Volume 30 Number 3
Neonatal skin injury scales: a scoping review with narrative synthesis protocol
Stephanie Hall, Deanne August, Fiona Coyer and Nicole Marsh
Keywords skin injury, scoping review, neonatal, severity scales
For referencing Hall S et al. Neonatal skin injury scales: a scoping review with narrative synthesis protocol. Wound Practice and Research 2022; 30(3):175-178.
DOI
https://doi.org/10.33235/wpr.30.3.175-178
Submitted 6 January 2022
Accepted 9 March 2022
Abstract
Background Mechanical skin injuries have been a neonatal complication since the 1980s but a number of factors affect their assessment, particularly the assessment of injury severity. Whilst there is a single severity and classification system used to assess adult pressure injuries within Australia, there are no neonatal-specific standards for injury assessment. Unfortunately, neonates sustain skin injuries frequently and, whilst there are some similarities to adult injuries, the maturity of neonatal skin puts the application of adult injury scales into question. In addition, several severity systems are currently utilised, thus outcomes for skin injury prevention or management strategies are difficult to compare.
Aims This review will investigate severity scales used for neonatal skin injury. Secondly, this review will determine the (i) characteristics of severity scales such as ordinal or categorical groupings and (ii) assessment of scale validation for population.
Methods This scoping review will utilise the PRISMA-ScR framework and the 2015 Joanna Briggs Institute methodology. The electronic medical databases chosen are PubMed, CINAHL, COCHRANE Central and Scopus. Publications from within the last 20 years will be included to ensure the scales and the neonatal population reflect the timepoint when neonatal skin care became a safety and quality outcome as recognised by international seminal works.
Ethics and dissemination
As the data (i.e. journal articles) used is publicly available, ethical approval was not required for this scoping review. A peer-reviewed journal and conference presentation will then be used to disseminate findings.
Introduction
Mechanical force skin injuries have been identified in the literature as a neonatal complication since the 1980s1,2. Neonates are particularly vulnerable to skin injuries related to hospitalised care, with a variety of severities identified in previous studies3. Some of these injuries have lasting impacts such as scarring which affects movement and self-esteem for the individual affected4,5. Therefore the severity of tissue damage sustained is equally as important as the frequency and location of injuries. In Australia, adult pressure injuries are reported and compared using an internationally accepted system, the National Pressure Ulcer Advisory Panel (NPUAP) injury classification system6. The NPUAP injury staging system has been co-endorsed by the European Pressure Ulcer Advisory Panel and the Pan Pacific Pressure Injury Alliance for all populations, including neonates. However, while neonates sustain skin injuries from a number of mechanical forces similar to adults, the maturity of neonatal skin puts the application of adult injury scales into question1,2,7. A mechanical skin injury is defined as a distortion or injury of the integrity of the skin and/or underlying integumentary structures by external motion3,6. Common forces associated with skin injury for the neonatal population include pressure (a source exerting force on the skin), friction (resistance created by surfaces moving between skin and surface), shearing (still outer layers with inner layers moving transversely), tear (blunt force resulting in separation of skin layers)8,9 and stripping (when adhesive is more strongly bonded to the skin than the intradermal bonds, this is known as medical adhesive-related skin injury [MARSI])10. In a neonatal hospital setting, mechanical force skin injuries can occur from a single adhesive removal or from medical devices required for life-saving treatment, hence the paradox that those most susceptible to injury are also those in need of interventions11,12.
Currently, there is no neonatal-specific standard of injury assessment including severity3,5. Premature skin is particularly at risk for injury related to weak cohesion in the dermo-epidermal junction and is oedematous, making it less stable and more prone to skin layer separation13. Neonatal skin layer thickness ranges from 0.9–1.2mm and has increasing strength/presence of collagen bonds as gestational age rises13,14. A consequence to this is that as the gestational age of neonates decreases, there is a greater prevalence of mechanical skin injuries. As neonates sustain skin injuries from a different and more diverse set of mechanical forces, this puts the direct application of adult injury scales into question3,14.
Skin injury severity tools are primarily used by nursing staff as a part of their patient care and assessment and inform the creation of treatment guidelines15. Several severity systems are currently utilised in the assessment of neonatal skin injury, many of which are unvalidated or modified from adult tools11,16,17. Without a clear understanding of the number and source of current severity systems, it is not possible to determine how applicable these tools are to the accuracy of injury severity. Furthermore, with a number of systems available and used, outcomes for skin injury prevention or management strategies are difficult to compare.
To our knowledge this is the first scoping review to investigate the range of different neonatal skin injury severity scales. The aim of this scoping review will be to identify neonatal skin injury severity scales and report differences in their approach severity or classification. Two additional objectives will be undertaken: (i) identification of characteristics of severity scales such as ordinal or categorical groupings, and (ii) assessment of scale validation for population.
Outcome definitions
For the purposes of this scoping review, the following definitions will be used to determine outcomes.
- Neonate: considered any live born infant, cared for in a hospital/healthcare setting, until the time of discharge from a neonatal facility or unit or cared for in a specialised nursery setting.
- Skin injury: damage to skin or underlying tissue as a result of a single or combination of mechanical forces (such as pressure or stripping) acquired in relation to or as a result of hospital care.
- Severity, staging or classification: a grouping or ordinal categorisation that separates and identifies the level of damage.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews (PRISMA-ScR) extension (Appendix 1) and 2015 Joanna Briggs Institute (JBI) for scoping reviews will be used as framework for this review with narrative synthesis with quality assessment using the Mixed Methods Appraisal Tools (MMAT)18–20. This scoping review protocol has been registered at Open Science Framework (OSF) (htpps://osf.io/jqkxf ) (6/01/2022).
Eligibility criteria
As this review aims to identify the current neonatal skin injury severity scales for subjects in the hospital setting, the decision was made that the population of interest would be restricted. Specifically, hospitalised neonates obtain mechanical force skin injuries related to life-saving care and monitoring interventions, thus we will exclude hospitalised neonates being cared for by their parents5. Peer-review publications from within the last 20 years will be included to ensure the scales and the neonatal population reflect the timepoint when neonatal skin care became an internationally recognised safety and quality outcome21.
Inclusion
- Publications that report mechanical force, neonatal skin injuries.
- Original publications.
- Publications in English.
- Cohort design (prospective or retrospective), quasi-experimental, randomised control trial, interrupted time series, systematic reviews, qualitative research, case series and studies.
- National and international evidenced-based guidelines.
- Publications from within the last 20 years.
Exclusion
- Hospitalised neonates being cared for by their parents or in a maternity/birth setting.
- Publications reporting on the primary cause of skin injuries not related to mechanical force (such as infection, chemical burn).
- Publications that report mixed populations such as paediatrics with neonates included, when a neonatal subset cannot be identified.
- Conference abstracts, letters to the editor, lab and vivo studies.
Information sources
The databases PubMed, CINAHL, COCHRANE Central and Scopus will be searched January 2001 to December 2021. Additionally, consultation will be undertaken with experts in the field and a reference list of all publications will be reviewed for additional articles. Initial key words included neonat* OR infant* with skin injury, skin break, skin trauma, pressure injury. These were expanded into search strings using additional key words and medical subject headings (MeSH).
Searching
A search of electronic databases will be conducted for the period January 2001 to December 2021 for the peer-review published literature. The first step will be searching the identified databases, followed by the screening of abstracts against the selection criteria. The third step will involve the extraction of data and narrative synthesis of findings, with critical analysis reported using the MMAT for quality assessment. Databases searched will include PubMed, CINAHL, COCHRANE Central and Scopus databases. Additionally, consultation will be undertaken with experts in the field and a reference list of all publications will be reviewed for additional articles. Initial key words included neonat* OR infant* with skin injury, skin break, skin trauma, pressure injury. These were expanded into search strings using additional key words and medical subject headings (MeSH) (Appendix 2).
Key search terms will include:
(“Soft Tissue Injuries”[Mesh] OR “skin injury” OR “skin injuries” OR “skin break” OR “skin breakdown” OR “skin trauma” OR “pressure injury” OR “pressure injuries” OR “mechanical injury” OR “mechanical injuries” OR “friction” OR “shear” OR “stripping”) AND (“severity scale” OR “severity scales” OR “scale” OR “scales” OR “assessment” OR “assessments” OR “classification” OR “classifications” OR “staging” OR “Severity of Illness Index”[Mesh]) AND (“Infant, Newborn”[Mesh] OR “neonate” OR “neonates” OR “neonatal” OR “infant” OR “infants” OR “infant’s” OR “infancy” OR “infantile” OR “newborn” OR “newborn’s” OR “newborns” OR “new born”) AND (2001:2021[dp]).
Selection of sources
Eligible publications will be reviewed by two independent researchers (SH, DA) who will undertake assessment of the titles and abstracts, and any disagreement will be reviewed by a third researcher (NM). Articles identified will be uploaded into a reference manager (Endnote X9), combined, and searched for duplicates. After the removal of duplicates, titles and abstracts of articles will be inputted into Endnote x9 for screening against the inclusion and exclusion criteria. Full text copies of studies deemed eligible will then be independently reviewed and assessed, with differences in opinion reviewed by the third author.
Data charting process
After full text articles are identified, data will be entered into purpose-built data collection tool in Excel by a research assistant, a study author (S, DA, NM, FC) will check and verify data entered. If extracted information is unclear from these reviews, a clarification will be sought from the contact author for individual publications. Extracted data will include study design (e.g. systematic review), population (e.g. preterm or term), study intervention (if pertinent), country, year, and components of the severity scales (e.g. (i) and (ii) scoping review sub-objectives). Further details of the domains and individual attributes, scale (e.g. colour, numerical rating) collection method will also be extracted.
Assessment of the validation of scales will be valuable for several reasons. Firstly, skin maturation differs greatly between gestational ages of neonates, with many requiring hospital care having only a few layers of stratum corneum and an underdeveloped dermis13. Therefore, it is likely that scales developed for adults, paediatrics or even term babies may be inappropriate to categorise skin injury severity and thus the level of evidence and source of data for the scale is important.
Discussion
The neonatal population comprises a diverse range of skin structural maturities distinct from paediatric and adult skin, with additional limitations in functional maturation of the skin13,14. These limitations include differences in depth of skin layers such that a term neonate has only 1.2mm of skin compared to an adult with 2.1mm13,14. Therefore the identification of of the original population the scale was sourced from is important to consider the underlying assumptions about skin physiology and injury depth.
Skin injury severity scales have a direct impact on neonatal care and the development of best practice guidelines for this population11,22. Outcomes measured by injury scales can affect decisions for treatment wishing to avoid injury and the management choices of injuries sustained23. However, while severity scales can improve consistency of nursing skin assessment, scales can be subjective24, with limitations to the evaluation of depth and colour by the human eye found particularly for the neonatal population25,26. With the risk of depth interpretation, there are additional complexities in multiple clinicians’ assessments and subsequent assessments onsite and between facilities. Without uniformity of skin injury assessment, treatment practices continue to be proposed as best practice without measurable comparators. For example, it is difficult to determine best practice to avoid nasal injury from airway devices when injuries can be reported at three or four stages of injury27,28. Furthermore, without using the same scale or equivalency between the scales it is not possible to accurately compare injury rates between hospitals to guide efficacy of interventions and best practice.
The future of skin care and injury management in acutely unwell neonates depends on the development and review of best practice evidence. Scoping the current literature and addressing the objectives of this review will allow for improved understanding of neonatal skin injury assessment tools to add to future guidelines and research on this topic.
Acknowledgements
Natalie Barker, librarian University of Queensland, for her guidance in search terms.
Conflict of interest
DA reports speaker fees provide to Griffith University from 3M, consultancy work for the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) and conference travel from Johnson and Johnson Pacific with ATORA Neonatal Nurses College of Aotearoa (NNCA) Neonatal Nurses Society all unrelated to this project. NM reports that Griffith University has received on her behalf speaker fees from 3M, investigator-initiated research grants from Becton Dickinson, Eloquest and Cardinal Health, and a consultancy payment from Becton Dickinson for clinical feedback. SH and FC declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable
Funding
The authors received no funding for this study
Author contribution
All authors co-developed the research question, protocol and manuscript content.
Author(s)
Stephanie Hall*1,2, Deanne August1,3,4, Fiona Coyer2,5,6 and Nicole Marsh3,7
1Neonatal Unit, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
2School of Nursing, Queensland University of Technology, Brisbane, QLD, Australia
3Nursing and Midwifery Research, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
4School of Nursing, Midwifery and Social Work, University of Queensland, Brisbane, QLD, Australia
5School of Nursing, Centre for Healthcare Transformation, Faculty of Health, Queensland University of Technology, Brisbane, QLD, Australia
6Intensive Care Services, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
7School of Nursing, Griffith University, Brisbane, QLD, Australia
*Corresponding author Email stephanie.hall2@health.qld.gov.au
References
- August DL, New K, Ray R, Kandasamy Y. Frequency, location and risk factors of neonatal skin injuries from mechanical forces of pressure, friction, shear and stripping: a systematic literature review. J Neonat Nurs 2018;24(4):173–80.
- August DL, Ray RA, Kandasamy Y, New K. Neonatal skin assessments and injuries: nomenclature, workplace culture and clinical opinions-Method triangulation a qualitative study. J Clin Nurs 2020;29(21–22):3986–4006.
- August DL, Kandasamy Y, Ray R, Lindsay D, New K. Fresh perspectives on hospital acquired neonatal skin injury prevalence from a multicentre study: length of stay, acuity and incomplete course of antenatal steroids. J Perinat Neonat Nurs 2021;35(3).
- Cartlidge P, Fox P, Rutter N. The scars of newborn intensive care. Early Human Dev 1990;21(1):1–10.
- August DL, Marceau J, Benton J. Neonatal skin and wound care. In: Mannix T, V Kain, editors. Neonatal nursing in Australia and New Zealand: principles for practice. 1st ed. Chatswood, NSW: Elsevier; 2018. p. 359–80.
- Haesler E, editor. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. EPUAP/NPIAP/PPPIA: 2019.
- August DL, Ireland S, Benton J. Silver-based dressing in an extremely low-birth-weight infant: a case study. J WOCN 2015;42(3):290–3.
- Campbell JL, Coyer FM, Osborne SR. The skin safety model: reconceptualizing skin vulnerability in older patients. J Nurs Scholarsh 2016;48(1):14–22.
- LeBlanc K, Baranoski S. International Skin Tear Advisory Panel: putting it all together, a tool kit to aid in the prevention, assessment using a simplified classification system and treatment of skin tears. WCET J 2014;34(1):12–27.
- McNichol L, Lund C, Rosen T, Gray M. Medical adhesives and patient safety: state of the science: consensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J WOCN 2013;40(4):365–80; quiz E1–2.
- Broom M, Burton W, Ehrlich L, Dunk A, Abdel-Latif M. Developing an Australian skin risk assessment and management tool for neonates. Wound Prac Res 2017;25(1):15.
- Nist MD, Rodgers EA, Ruth BM, Bertoni CB, Bartman T, Keller LA, et al. Skin rounds: a quality improvement approach to enhance skin care in the neonatal intensive care unit. Adv Neonat Care 2016;16 Suppl 5S:S33-S41.
- August D, van der Vis KM, New K. Conceptualising skin development diagrammatically from foetal and neonatal scientific evidence. J Neonat Nurs 2019;25(6):311–4.
- Visscher MO, Burkes SA, Adams DM, Hammill AM, Wickett RR. Infant skin maturation: preliminary outcomes for color and biomechanical properties. Skin Res Technol 2017.
- Curley MA, Hasbani NR, Quigley SM, Stellar JJ, Pasek TA, Shelley SS, et al. Predicting pressure injury risk in pediatric patients: the Braden QD Scale. J Pediatr 2018;192:189–95. e2.
- Chen CY, Chou AK, Chen YL, Chou HC, Tsao PN, Hsieh WS. Quality improvement of nasal continuous positive airway pressure therapy in neonatal intensive care unit. Pediatr Neonat 2016;58(3):229–35.
- Imbulana DI, Manley BJ, Dawson JA, Davis PG, Owen LS. Nasal injury in preterm infants receiving non-invasive respiratory support: a systematic review. Arch Dis Child Fetal Neonat Ed 2018;103(1):F29-F35.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annal Int Med 2018;169(7):467–73.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evidence Imp 2015;13(3):141–6.
- Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educat Inform 2018;34(4):285–91.
- Lund C, Osborne JW, Kuller J, Lane AT, Lott JW, Raines DA. Neonatal skin care: clinical outcomes of the AWHONN/NANN evidence-based clinical practice guideline. Association of Women’s Health, Obstetric and Neonatal Nurses and the National Association of Neonatal Nurses. JOGNN 2001;30(1):41–51.
- Newnam KM, McGrath JM, Salyer J, Estes T, Jallo N, Bass WT. A comparative effectiveness study of continuous positive airway pressure-related skin breakdown when using different nasal interfaces in the extremely low birth weight neonate. App Nurs Res 2015;28(1):36–41.
- Amer Y, Bridges C, Marathe K. Epidemiology, pathophysiology, and management strategies of neonatal wound care. NeoRev 2021;22(7):e452-e60.
- Stausberg J, Lehmann N, Kröger K, Maier I, Niebel W. Reliability and validity of pressure ulcer diagnosis and grading: an image-based survey. Int J Nurs Stud 2007;44(8):1316–23.
- Meszes A, Tálosi G, Máder K, Orvos H, Kemény L, Csoma ZR. Lesions requiring wound management in a central tertiary neonatal intensive care unit. World J Pediatr 2016:1–8.
- August D, Hitchcock I, Tangney J, Ray RA, Kandasamy Y, New K. Graduated colour tape measure: development and demonstration of this tool in a case series of neonatal skin injuries. J Tissue Viabil 2019;28(3):133–8.
- Fischer C, Bertelle V, Hohlfeld J, Forcada-Guex M, Stadelmann-Diaw C, Tolsa JF. Nasal trauma due to continuous positive airway pressure in neonates. Arch Dis Child Fetal Neonat Ed 2010;95(6):F447–51.
- Fujii K, Sugama J, Okuwa M, Sanada H, Mizokami Y. Incidence and risk factors of pressure ulcers in seven neonatal intensive care units in Japan: a multisite prospective cohort study. Int Wound J 2010;7(5):323–8.
Appendix 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist
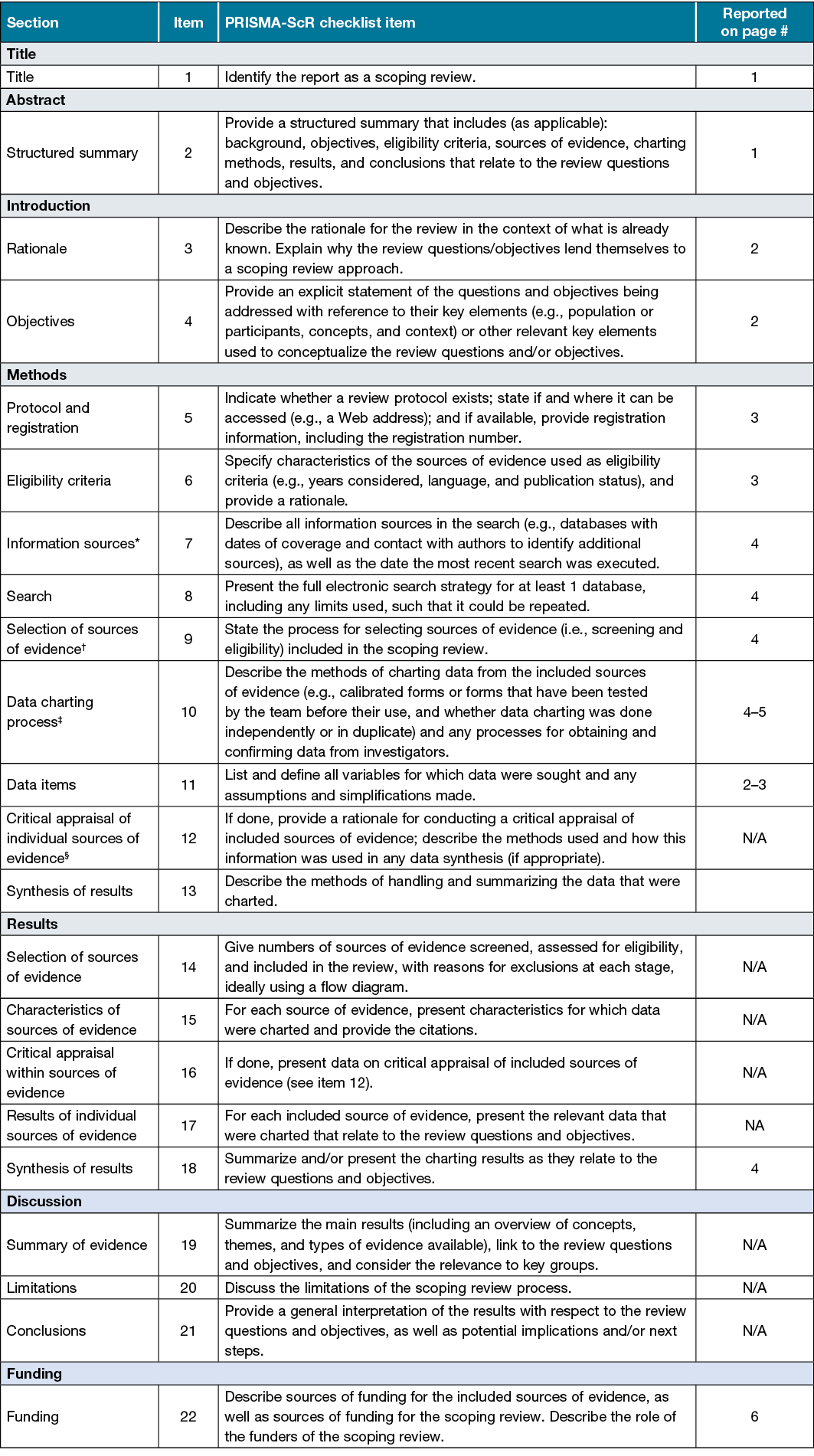
JBI = Joanna Briggs Institute; PRISMA-ScR = Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews.
* Where sources of evidence (see second footnote) are compiled from, such as bibliographic databases, social media platforms, and Web sites.
† A more inclusive/heterogeneous term used to account for the different types of evidence or data sources (e.g., quantitative and/or qualitative research, expert opinion, and policy documents) that may be eligible in a scoping review as opposed to only studies. This is not to be confused with information sources (see first footnote).
‡ The frameworks by Arksey and O’Malley (6) and Levac and colleagues (7) and the JBI guidance (4, 5) refer to the process of data extraction in a scoping review as data charting.
§ The process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. This term is used for items 12 and 19 instead of “risk of bias” (which is more applicable to systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (e.g., quantitative and/or qualitative research, expert opinion, and policy document).
From: Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann Intern Med 2018;169:467–473. doi: 10.7326/M18-0850



