diabetic sunflower oil groups (p<0.05) (Tables 1 & 3; Figure 7).
Table 2. Wound healing parameters on day 7 according to the different groups
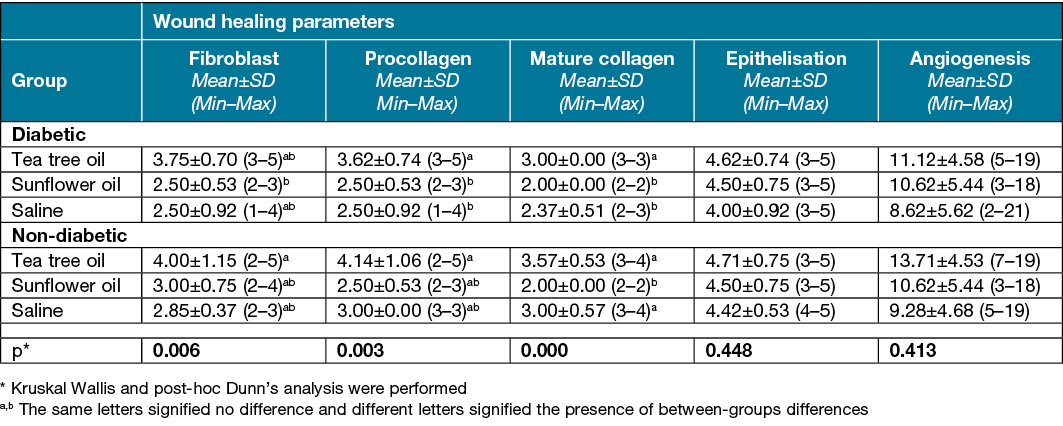
Table 3. Wound healing parameters on day 15 according to the different groups
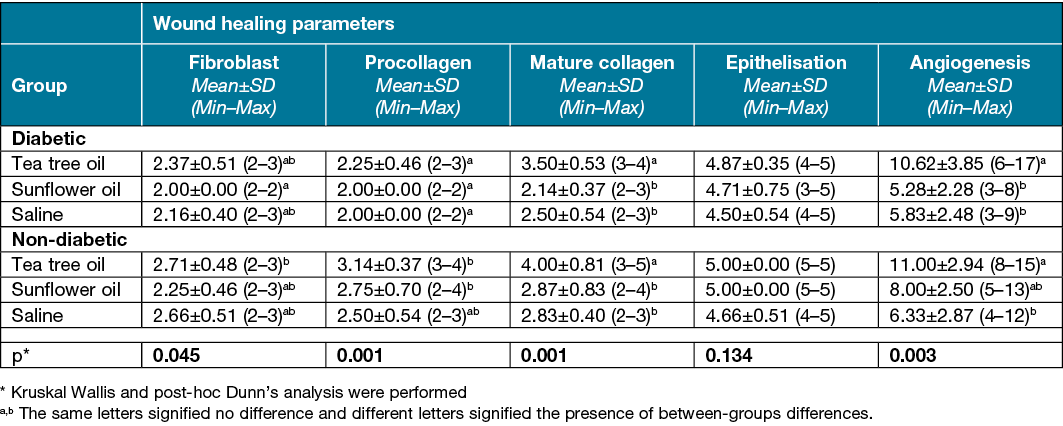
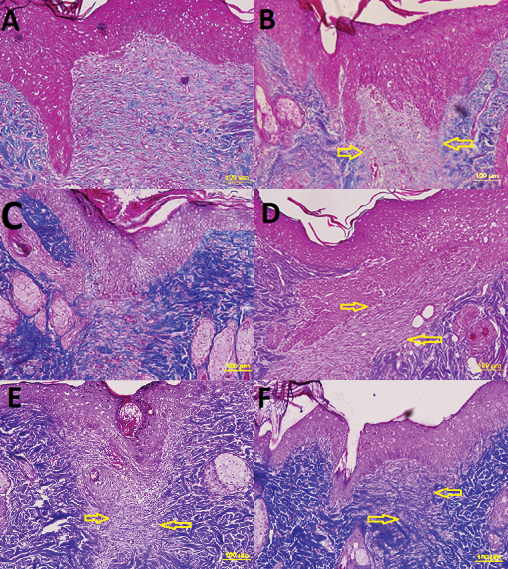
Figure 7. View of day 15 (x20 Masson’s trichrome) in the following groups; yellow arrows indicate procollagen density. Scores are also listed below:
A=non‑diabetic tea tree oil. Score: very good (5)
B=diabetic tea tree oil. Score: good (enough) (4)
C=non‑diabetic sunflower oil. Score: insufficient (2)
D=diabetic sunflower oil. Score: moderate (3)
E=non‑diabetic saline. Score: moderate (3)
F=diabetic saline. Score: insufficient (2)
Angiogenesis
While there was no significant difference between the groups in terms of angiogenesis on day 7 of the study (p>0.05), it was found that the non‑diabetic tea tree oil group had a statistically higher angiogenesis amount than the diabetic and non‑diabetic saline groups and the diabetic sunflower oil group on day 15 (p<0.05) (Tables 2 & 3).
Epithelisation level
On day 3 of the study, the epithelisation level was found to be statistically significantly higher in the non‑diabetic tea tree oil group than the diabetic saline group (p<0.05) (Table 1). No significant difference was found in terms of epithelisation levels on day 7 and 15 of the study (p>0.05) (Tables 2 & 3).
Discussion
In one study, it was reported that the toxicity of tea tree oil applied to the skin is limited and anti-inflammatory components penetrate the vascular dermis to regulate inflammatory processes28. Similarly, it has been shown that tea tree oil can selectively regulate cell function, particularly monocyte activity, during inflammation and control inflammatory responses to foreign antigens in the skin following topical application29. In our study, the inflammatory cell density of the non‑diabetic tea tree oil group was found to be statistically significantly higher than the diabetic saline group. Considering the effect of inflammatory cell density on wound healing, tea tree oil can therefore be considered to have a positive effect on wound healing based on this finding in our study.
Fibroblasts are one of the most dominant cell types in the proliferation stage of wound healing30,31. Collagen synthesis and granulation tissue formation is required to increase the number of fibroblasts. During the proliferation phase, some cells in the wound area differentiate into fibroblasts via chemical signals, and fibroblast migration to the wound site begins32. As the wound approaches the maturation stage, there is a decrease in the number of fibroblasts in the wound area33. On day 7 and 15 of the study, a significant difference was found between the groups in favour of the non‑diabetic tea tree oil group.
Collagen formed by the activity of fibroblast cells is the main macro molecule of connective tissue34. Collagen synthesis begins as procollagen 4–5 days after injury and is converted to mature collagen approximately 2 weeks later33. In our study, there was a statistical difference in favour of the non‑diabetic tea tree oil group in terms of procollagen and mature collagen density on days 3, 7 and 15 of the study. At the same time, it was determined that the procollagen density of the non‑diabetic tea tree oil and non‑diabetic sunflower oil groups were higher on day 3 and 15 than the diabetic tea tree oil and diabetic sunflower oil groups. Impaired functioning of the inflammatory process in diabetes affects the other stages of the healing process, reducing collagen production33. In line with this literature, it was found that procollagen and mature collagen concentrations were lower in the diabetic group than the non‑diabetic group.
As part of granulation tissue formation, vascular endothelial growth factor and fibroblast growth factor contribute to endothelial cell proliferation and vascular formation32. The newly formed vessels play an important role in healing, facilitating the transportation of oxygen and nutrients to the wound site. In patients with diabetes and vascular disease, the newly formed capillary system is inadequate for this purpose, resulting in delayed wound healing34. In our study, while there was no significant difference between the groups in terms of angiogenesis on day 7 of the study, it was found that the non‑diabetic tea tree oil group had a statistically higher angiogenesis amount than the diabetic and non‑diabetic saline groups and the diabetic sunflower oil group on day 15.
The epithelisation process starts immediately after the injury and continues until the maturation phase10. Proliferation and migration of epithelial cells provide closure of the wound surface and, with completion of epithelisation, the wound is protected from dehydration and infections35. In the study by Sanaei et al.36, the Berula angustifolia plant was applied topically to the incisional and excisional surgical wound in diabetic rats, and the epithelisation level was found to be significantly better compared to the control groups. In our study, on day 3 of the study, the epithelisation level was found to be statistically significantly higher in the non‑diabetic tea tree oil group than the diabetic saline group.
Studies have shown that some herbal products accelerate wound healing and prevent complications in wound models23,24. Although there are extensive studies showing the antibacterial, antifungal and antiviral effects of tea tree oil, one of the products used for this purpose, there is limited literature information on its effectiveness on wound healing12,37. A study conducted by Edmonson et al.38 found that tea tree oil was effective on wound healing in 8 of 11 patients, reducing wound size. In the study by González-Palomares et al.14 it was reported that tea tree oil has a significant beneficial effect on wound healing and especially on cell proliferation and muscle function in rats with a diabetic ulcer. In another study, a dressing was applied with a mixture of rosemary oil and tea tree oil, and the applied mixture increased the percentage of wound contraction and reduced oxidative stress in the wound area15. Similar to the literature, in our study, tea tree oil has been found to positively affect inflammatory cell density, collagen and procollagen density, angiogenesis and epithelisation. Although it has been stated in previous studies that tea tree oil regulates inflammatory reaction28,29, it is not known how it affects epithelisation, angiogenesis and collagen formation. However, we think that this may be due to the cytokines and growth factors secreted from inflammatory cells.
Limitations and recommendations for future research
In this study parameters such as inflammatory cell, fibroblast, procollagen and mature collagen density as well as vascularisation number were evaluated by H+E and Masson’s trichrome staining. Due to the financial limitations, it was not possible to use immunohistochemical methods which can measure the healing parameters more objectively. Therefore, for example, the fibroblast density on day 3 and the number of inflammatory cells on days 7–15 could not be analysed. Furthermore, it is recommended that the repetition of a similar study be performed using immunohistochemical analysis. It is also recommended to further investigate the components of tea tree oil and conduct advanced studies to understand the mechanism of action of the active substance which has a healing effect.
Conclusion
In conclusion, it has been determined that tea tree oil has an accelerating effect on wound healing and is an alternative method that can be used in wound dressing. In addition, our study is the first to demonstrate the effect of tea tree oil on wound healing in an acute wound. For this reason, it is thought to contribute to the literature.
Author contributions
YS contributed to the conception, design, acquisition, analysis and interpretation, drafted the article, critically revised the article, gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. GNC contributed to conception, design, acquisition, analysis and interpretation, drafted the article, critically revised the article, gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. AL contributed to conception, design, acquisition, analysis and interpretation. SÖ contributed to conception, design, acquisition, analysis and interpretation.
Ethics statement
Written approval (Certificate of approval no.16/122) was obtained from Erciyes University’s local ethics committee of animal experiments.
All procedures performed on the animals were in compliance with the Helsinki Declaration and the Guide for Care and Use of Laboratory Animals. All efforts were made to minimise animal suffering.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article. No ghost writers were used to write this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article – Erciyes University Lecturer Training Program.
Author(s)
Yeliz Sürme1*, Gülsüm Nihal Çürük2, Ayça Lekesizcan and Saim Özdamar3
1Department of Surgery Nursing, Faculty of Health Sciences, Erciyes University, Kayseri, Turkey
2Department of Internal Disease Nursing, Faculty of Health Sciences, İzmir University of Economics, İzmir, Turkey
3Department of Histology Embryology, Faculty of Medicine, Pamukkale University, Denizli, Turkey
*Corresponding author Email yelizcucuk@hotmail.com
References
- Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabetic Med 2006;23:594–608. doi:10.1111/j.1464-5491.2006.01773.x
- Guo S, Dipietro LA, Dipietrio L. Factors affecting wound healing. J Dental Res 2010;89:219–229. doi:10.1177/0022034509359125
- Jones J. Examining the multifactorial nature of wound infection. Wounds Essent 2012;2:90–97.
- Zhang Y, Zheng Q, Wang S et al. Diabetes mellitus is associated with increased risk of surgical site infections: a meta-analysis of prospective cohort studies. Am J Infect Cont 2015;43:810–815. doi:10.1016/j.ajic.2015.04.003
- Sivrikoz E, Karamanos E, Beale E, Teixeira P, Inaba K, Demetriades D. The effect of diabetes on outcomes following emergency appendectomy in patients without comorbidities: a propensity score-matched analysis of national surgical quality improvement program database. Am J Surgery 2015;209:206–211. doi:10.1016/j.amjsurg.2014.03.015.
- Mickelson MA, Mans C, Colopy SA. Principles of wound management and wound healing in the exotic pets. Vet Clinic North Am: Exotic Animal Practice 2016;19(1):33–53. doi:10.1016/j.cvex.2015.08.002.
- Yao K, Bae L, Yew, WP. Post-operative wound management. Aust Family Physic 2013;42:867–870.
- Dorai AA. Wound care with traditional, complementary and alternative medicine. Indian J Plastic Surg 2012;45(2):418–424. doi:10.4103/0970-0358.101331
- Thakur R, Jain N, Pathak R, Singh Sandhu S. Practices in wound healing studies of plants. Evid-Based Complement Altern Med 2011;1–17. doi:10.1155/2011/438056
- Woollard AC, Tatham KC, Barker S. The influence of essential oils on the process of wound healing: a review of the current evidence. J Wound Care 2007;6(16):255–257. doi:10.12968/jowc.2007.16.6.27064
- Tezgül Çakır N, Kaleağası S, Kökdi G. A promising antimicrobial: tea tree oil. Ankara Eczacılık Fakültesi Dergisi 2005;34:315–327.
- Falci SPP, Teixeira MA, Chagas PF et al. Antimicrobial activity of melaleuca sp. oil against clinical isolates of antibiotics resistant Staphylococcus aureus. Acta Cirúrgica Brasileira 2015;30:401–406. doi:10.1590/S0102-865020150060000005
- Chin K, Cordell B. The effect of tea tree oil (Melaleuca alternifolia) on wound healing using a dressing model. J Altern Complement Med 2013;19, 942–945.
- González-Palomares L, Gómez-Barroso M, Vargas-Vargas MA, et al. Beneficial effects of the Melaleuca alternifolia essential oil in the treatment of diabetic ulcers. FASEB J 2020;34(1):1–1. doi:10.1096/fasebj.2020.34.s1.06404
- Labib R.M, Ayoub I.M, Michel H.E et al. Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PloS One 2019;14(9):e0219561.
- Akdemir N, Akkuş Y. Rehabilitasyon ve Hemşirelik. Hacettepe Üniversitesi Hemşirelik Fakültesi Dergisi 2006;13, 82–91.
- Surme Y, Tekinsoy Kartın P, Çürük N. Knowledge and practices of nurses regarding wound healing. J PeriAnesthes Nurs 2018;33(4):471–478. doi:10.1016/j.jopan.2016.04.143
- Aune E, Struksnes S. Home care nurses’ experience of providing health-care to patients with hard-to-heal wounds. J Wound Care 2019;28(3):178–187. doi:10.12968/jowc.2019.28.3.178
- Dutton M, Chiarella M, Curtis K. The role of the wound care nurse: an integrative review. Br J Commun Nurs 2014;19(3):39–47. doi:10.12968/bjcn.2014.19.sup3.s39
- Erbaş O. Deneysel diyabet modelleri. FNG & Bilim Tıp Dergisi 2015;1(1):40–42. doi:10.5606/fng.btd.2015.008
- Silva CJ, Barbosa LCA, Maltha CRA, Pinheiro AL, Ismail FMD. Comparative study of the essential oils of seven Melaleuca (Myrtaceae) species grown in Brazil. Flavour Fragrance J 2007;22(6):474–478. doi:10.1002/ffj.1823
- Pazyar N, Yaghoobi R, Bagherani N, Kazerouni A. A review of applications of tea tree oil in dermatology. Int J Dermatol 2013;52(7):784–790.
- Teoh S.L, Latiff A, Das S. The effect of topical extract of Momordica charantina on wound healing in non diabetic rats and in rats with diabetes induced by streptozotocin. Clin Experiment Dermatol 2009;34:815–822. doi:10.1111/j.1365-2230.2008.03117.x
- Altıparmak M, Eskitaşoğlu T. Comparison of systemic and topical Hypericum perforatum on diabetic surgical wounds. J Investigat Surg 2017;1–9. doi:10.1080/08941939.2016.1272654
- Sultana J, Rahman Molla M, Kamal M, Shahidullah M, Begum F, Bashar A. Histological differences in wound healing in Maxillofacial region in patients with or without risk factors. Bangladesh J Pathol 2009;24:3–8. doi:10.3329/bjpath.v24i1.2874
- Gal P, Kilik R, Mokry M et al. Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: standardization of semi-quantitative and quantitative histological assessments. Veterinarni Medicina 2008;53:652–659.
- Ross MH, Pawline W. Histology – a text and atlas / with correlated cell and molecular biology [Trans. Baykal B, ed.]. 6th ed. Palme Publishing; 2014.
- Hart PH, Brand C, Carson CF et al. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res 2000;49(11):619–626.
- Brand C, Ferrante A, Prager RH et al. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm Res 2001;50(4):213–219.
- Qing C. The molecular biology in wound healing & non‑healing wound. Chin J Traumatol 2017;20(4):189–193. doi:10.1016/j.cjtee.2017.06.001
- Ho J, Walsh C, Yue D, Dardik A, Cheema U. Current advancements and strategies in tissue engineering for wound healing: a comprehensive review. Adv Wound Care 2017;6(6):191–209. doi:10.1089/wound.2016.0723
- Monaca JL, Lawrence WT. Acute wound healing an overview. Clini Plastic Surg 2003;30:1–12. doi:10.1016/s0094-1298(02)00070-6
- Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care 2013;22(8):407–412. doi:10.12968/jowc.2013.22.8.407
- Sinno H, Prakash S. Complements and the wound healing cascade: an updated review. Plastic Surg Int 2013;2013:1–8. doi:10.1155/2013/146764
- Tokeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harbor Perspective Med 2015;5:1–12. doi:10.1101/cshperspect.a023267
- Sanaei N, Mohammadi R, Raisi A, Zarei L. Extract of Berula angustifolia (L.) mertens enhances wound healing in streptozotocin induced diabetic rats. Wounds 2018;30:242–248.
- Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules 2018;23:1–23. doi:10.3390/molecules23092392
- Edmondson M, Newall N, Carville K, Smith J, Riley T, Carson C. Uncontrolled, open-label, pilot study of tea tree (Melaleuca alternifolia) oil solution in the decolonisation of methicillin-resistant Staphylococcus aureus positive wounds and its influence on wound healing. Int Wound J 2011;8:375–384. doi:10.1111/j.1742-481X.2011.00801.x



