Volume 30 Number 2
A prospective single centre case series on the use of epidermal grafts and adjunct technologies to facilitate wound healing in a vascular surgical patient population
Frank P Guerriero and Christopher L Delaney
Keywords biodegradable temporising matrix, epidermal skin grafting, vascular diseases
For referencing Guerriero FP and Delaney CL. A prospective single centre case series on the use of epidermal grafts and adjunct technologies to facilitate wound healing in a vascular surgical patient population. Wound Practice and Research 2022; 30(2):99-107.
DOI
https://doi.org/10.33235/wpr.30.2.99-107
Submitted 9 September 2021
Accepted 11 November 2021
Abstract
Epidermal grafting (EG) using a novel epidermal harvesting system (the CELLUTOMETM System) offers an alternative method to classic surgical approaches of assisted wound closure. Use of this device requires minimal training, leaves a nominal donor site injury, and may be employed in non-operating theatre environments, lending to consideration for use in a high-risk vascular surgical cohort.
This single centre series observed the application of EG to lower limb wounds in vascular surgical patients with aetiological barriers to wound healing, including peripheral arterial disease (PAD), venous insufficiency and diabetic neuro-ischaemia. Complete wound healing was established as the primary outcome measure, with time to 100% epithelialisation as a secondary outcome measure.
Ten consecutive patients underwent EG via the CELLUTOMETM Epidermal Harvesting System for treatment of wounds related to a variety of vascular surgical aetiologies. Complete wound healing was observed in seven out of ten patients, significant wound surface area reduction was seen in one patient, with the remaining two patients failing to respond, requiring further surgery to facilitate wound healing. These results suggest that EG via the CELLUTOMETM System offers a viable option for assisted wound closure of wounded vascular surgical patients in whom avoidance of general anaesthesia may be desirable.
Introduction
Patients suffering from vascular disease present unique challenges to any clinician seeking to facilitate wound healing in this cohort. Vascular disease impairs wound healing due to impairment of tissue oxygenation, compromised supply of nutrients, chronic inflammation and poor removal of metabolic waste products1. Failure to heal a wound may lead to a life-threatening infection or limb amputation, outcomes which are particularly prevalent in the vascular surgical cohort. Traditional methods of assisted wound closure (defined as 100% epithelialisation), such as skin grafting or autologous flaps, require an operating theatre and often necessitate general anaesthesia; they are often avoided in this cohort due to high risk of systemic complications and failure related to high burden of comorbidities and compromise to healing capacity2.
Epidermal grafting (EG) is a method that serves as a potentially suitable wound closure solution for the vascular surgical cohort due in part to its minimal impact on the donor site. Historically, methods were limited to clinicians with formal surgical skills and techniques that were time consuming and unreliable3,4.
The CELLUTOMETM Epidermal Harvesting System (3M, San Antonio, USA) offers clinicians an automated method of harvesting epidermal tissue in a consistent and reliable manner. The device is able to be operated by clinicians with minimal surgical training, incites minimal procedural pain or donor site trauma, and can be employed at the patient bedside or outpatient setting. As the use of this device does not require anaesthesia nor operating theatre support, it is a very attractive proposition for assisting wound healing in the vascular surgical population, potentially decreasing the risk of surgical complications related to a high burden of comorbidities5. The effectiveness of using EG to facilitate wound healing in this high risk patient population has not previously been assessed.
This series reported on the application of the CELLUTOMETM System to a variety of lower limb wounds in a cohort of vascular disease patients with aetiological barriers to wound healing, including peripheral arterial disease (PAD), venous insufficiency and diabetic neuro-ischaemia.
Materials and methods
A prospective case series was undertaken from April 2019 to September 2020 at a tertiary metropolitan vascular surgical service in South Australia. Patients were followed up until complete epithelialisation was observed for a maximum of 26 weeks.
Patient selection criteria
Initial patient screening considered adult patients being treated by the vascular surgical department at our institution for wounds related to:
- Stalled healing secondary to chronic underlying aetiology (venous hypertension/insufficiency, PAD or diabetes).
- Delayed closure due to significant tissue deficit arising from surgery (fasciotomy, amputation or surgical debridement).
Patient selection for application of EG was based on the following criteria:
- Wound superficial in depth (no deeper than dermis level of tissue deficit).
- Wound bed containing 80% or greater granulation tissue volume.
- Wound not acutely infected, nor with signs of active tissue infection (clinical opinion of treating vascular surgery medical officer, nurse practitioner as defined by the International Wound Infection Institute, Wound infection in clinical practice document6).
- Wound situated in the lower extremity (lower limbs).
- Wound present on a limb with no acute ischaemia.
- Wound healing predicted to take longer than 6 weeks or more (clinical opinion of treating nurse practitioner or vascular surgeon).
A flow chart outlining the case selection criteria, relevant aetiology and outcomes of EG application is provided in Figure 1.
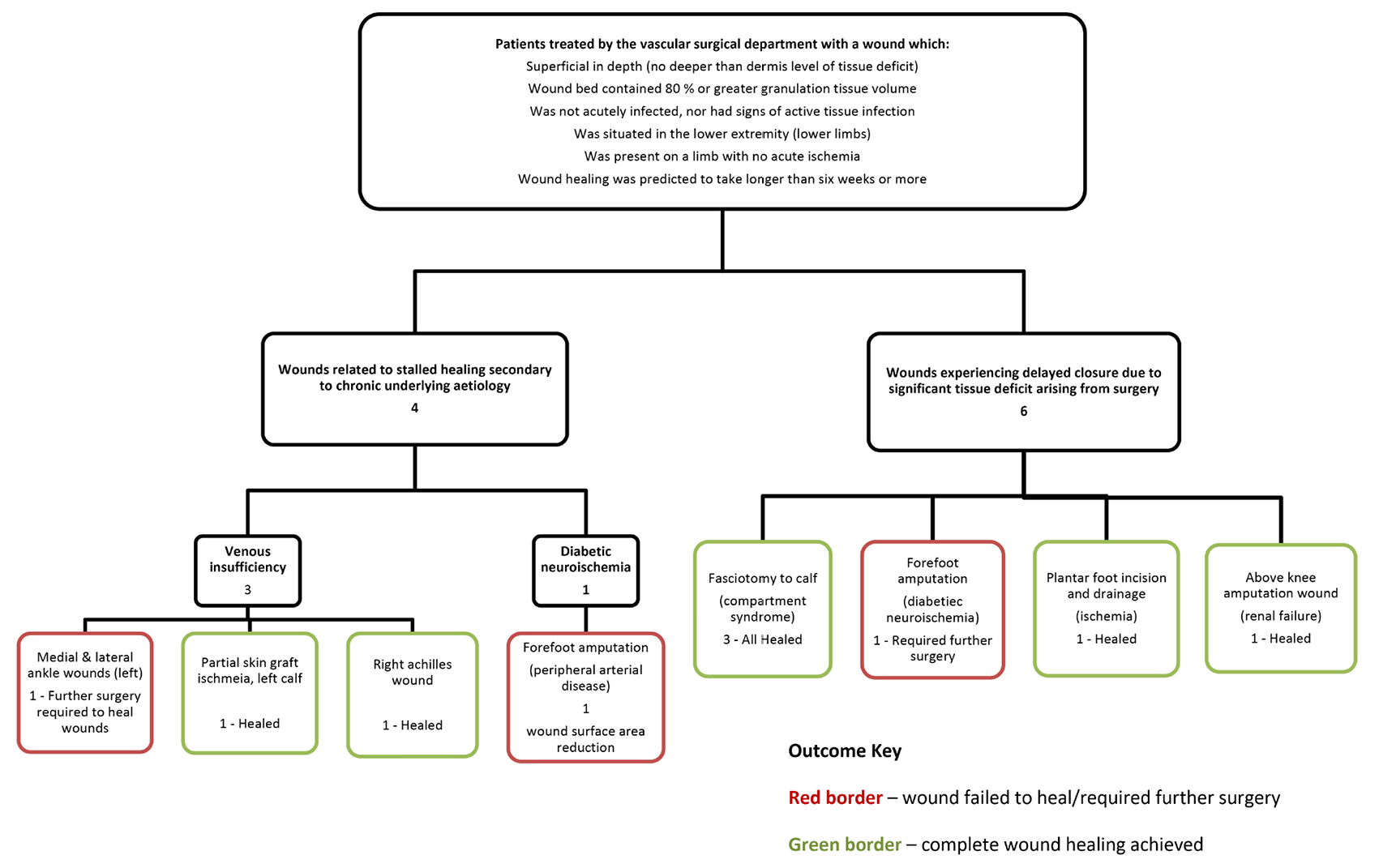
Figure 1. Case selection flow chart
Technique for application
An internal guideline was established based on manufacturer recommendations. In brief, this included guidelines on the donor site selection (an area of skin that would facilitate a 5x5cm plane of flat surface area where a harvesting device could be placed, typically the medial thigh or medial bicep), the donor site preparation (hair removal, topical antiseptic), the recipient wound bed preparation, and post-procedure management (dressings and topical antiseptics)7. Wounds were assessed to be free from infection or non-viable tissue (defined as absence of peri-wound erythema, absence of slough/non-viable tissue and absence of purulent exudate or malodour) prior to application of the EG.
The CELLUTOMETM System was employed in accordance with the above guideline with a minimum harvest device activation of 30 minutes to facilitate 95–100% EG formation in the target harvest site. Donor sites were covered with a silicone adhesive foam dressing; this dressing was left in place for 7 days post-procedure, after which the site was inspected and redressed with the same dressing for a further 7 days. The donor site was left undressed at 14 days post-procedure.
Transfer of harvested epidermal tissue from donor site to the target recipient wound was facilitated using an adhesive silicone dressing. This ‘transfer dressing’ was left in place for a minimum of 7 days, with a maximum of 14 days depending on prevalence of epithelial tissue and normalised advancing wound edges. Selection of secondary dressings and frequency of dressing changes were based on volume of wound exudate. Compression bandaging was employed to manage oedema in the setting of venous insufficiency.
All participants were appropriately consented and the EG procedure was performed in a variety of settings. These included as an inpatient (at the bedside), in rehabilitation (also at the bedside), and as an outpatient (in an outpatient treatment area). In all instances the procedure was performed outside of a formal operating theatre setting, and no anaesthetic support was required for any of these procedures, nor was any analgesia required or provided for the procedure. Participants were questioned about procedural-related pain post-procedure. For all cases the EG procedure was performed by a single operator, a nurse practitioner who had received initial instruction and inaugural case supervision by a representative from the CELLUTOMETM System manufacturer.
Adjunct technologies
This case series featured two cases where EG technology was used post-application of a novel tissue regeneration matrix – NovoSorb® Biodegradable Temporising Matrix (BTM). BTM is a synthetic bioabsorbable polyurethane dermal matrix that serves to facilitate granulation tissue proliferation beneath a sealed protective membrane. After debridement of non-viable tissue, application and securing in place using sutures or staples, BTM is designed to integrate into the wound bed as cells and tissues migrate through its structure. When clinically indicated, delamination of the sealing membrane is performed once a vascularised dermis is prevalent. The resulting vascularised dermis facilitated by the BTM scaffold allows for a tissue surface that is a suitable recipient for EG in order to facilitate skin closure. BTM is typically used to facilitate tissue regeneration in instances of significant tissue deficits and/or when a patient has exposed sub-fascial structures such as tendons or muscle8.
Topical negative pressure wound therapy (NPWT) provides negative pressure to the wound bed through a polyurethane foam or gauze interface connected to an electric negative pressure pump. NPWT can decrease tissue deficit volumes and increase the rate of wound healing when compared with non-negative pressure wound dressing controls9.
For this case series, NPWT was utilised as an adjunct therapy to bolster the harvested EG placed in the wound bed and to assist with management of wound exudate. When employed, the NPWT was left in place for the first 7 days post-EG application, after which the dressing would be removed, ensuring not to disturb or lift the EG transfer dressing from the wound bed. Following the initial 7 days of NPWT therapy, NPWT would either be reapplied for a further 7 days or discontinued and replaced with conventional wound dressings. NPWT was always discontinued at the 14-day mark for any case where this technology was employed.
Outcomes
Complete wound closure was the primary outcome, defined as 100% epithelialisation (intact skin regeneration over the original area of wounding, without exudate)10. Time to complete wound healing and number of EG failures were established as secondary outcome measures. EG failure was defined as a wound bed with any one of the following parameters:
- Absence of new epithelial tissue at a timeframe of 2 weeks post-EG application.
- Return to theatre for debridement or surgical revision of wound bed post-EG application.
- Wound bed surface area increase post-EG application.
- <10% wound bed surface area decrease at 2 weeks post-EG application.
Wound assessments related to outcomes measures were performed by a nurse practitioner specialised in wound management and vascular surgery.
Data collected
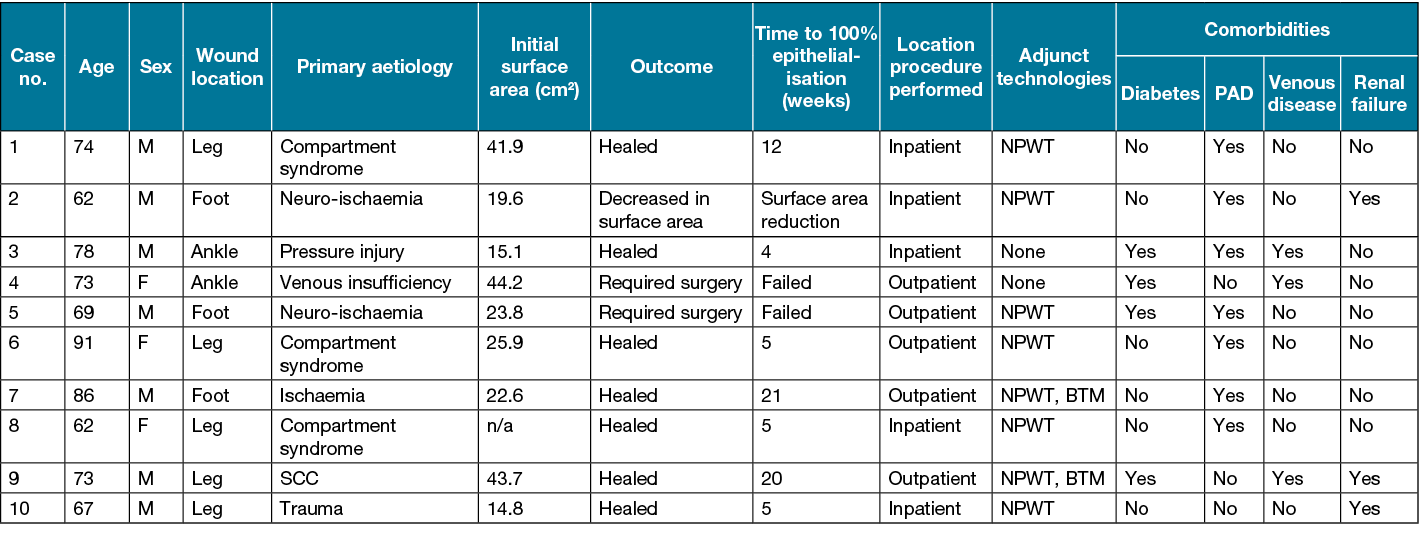
Details on patient age, sex, wound aetiology, wound location, pre-procedure wound surface area, donor harvest site, time to complete wound healing, primary aetiology, adjunct technologies used, intra- and post-procedure analgesia usage, and comorbidities related to compromised wound healing (diabetes, PAD, venous disease and renal failure) were collected (Table 1).
Clinical photography of patient wounds was obtained from all cases. Consent to publish de-identified clinical photographs and case details was obtained from all cases.
Results
Data were collected from a total of ten patients treated for wounds arising from a variety of vascular aetiologies. Most patients were male (seven out of ten) with an average age of 73.5 years. All of the 10 cases are outlined in more detail in Table 1.
Primary outcome – wound closure
Complete wound closure was observed in seven out of ten cases. Failure of EG was observed in two cases – the absence of new epithelial tissue was observed in the forefoot amputation case (Case 5) and an increase in wound surface area was observed in the venous ankle leg ulcer case (Case 4). The final failed case (Case 2) experienced an inconclusive outcome, in that there was a significant decrease in wound surface area observed in a 6-month period; however, the wound failed to completely epithelialise within the 26-week maximum follow-up timeframe (Figure 2). This case is described in more detail in the case study section below.
The average number of weeks to complete wound healing from initial EG application was 10.7 weeks (range: 4–21 weeks).
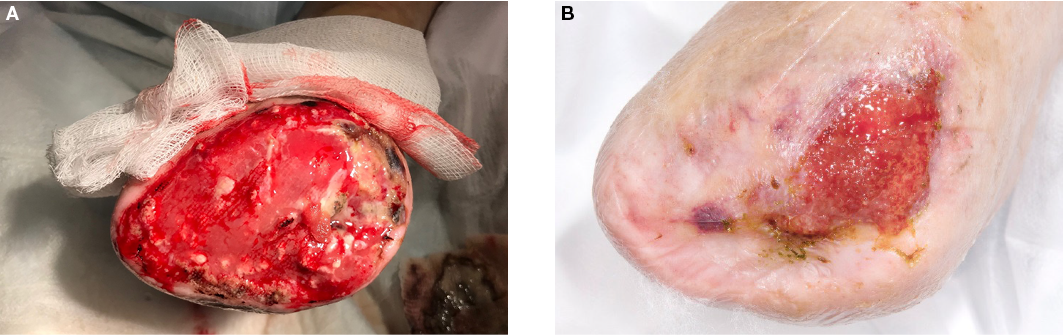
Figure 2. Case 2
A=right forefoot amputation with exposed proximal metatarsal in a patient with severe PAD
B=wound healing observed at 12 weeks post-EG application
Primary wound aetiology
Fasciotomy secondary to compartment syndrome arising from reperfusion post-acute limb ischaemia was the most common wound aetiology (n=3), with trauma (n=1) and squamous cell carcinoma (SCC) (n=1) as the causes for the remaining leg wounds. Ischaemia or diabetes-related neuro-ischaemia were the primary aetiologies for all foot wounds (n=3). The two ankle wounds were attributed to pressure injury or venous insufficiency as primary causes.
Comorbid contributors to poor wound healing
PAD was observed to be the most common comorbidity (n=7) in this trial sample; however, nearly all (n=6) PAD patients underwent a peripheral revascularisation procedure prior to EG application. Half of the six PAD patients who had undergone a revascularisation procedure continued to experience residual tissue ischaemia, defined by post-procedural toe pressures lower than 50mmHg (prognostic for poor wound healing)11. Of the three patients with residual tissue ischaemia post-revascularisation, only one progressed to complete wound healing (Case 7). Complete wound healing was observed in the remaining four patients with PAD, three of whom were successfully treated with revascularisation procedures and one who did not undergo a revascularisation procedure. Of the two PAD patients with failed EG, one was additionally diabetic (Case 5) and the other had renal failure (Case 2).
Complete wound healing was observed in two of the three cases with venous disease, with all venous disease cases additionally suffering from diabetes mellitus and one with PAD.
All three of the patients who failed to heal their wound following EG application had a minimum of two comorbid contributors to poor wound healing (Table 1).
Anatomical location of wounds
All EG procedures were performed on the lower limbs, with the majority (n=9) performed below the knee. Of the three wounds treated on the foot (below the ankle), only one successfully progressed to complete wound epithelialisation (Case 7). This successful case, with tissue loss secondary to ischaemia, had undergone adjunctive application of BTM prior to EG application and is described in more detail in the case study section below. Of the remaining two non-healing foot wound cases, Case 5, a diabetic with a neuro-ischaemia-related forefoot wound, required additional surgical tissue debridement and further revascularisation to facilitate wound healing post-EG application. Case 2 had an ischaemic foot wound that experienced some surface area decrease post-EG application; however, this never progressed to complete wound healing in the follow-up timeframe (Case 2, Figure 2). Only one of the two ankle wounds progressed to complete wound healing, an Achilles wound secondary to pressure injury (Case 3). The remaining ankle case (Case 4), a venous leg ulcer wound with chronic bacterial colonisation, required surgical debridement, long-term antibiotics and a split-thickness skin graft (STSG) to facilitate wound healing.
Adjunct therapies
NPWT was utilised as an adjunct therapy in all but two cases (Case 3 & 4). NPWT was utilised for pre-EG application wound bed preparation for all wounds related to surgery as the primary cause of tissue deficit (n=8, Cases 1–2 & 5–10). NPWT was utilised as a post-EG application adjunct therapy for seven out of ten cases.
Case 1 did not receive post-EG procedure NPWT as this was our first case and wound exudate was deemed to be minimal at time of EG application. A high to moderate volume of exudate was observed following EG application for Case 1 necessitating frequent dressing changes which is sub-optimal in the first 7 days post-EG application. Therefore, we adopted use of NPWT as a standard post-EG application practice post this case (where not contraindicated). Case 3 did not receive NPWT prior to EG application as their wound was chronic in nature, superficial in depth and the tissue was deemed suitable for EG application. Case 4 did not receive NPWT pre- or post-EG application as the wound was chronic in nature, superficial in depth and NPWT was deemed a potential tripping hazard due to anatomical location and compromised ambulatory capacity.
BTM wound scaffold was employed in two cases, where patients had large, deep tissue deficits secondary to their primary surgical intervention. Case 7 had BTM applied to address a large left foot plantar deficit with exposed tendon. Case 9 had BTM applied to deep tissue deficits of the medial and lateral left leg following debridement of failed STSG. Both BTM cases utilised NPWT as adjunct therapy immediately post-application of BTM (intra-operatively). Both cases are outlined in more detail in the case study section below.
EG procedure setting
EG application procedure was performed in the outpatient and inpatient settings, with even distribution for each setting. Inpatient EG application was performed at the patient bedside; this occurred in both multi-bed and single bed wards. Outpatient EG application was performed in a treatment room clinic setting. EG failure was observed in two cases out of the five performed in the outpatient setting and partial success observed in only one of the acute bedside settings.
Donor site outcomes
One hundred percent of the donor sites were observed to be completely healed within a 6-week timeframe, with no scarring observed in all cases.
Procedural-related pain
There were no documented additional analgesia requirements related to intra- or post-EG procedure pain. All EG procedures were performed without anaesthetic support.
Case studies
Case 7
Case 7 was an 86-year-old male with critical limb ischaemia who presented with left foot plantar gangrene. The patient was re-perfused with an angiographic procedure with moderate success (left hallux toe pressure of 23mmHg post-procedure and a high risk of proximal amputation). His left plantar wound was debrided, leaving him with a large plantar tissue deficit including exposed plantar tendons. His wound was initially treated with NPWT, which failed to facilitate granulation over the exposed tendons. BTM wound scaffold was applied to the wound bed 8 days post-initial debridement. The BTM was observed to undergo approximately 75% tissue integration with granulation tissue observed above all but the distal portions of the exposed tendons after a period of 24 days (Figure 3). This wound was dressed with conventional dressings post-BTM delamination performed 24 days after application, where further granulation tissue was observed. EG application was performed at 46 days post-initial debridement (once wound bed was >90% granulation tissue). Complete epithelialisation was observed at 144 days (20.5 weeks) post-EG application. This timeframe should be considered in the context of significant PAD and the fact that the patient was weight bearing on this limb for the majority of time post-EG application.
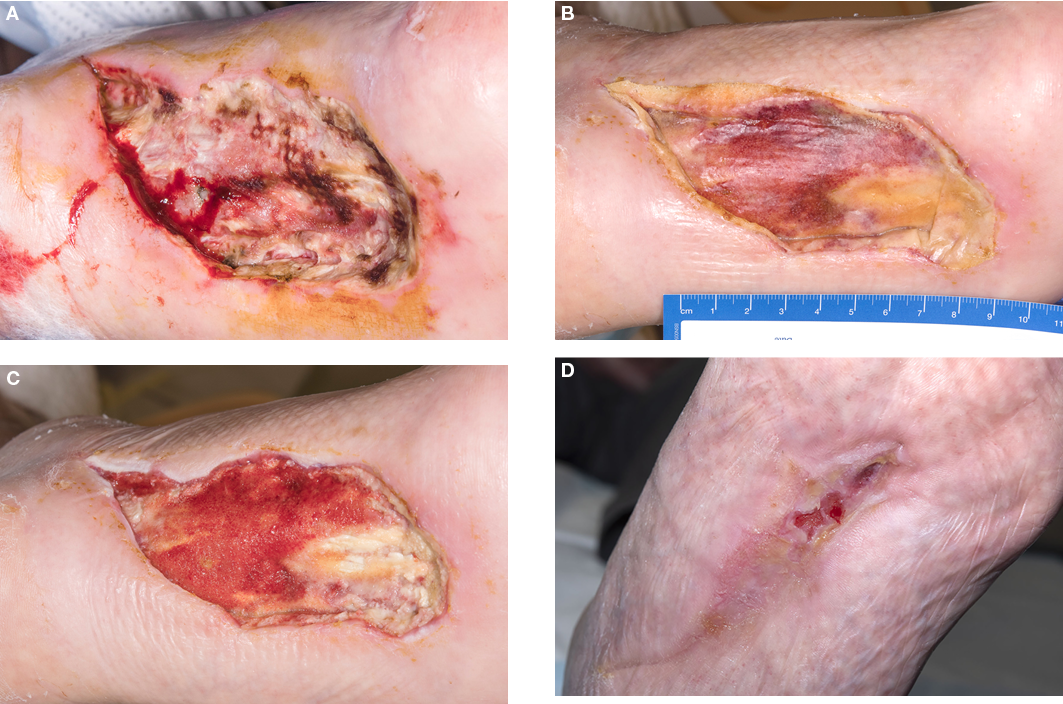
Figure 3. Case 7
A=necrotic left foot plantar wound post-surgical debridement
B=left foot plantar wound with NovoSorbTM BTM in situ
C=wound bed post-NovoSorbTM BTM delamination, 46 days post-initial debridement
D=wound almost completely epithelialised 19 weeks post-initial debridement
Case 2
Case 2 was a 62-year-old male with an extensive cardiovascular history including severe PAD resulting in previous left above knee amputation and, more recently, a right forefoot amputation (Figure 2). The forefoot amputation was quite proximal but demonstrated an initial 90% granulating wound bed with exposed bone on the medial aspect of the wound bed. EG application was implemented to expedite wound healing and to see if granulation tissue would cover the remaining exposed bone. The initial wound surface area was 19.6cm2 prior to EG application. The wound demonstrated a 63.7% surface area decrease (7.1cm2) at 12 weeks post-EG application. There was granulation tissue present in approximately 99% of the wound bed at the 12-week mark, with only a small portion of exposed bone remaining visible.
Case 9
A 73-year-old male underwent a wide excision and STSG of a chronic ulcer on his left anterior shin with underlying SCC. Unfortunately, there was only partial take of the STSG, failure of which was attributed to the significant chronic venous insufficiency, previously treated with iliac vein stent 5 weeks prior to diagnosis of SCC in his chronic left leg ulcer. The patient was subsequently left with wounds on the medial and lateral side of the partially taken STSG. Wound sizes measured 43.7cm2 (lateral) and 61.6cm2 (medial) (Figure 4).
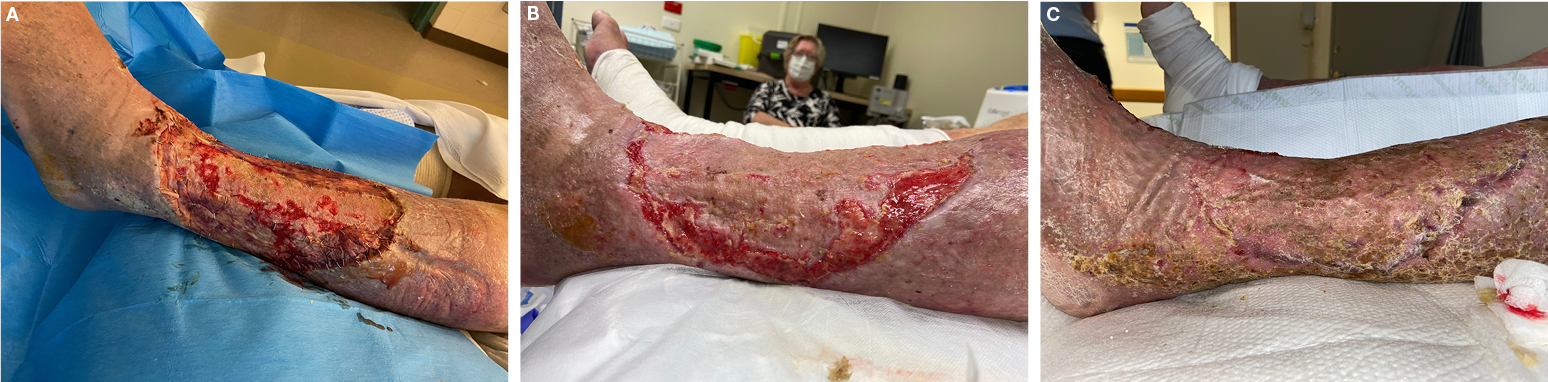
Figure 4. Case 9
A=wound bed with NovoSorbTM BTM in place, post-debridement of failed STSG on background of chronic venous insufficiency
B=wound bed on left lateral leg post-NovoSorbTM BTM delamination, pre-application of EG
C=wound bed demonstrating 100% epithelialisation at day 120 post-EG application
The patient underwent debridement and application of BTM to the failed STSG sites, with 80% integration observed at 30 days post-BTM application. The resulting granulation tissue received EG application 4 days post-BTM delamination. Complete epithelialisation of the lateral wound was observed at day 140 post-EG application. At 140 days post-EG application, the medial wound had an observed reduction of wound surface area of 95.7% measuring only 2.6cm2, with a healthy granular wound bed.
Discussion
This real-world experience of EG in a heterogenous group of patients with vascular disease has provided results which suggest safety and efficacy of the harvesting technique. Our results are comparable to those of Hachach-Haram et al.’s12 multicentre study of 35 plastic surgery patients. Our current series has shown a favourable trend of EG harvested by the novel CELLUTOMETM System assisting to overcome the additional barriers to wound healing created by vascular diseases; this is an important finding and warrants further investigation in this high risk group of patients in which avoidance of general anaesthesia is desirable.
Infection and excess pedal biomechanical pressures were identified as factors contributing to EG failure. The EG failure observed in one of two venous disease patients was attributed to a refractory chronic Pseudomonas tissue colonisation. Our experience in this series has reinforced the need to ensure the wound bed is free from infection prior to EG application. Performance, therefore, of a pre-procedure wound swab and use of topical antimicrobial dressings post-procedure are key practice recommendations arising from our experience. These recommendations align with those detailed in the case series published by Hachach-Haram et al., in 201712.
The remaining EG failure was secondary to shearing forces due to 50% of the treated wound bed surface being on the plantar aspect of the foot (Case 5). Shearing forces are an established mechanism of injury in the aetiology of neuropathy in diabetic patients13 and, therefore, an EG recipient site exposed to unrelieved biomechanical forces is a potential contraindication of EG.
This study observed EG application in both acute inpatient bedside and outpatient clinic settings, where the minimal equipment requirements and small physical footprint of the harvesting device made for easy use in either setting, reinforcing the flexibility and operating time-saving potential of this device, as experienced in other studies11,14. The minimal space and equipment requirements of the CELLUTOMETM System lend to potential savings in patient bed-days and implementation costs when compared to traditional surgical alternatives such as STSG14. Furthermore, the previously reported benefits of timely implementation of tissue harvest, pain-free procedure and rapid donor site healing, related to use of the novel CELLUTOMETM System EG device11,14–16, were similarly observed in this case series.
Finally, we highlight the success of the combination of two novel wound healing technologies. Both Case 7 and 9 (Table 1) had wounds treated with a combination of BTM to facilitate initial wound bed granulation (in both cases over exposed tendon), NPWT and, finally, EG to facilitate epithelialisation. Our findings suggest that the combination of a biodegradable temporising matrix and EG offers a unique tissue regeneration and wound closure solution in the setting of vascular disease, warranting further study of combination of these technologies.
Table 1. Descriptive characteristics
Conclusion
The favourable results in this small case series presents early promising signs that EG in a vascular disease cohort is a viable wound closure option.
Our observations align with existing evidence11,13–16 that the CELLUTOMETM System is an easy to use method of harvesting EG which can be employed in either the outpatient or inpatient setting, does not require operating theatre or anaesthetic support, and therefore has capacity to save on bed-days and operating theatre time related to surgical wound closure.
To the best of our knowledge, we are the first to use EG post-tissue proliferation using the BTM wound scaffold as a combination of technologies to promote wound closure in the setting of vascular disease. Our favourable outcomes provide an exciting new prospect for wound healing in a notoriously difficult cohort. A larger case series further studying the combination of these technologies and their impact on wound closure in the setting of vascular disease is warranted.
Acknowledgements
We would like to thank the staff of the Flinders Medical Centre Media and Illustration Unit for their clinical photographic contributions to this paper. We also acknowledge the use of the CELLUTOMETM System and would like to acknowledge the use of Adaptic Touch® (Systagenix, UK) as the silicone adhesive dressing utilised for capture and transfer for the EG material. We acknowledge the use of VAC® Therapy (3M, San Antonio, TX, USA) as the NPWT device utilised in all cases described in this case series. We acknowledge the use of NovoSorbTM Biodegradable Temporising Matrix® (PolyNovo Biomaterial Pty Ltd, Port Melbourne, VIC, Australia) as the wound scaffold utilised to promote tissue granulation and wound bed preparation in the cases referenced in this paper.
Conflict of interest
Frank Guerriero has previously received honorariums for education work provided to the CELLUTOMETM System manufacturing company 3M+KCI, San Antonio, USA. In October 2019 The Flinders Medical Centre Department of Vascular and Endovascular Surgery was awarded an unconditional education grant from KCI Medical, San Antonio for research in the field of tissue regeneration.
Funding
The unit has received a grant from the manufacturer of CELLUTOME however this was a unconditional education grant related to an alternate project and was provided post the commencement of this case series project. The FMC Dept. vascular and endovascular surgery has not undertaken any kind of formally sponsored trial by 3M/KCI.
Ethics statement
Consent to publish de-identified clinical information was obtained from all case series participants. Ethics approval for this project was provided by the Southern Adelaide Local Health Network, Office for Research.
Author(s)
Frank P Guerriero*1 and Christopher L Delaney2
1Flinders University, SA, Australia Department of Vascular and Endovascular Surgery, Flinders Drive, Flinders Medical Centre, Bedford Park, SA 5042, Australia
2Flinders University, SA, Australia
*Corresponding author Email frank.guerriero@sa.gov.au
References
- Guo S, DiPietro LA. Factors affecting wound healing. J Dental Res 2010;89(3):219–229.
- Stankiewicz M, Coyer F, Webster J, Osborne, S. Incidence and predictors of lower limb split-skin graft failure and primary closure dehiscence in day-case surgical patients. Dermatol Surg 2015;41(7):775–783.
- Kiistala U, Mustakallio KK. In-vivo separation of epidermis by production of suction blisters. Lancet 1964;1:1444–1445.
- Angeletti F, Kaufmann. Suction blister epidermal graft (SBEG) – an easy way to apply this method. J German Soc Dermatol 2019;17(4)468–471.
- Moridzadeh R S, Sanaiha Y, Madrigal J, Antonios J, Benharash P, Baril DT. Medical complexity of patients by surgical specialty: who operates on the sickest patients? Abstract from the 2019 Western Vascular Society Annual Meeting 2019;70(3):E91–92.
- International Wound Infection Institute (IWII). Wound infection in clinical practice. Wounds International 2016.
- Schultz G, Sibbald G, Falanga V, Ayello E, Dowsett C, Harding K, Romanelli M, Stacey MS, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28.
- Wagstaff MJD, Salna IM, Caplash Y, Greenwood JE. Biodegradable Temporising Matrix (BTM) for the reconstruction of defects following serial debridement from necrotising fasciitis: a case series. Burns Open 2019;3(1):12–30.
- Expert Working Group. Vascuum assisted closure: recommendations for use. Int Wound J 2008;5(s4): iii–19.
- Edmonds M, Lazaro-Martinez JL, Alfayate-Garcia JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patient with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind randomised controlled trial. Lancet Diabetes Endocrinol 2018;6:186–96.
- Hachach-Haram N, Bystrzonowski N, Kanapathy M, Smith OJ, Harding K, Mosahebi A, Richards T. A prospective, multicentre study on the use of epidermal grafts to optimise outpatient wound management. Int Wound J 2017;14:241–249.
- Sonter JA, Ho A, Chuter VH. The predictive capacity of toe blood pressure and the toe brachial index for foot wound healing and amputation: a systematic review and meta-analysis. Wound Practice Res 2016;22(4):208–220.
- Yavuz M. Plantar shear stress distributions in diabetic patients with and without neuropathy. Clin Biomechan (Bristol, Avon) 2014;Feb 29(2):223–229.
- Smith OJ, Edmondson SJ, Bystrzonowski N, Hachach-Haram N, Kanapathy M, Richards T, Mosahebi A. The CelluTome epidermal graft-harvesting system: a patient reported outcome measure and cost evaluation study. Int Wound J 2016;14:555–557.
- Serena T, Francius A, Taylor C, MacDonald J. Use of a novel epidermal harvesting system in resource-poor countries. Adv Skin Wound Care 2015;28(3):107–112.
- Richmond NA, Lamel SA, Braun LR, Vivas AC, Serena T, Kirsner R. Epidermal grafting using a novel suction blister-harvesting system for the treatment of pyoderma gangrenosum. JAMA Dermat 2014;150(9):999–1000.



