Volume 30 Number 3
eHealth interventions for the prevention of pressure injuries: a scoping review protocol
Anna J Rose, Arif Kamil, Elysa Roberts, Alexandra Hopson and Peta E Tehan
Keywords pressure injury, scoping review, eHealth
For referencing Rose et al. eHealth interventions for the prevention of pressure injuries: a scoping review protocol. Wound Practice and Research 2022; 30(3):179-184.
DOI
https://doi.org/10.33235/wpr.30.3.179-184
Submitted 28 February 2022
Accepted 16 May 2022
Abstract
Background Pressure injuries (PI) are an important quality and safety issue in healthcare, with an annual cost of treating PI in Australia estimated to exceed $A983 million. Electronic health (eHealth) interventions are increasingly being used to assist consumers and services in the prevention and management of PI. Digital health or eHealth involves health services and information delivered or enhanced through the internet and related technologies. This scoping review aims to: identify and map existing eHealth interventions for the prevention of PI; map learnings from these studies related to the non-adoption, abandonment and challenges to the scale-up, spread and sustainability of these devices; and determine gaps in research related to the use of eHealth interventions in the prevention of PI.
Method A scoping review will be guided by the methodology outlined by Arksey and O’Malley and the PRISMA-ScR framework. MEDLINE, CINAHL, ScienceDirect and the Cochrane library databases will be systematically searched using a piloted search strategy. Two reviewers will independently screen all titles, abstracts and full text articles. Any conflicts will be resolved by a third author. Consultations with authors holding clinical expertise in PI prevention and wound care will further inform the synthesis and reporting of findings. Findings will be presented in tabular and narrative format.
Results This scoping review will assist in the translation of existing clinical practice guidelines into practice. By harnessing the power of technology and data, effective eHealth solutions will facilitate greater client and carer involvement and earlier intervention in PI by supporting clinicians to tailor care to an individual’s needs and preferences and providing the right intervention at the right time.
Introduction
Pressure injuries (PI) are a global health issue. They are defined as “localised damage to the skin and/or underlying tissue, as a result of pressure or pressure in combination with shear. PI usually occur over a bony prominence but may also be related to a medical device or other object”1. They can be a painful, costly and often preventable healthcare complication1. PI are an important quality and safety issue in healthcare, highlighted by their inclusion within the Australian national safety standards2. The prevalence of PI remains a concern, affecting multiple service settings (1.1–26.7% in hospital settings; 6–29% in community settings; 7.6–53.2% in the nursing home setting) with significant healthcare implications and consequences3. Hospital length of stay and readmission rates are greater in individuals who develop a PI than those who remain PI free4–6. At the national level, the annual cost of treating PI in Australia was estimated to be A$983 million in 2012/137. The prevention of PI is a rapidly developing area of research. However, the evidence base supporting the use of technology in the prevention of PI has not yet been systematically reviewed and mapped.
A variety of population groups are deemed at high risk of PI. Those with spinal cord injury are at greatest risk for developing PI due to the combination of immobility, loss of sensation and other physiological factors including alteration to vascular supply, temperature control and autonomic response, and nutritional status8,9. Ageing also increases the risk of PI due to biological changes to the skin such as decreased cross-links of collagen fibres, the flattening of the dermal-epidermal junction and decreased sebum production which renders the skin increasingly fragile, dry and inflexible10,11. Further physiological changes in ageing such as weight loss, reduced muscle strength, and reduced physical activity also places the older person at an increased risk of developing a PI12. Diabetes populations can also be at increased risk, particularly when individuals have complications such as neuropathy and/or peripheral arterial disease13.
Interventions to reduce the incidence of and manage patients with PI tend to focus on pressure re-distribution to reduce the magnitude and duration of mechanical load14. This is generally achieved by repositioning body parts and implementing dynamic surfaces that help to redistribute pressure15. There are a range of interventions available; however, these have varying degrees of effectiveness and there is a lack of cohesiveness in their implementation16. eHealth interventions are increasingly being utilised in PI prevention through modalities such as mobile phone applications that enable timely access to education, remote consultation with health professionals, and social and emotional support from other peers17–21. In community settings, where there is less opportunity for healthcare professionals to view the skin, and where clients may be reluctant to share signs and symptoms of PI online due to modesty issues, supporting client and carers to express care needs early is essential22–25. Early detection of PI is known to lead to reduced costs to health services and impact on individual client wellbeing26,27.
To better support those at risk of PI, who are often managing competing health needs, health promotion efforts must provide education and actionable strategies that enable client engagement in care processes1,28. Building a sense of self-efficacy and social support to initiate and maintain desired self-care strategies has been found to have some benefit in the management of cancer, multiple sclerosis and obesity29–32. eHealth interventions may have an important role in addressing these issues. eHealth is defined as:
... an emerging field in the intersection of medical informatics, public health and business, referring to health services and information delivered or enhanced through the Internet and related technologies. In a broader sense, the term characterizes not only a technical development, but also a state-of-mind, a way of thinking, an attitude, and a commitment for networked, global thinking, to improve healthcare locally, regionally, and worldwide by using information and communication technology33.
The setting or context for adopting eHealth interventions is another important consideration. The Australian healthcare system, for instance, is a mix of public and private health services. While this hybrid system is successful at offering a high standard of care, there remain areas for further improvement. For example, the fragmentation of care remains a persistent issue within the Australian healthcare system and the further privatisation of healthcare services, as well as necessary reforms to public agencies such as the national disability insurance scheme, have compounded these issues, creating barriers to centralised information sharing and lessening equity of access34,35.
However, technological innovations being explored in private healthcare settings offer opportunities and resources that can support preventative approaches when applied to the public health system. Notably, a thoughtfully designed eHealth intervention could enable clients and carers to participate more effectively in injury prevention and facilitate greater collaboration among multidisciplinary healthcare teams. As the development of new eHealth interventions can be costly, it is important to first identify existing interventions that have the potential to be applied in the Australian context and are fit-for-purpose, meeting the specific needs of local stakeholders that are currently being underserved. Further, the identification of strategies that may support the implementation of eHealth interventions is necessary in averting the potential to compromise the quality, safety and efficiency of patient care if they are not implemented effectively36,37. Conversely, abandonment of eHealth interventions is described in the literature; the need for user-centred design has been identified as a way to support the implementation of eHealth interventions and reduce the likelihood of its abandonment38,39. Thus, it is also necessary to broaden and focus our understanding of the barriers to implementation and best approaches to incorporate eHealth into PI prevention by learning from those at the forefront of research in this field17–21,40.
Some well-documented challenges in the use of eHealth interventions include non-adoption and subsequent abandonment by clients and clinicians, as well as potential failures to become part of mainstream practice and sustain such interventions within and across services37. The need to capture the complex interplay of factors that contribute to the challenges of integrating eHealth into everyday healthcare is widely recognised with frameworks available that assist in the development, implementation and evaluation of eHealth interventions37. Furthermore, co-design has been identified as a useful approach in the development of supports for self-management of various health conditions that better meet patient, carer and clinician need41–45. Co-design approaches enable design based on the end users’ situations, needs and interests, and can help to ensure access for those from diverse socioeconomic backgrounds46,47.
To enable co-design approaches to the development of an eHealth intervention it is necessary to synthesise and evaluate research undertaken to date and use this as a foundation for involving consumers in the design and/or implementation of eHealth solutions within local contexts. The Nonadoption, Abandonment, and challenges to the Scale-up, Spread, and Sustainability (NASSS) framework is designed to assist in theorising and evaluating healthcare technologies37. The framework includes the following seven domains: the condition or illness; the technology; the value proposition; the adopter system (comprising professional staff, patient and lay caregivers); the organisation(s); the wider (institutional and societal) context; and the interaction and mutual adaptation between all these domains over time, and is not intended to be used as a checklist rather should be used reflexively to guide conversations and help generate ideas37. Therefore, it is an appropriate framework to guide the collation and consideration of current eHealth devices used in PI prevention and the factors influencing their successful adoption over time.
Objectives
This review will generate a knowledge base, built on existing literature and expanded through the identification of gaps in knowledge, to assist in the development of new eHealth interventions and ensure their successful implementation and sustained use in the prevention of PI. The current review will also help to identify key topics and questions to explore with consumers (clients and clinicians) when developing an eHealth intervention that aims to support enhanced self-management of PI prevention42. The research question that will be answered in this scoping review is “What eHealth interventions are available to prevent pressure injury in individuals at risk?
The scoping review will address the following aims:
- Identify and map research evidence regarding the use of eHealth interventions for the prevention of PI in individuals at risk of PI.
- Describe considerations given to the design, development, implementation, scale up, spread and sustainability of existing eHealth interventions by mapping included studies using the seven domains of the NASSS framework37.
Methods
This scoping review will be conducted using the scoping review framework articulated by Arksey and O’Malley48 and will be reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist49. Consultations with team members with expertise in PI prevention and wound care will inform the synthesis and reporting of findings. Quantitative and qualitative findings will be presented in tabular and narrative format.
The protocol for this scoping review is registered with the Open Sciences Framework (OSF) registry (https://doi.org/10.17605/OSF.IO/D6WHK). Prior to registration, the protocol was reviewed by three researchers with assistance provided by a university librarian specialising in search strategies, and health and medical research.
Search strategy
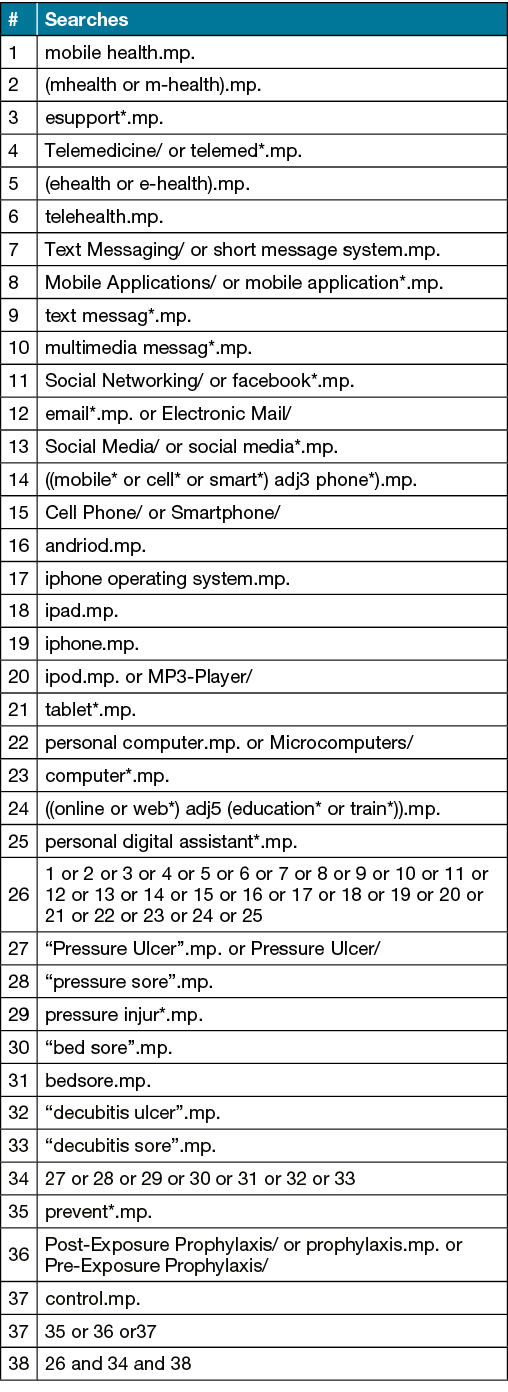
This study accessed databases from CINAHL, MEDLINE, ScienceDirect and the Cochrane Library. Search terms were derived for MEDLINE (Table 1) and later adjusted for other databases utilising database-specific search modifiers and Booleans as necessary. No language or date limiter was applied, with studies from the inception of each database until the date of the search being included. Individual reference lists from suitable articles will be manually searched for relevant sources appropriate to the aims of this study.
Table 1. MEDLINE search strategy
Inclusion and exclusion criteria
All types of original research studies, case studies and case series exploring the patient or practitioner experience of eHealth will be included. All adult patient populations which are at risk of PI and all service settings (i.e. hospital, community and residential aged care) will be included in the analysis. Studies that predominantly focus on other wound types (diabetic foot ulcer, skin tear, venous leg ulcer, arterial ulcer) will be excluded. Furthermore, studies that relate to the management of existing PI only as well as studies completed in children or those under 18 years will be excluded.
Study selection and data extraction
A plan for article storage, data collection and analysis has been piloted prior to searches being run to test the feasibility and acceptability of the planned approach48. For consistency and adopting efficient workflows, Covidence® will be used to store the articles and facilitate the team-based study screening and data extraction process. One author will conduct the literature search and export the title and abstract data into Covidence® (AR). Two authors will then screen the titles and abstracts for inclusion according to the criteria (AR and AK). If differences arise, a third party will judge the abstract for inclusion or exclusion (ER or AH or PT). Full texts of relevant studies will then be obtained, and two authors will screen these for relevance with differences judged by a third party. Articles deemed to satisfy the inclusion criteria will be included.
Data charting in the form of an Excel spreadsheet was formulated and piloted by AR and AK prior to its transfer into Covidence®. Data will be extracted by two authors independent of each other. Authors of papers that contained missing data or unclear information will be contacted based on provided contact information (where available). For each eligible study, the following data will be extracted from the full text article: author; year of publication, journal, title, country of study, setting, study design, participants, type of eHealth, intervention, comparator, PI stage, design, participant numbers, randomisation information, age (mean and range) and key quantitative (numeric) results and qualitative (textual) findings. Information relevant to the seven domains of the NASSS framework – the condition or illness, the technology, the value proposition, the adopter system (comprising professional staff, patient and lay caregivers), the organisation(s), the wider (institutional and societal) context, and the interaction and mutual adaptation between all these domains over time – will also be charted37.
Summarising, synthesising and reporting of results
The summarising and synthesising of the findings will be guided by the research aims. A content analysis approach will be used to analyse and synthesise any qualitative findings50,51. Extracted data will be presented in tables where the key findings will be synthesised and summarised in tables and narrative format. The discussion of the findings will be within the context of the current trends in the literature and implications for research, policy and practice. Clinical expertise will ensure that clinical relevance and compatibility is considered in the interpretation of the findings48,52. This systematic process will assist to inform future research needed to support the implementation of eHealth interventions at the local level.
Conflicts of interest
The authors declare no conflicts of interest.
Ethics statement
Ethics and dissemination: Approval from an ethics committee was not required as we are undertaking a review of published journal articles. Findings of the study will be disseminated through a peer reviewed journal, at conferences, and as part of follow-up future research projects involving a co-design approach.
Funding
This project was funded by a pilot grant from the School of Health Sciences at The University of Newcastle, NSW.
Author(s)
Anna J Rose1, Arif Kamil1, Elysa Roberts1, Alexandra Hopson2 and Peta E Tehan3,4*
1School of Health Sciences (Occupational Therapy), College of Health, Medicine and Wellbeing, University of Newcastle, NSW, Australia
2Hunter Spinal Cord Injury Service, Newcastle, NSW, Australia
3School of Clinical Sciences, Monash University, Melbourne, VIC, Australia
4School of Health Sciences (Podiatry), College of Health, Medicine and Wellbeing, University of Newcastle, NSW, Australia
*Corresponding author Email peta.tehan@newcastle.edu.au
References
- Haesler E, editor. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. EPUAP/NPIAP/PPPIA: 2019.
- Australian Commission on Safety and Quality in Health Care. Improvement guide standard 8: preventing and managing pressure injuries; 2012. Available from: http://www.safetyandquality.gov.au/wp-content/uploads/2012/10/Standard8_Oct_2012_WEB.pdf
- Graves N, Zheng H. Modeling the direct health care costs of chronic wounds in Australia. Wound Practice & Res 2014;22(1):20–7.
- Lyder CH, Wang Y, Metersky M, Curry M, Kliman R, Verzier NR, et al. Hospital-acquired pressure ulcers: results from the national medicare patient safety monitoring system study. J Am Geriatr Soc 2012;60(9):1603–8.
- Wilson JR, Arnold PM, Singh A, Kalsi-Ryan S, Fehlings MG. Clinical prediction model for acute inpatient complications after traumatic cervical spinal cord injury: a subanalysis from the Surgical Timing in Acute Spinal Cord Injury Study. J Neurosurg Spine 2012;17(1 Suppl):46–51.
- Wu Q, Ning G-Z, Li Y-L, Feng H-Y, Feng S-Q. Factors affecting the length of stay of patients with traumatic spinal cord injury in Tianjin, China. J Spinal Cord Med 2013;36(3):237–42.
- Nguyen K-H, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Aust Health Rev 2015;39(3):329–36.
- Marin J, Nixon J, Gorecki C. A systematic review of risk factors for the development and recurrence of pressure ulcers in people with spinal cord injuries. Spinal Cord 2013;51(7):522–7.
- Middleton J, Leong G, Mann L. Management of spinal cord injury in general practice: part 2. Aust Fam Phys 2008;37(5):321–38.
- Battersby M, Von Korff M, Schaefer J, Davis C, Ludman E, Greene SM, et al. Twelve evidence-based principles for implementing self-management support in primary care. Jt Comm J Qual Patient Saf 2010;36(12):561–70.
- Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineer 2021;8(5):63.
- Jaul E, Factor H, Karni S, Schiffmiller T, Meiron O. Spasticity and dementia increase the risk of pressure ulcers. Int Wound J 2019;16(3):847–51.
- Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70(10):2222–34.
- Hajhosseini B, Longaker MT, Gurtner GC. Pressure injury. Ann Surg 2020;271(4):671–9.
- Sprigle S, Sonenblum, S. Assessing evidence supporting redistribution of pressure for pressure ulcer prevention: a review. J Rehabil Res Dev 2011;48(3):11.
- Australian Wound Management Association. Pan pacific clinical practice guideline for the prevention and management of pressure ulcer; 2012. Available from: http://www.awma.com.au/publications/2012_AWMA_Pan_Pacific_Guidelines.pdf.
- Houlihan BV, Brody M, Everhart-Skeels S, Pernigotti D, Burnett S, Zazula J, et al. Randomized trial of a peer-led, telephone-based empowerment intervention for persons with chronic spinal cord injury improves health self-management. Arch Phys Med Rehabil 2017;98(6):1067–76.e1.
- Mercier HW, Ni P, Houlihan BV, Jette AM. Differential impact and use of a Telehealth intervention by persons with MS or SCI. Am J Phys Med Rehabil 2015;94(11):987–99.
- Olney CM, Vos-Draper T, Egginton J, Ferguson J, Goldish G, Eddy B, et al. Development of a comprehensive mobile assessment of pressure (CMAP) system for pressure injury prevention for veterans with spinal cord injury. J Spinal Cord Med 2019;42(6):685–94.
- Amann J, Fiordelli M, Brach M, Bertschy S, Scheel-Sailer A, Rubinelli S. Co-designing a self-management app prototype to support people with spinal cord injury in the prevention of pressure injuries: mixed methods study. JMIR Mhealth Uhealth 2020;8(7):e18018.
- Amann J, Fiordelli M, Scheel-Sailer A, Brach M, Rubinelli S. Opportunities and challenges of a self-management app to support people with spinal cord injury in the prevention of pressure injuries: qualitative study. JMIR Mhealth Uhealth 2020;8(12):e22452.
- Latimer S, Chaboyer W, Gillespie B. Patient participation in pressure injury prevention: giving patient’s a voice. Scand J Caring Sci 2014;28(4):648–56.
- Baron JS, Sullivan KJ, Swaine JM, Aspinall A, Jaglal S, Presseau J, et al. Self-management interventions for skin care in people with a spinal cord injury: part 2-a systematic review of use of theory and quality of intervention reporting. Spinal Cord 2018;56(9):837–46.
- Engelen M, van Dulmen S, Vermeulen H, de Laat E, van Gaal B. The content and effectiveness of self-management support interventions for people at risk of pressure ulcers: a systematic review. Int J Nurs Studi 2021;122:104014.
- Gourlan M, Pellechia A, Robineau S, Foulon B, Gault D, Lefort M, et al. “What pressure ulcers mean to me?” Representations of pressure ulcer in persons with spinal cord injury: a qualitative study. J Tissue Viab 2020;29(4):324–30.
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 2009;57(7):1175–83.
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care 2012;21(6):261–6.
- O’Connor T, Moore ZE, Patton D. Patient and lay carer education for preventing pressure ulceration in at-risk populations. Cochrane Database Syst Rev 2021;2:CD012006.
- Leahey TM, Huedo-Medina TB, Grenga A, Gay L, Fernandes D, Denmat Z, et al. Patient-provided e-support in reduced intensity obesity treatment: the INSPIRE randomized controlled trial. Health Psychol 2020;39(12):1037–47.
- Leavitt VM, Riley CS, De Jager PL, Bloom S. eSupport: feasibility trial of Telehealth support group participation to reduce loneliness in multiple sclerosis. Mult Scler 2020;26(13):1797–800.
- Zhu J, Ebert L, Liu X, Chan SW. A mobile application of breast cancer e-support program versus routine care in the treatment of Chinese women with breast cancer undergoing chemotherapy: study protocol for a randomized controlled trial. BMC Cancer 2017;17(1):291.
- Zhu J, Ebert L, Liu X, Wei D, Chan SW. Mobile breast cancer e-support program for Chinese women with breast cancer undergoing chemotherapy (part 2): multicenter randomized controlled trial. JMIR Mhealth Uhealth 2018;6(4):e104.
- Eysenbach G. What is e-health? J Med Internet Res 2001;3(2):E20-E.
- Malbon E, Carey G, Meltzer A. Personalisation schemes in social care: are they growing social and health inequalities? BMC Public Health 2019;19(1):805.
- Heinsch M, Tickner C, Kay-Lambkin F. Placing equity at the heart of eHealth implementation: a qualitative pilot study. Int J Equity Health 2022;21(1):38.
- Dendere R, Janda M, Sullivan C. Are we doing it right? We need to evaluate the current approaches for implementation of digital health systems. Aust Health Rev 2021. Dec (45), 778-781 doi 10.1071/AH20289.
- Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017;19(11):e367.
- Molina-Recio G, Molina-Luque R, Jiménez-García AM, Ventura-Puertos PE, Hernández-Reyes A, Romero-Saldaña M. Proposal for the user-centered design approach for health apps based on successful experiences: integrative review. JMIR Mhealth Uhealth 2020;8(4):e14376.
- Peiris D, Miranda JJ, Mohr DC. Going beyond killer apps: building a better mHealth evidence base. BMJ Global Health 2018;3(1):e000676.
- Bogie KM, Ho CH. Multidisciplinary approaches to the pressure ulcer problem. Ostomy Wound Manage 2007;53(10):26–32.
- Duckett S. What should primary care look like after the COVID‑19 pandemic? Aust J Prim Health 2020;26(3):207–11.
- Ayobi A, Stawarz K, Katz D, Marshall P, Yamagata T, Santos-Rodriguez R, et al. Co-designing personal health? Multidisciplinary benefits and challenges in informing diabetes self-care technologies. Proc ACM Hum-Comput Interact 2021;5(CSCW2):Article 457.
- Keely EJ, Archibald D, Tuot DS, Lochnan H, Liddy C. Unique educational opportunities for PCPS and specialists arising from electronic consultation services. Acad Med 2017;92(1):45–51.
- Jin K, Khonsari S, Gallagher R, Gallagher P, Clark AM, Freedman B, et al. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review and meta-analysis. Eur J Cardiovasc Nurs 2019;18(4):260–71.
- Ferguson J, Craig EA, Dounavi K. Telehealth as a model for providing behaviour analytic interventions to individuals with autism spectrum disorder: a systematic review. J Autism Dev Disord 2019;49(2):582–616.
- Latulippe K, Hamel C, Giroux D. Co-design to support the development of inclusive eHealth tools for caregivers of functionally dependent older persons: social justice design. J Med Internet Res 2020;22(11):e18399.
- Aidemark J, Askenäs L. Taking adaptable co-design action: flexible learning between health experts, end-users and technology experts in the early stage of eHealth design. In: Yoshitoshi M, Oliver H, editors. The Eleventh International Conference on eHealth, Telemedicine, and Social Medicine Athens, 25–28 February 2019; 2019: International Academy, Research and Industry Association (IARIA); 2019. p. 90–5.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8(1):19–32.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–73.
- Graneheim UH, Lindgren BM, Lundman B. Methodological challenges in qualitative content analysis: a discussion paper. Nurse Educ Today 2017;56:29–34.
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004;24(2):105–12.
- Sanson-Fisher RW. Diffusion of innovation theory for clinical change. Med J Aust 2004;180(S6):S55–6.



