Volume 30 Number 3
Complex wound healing in a complex patient
Clarissa A Young, Harriet K Semple and Gary M Kode
Keywords vasculitis, Biodegradable Temporizing Matrix, standard of care
For referencing Young CA et al. Complex wound healing in a complex patient. Wound Practice and Research 2022; 30(3): 169-174.
DOI
https://doi.org/10.33235/wpr.30.3.169-174
Submitted 22 March 2022
Accepted 16 May 2022
Abstract
Cutaneous vasculitis presents a significant challenge to wound care specialists with the potential to result in large areas of dermal necrosis complicated by multisystem pathology leading to poor physiological reserve and treatment options that arrest normal healing. Both surgical and conservative management needs to be flexible as plans can be derailed by the fluctuating course of disease. In this case the application of a synthetic dermal substitute, NovoSorb® Biodegradable Temporizing Matrix (BTM), and ongoing structured assessment using TIMERS and wound standards of care achieved unexpected positive results.
Introduction
This case study describes the multidisciplinary team approach to treating, managing and healing extensive wounds resulting from cutaneous vasculitis measuring 8% of total body surface area on bilateral upper limbs and the left lower limb where NovoSorb® Biodegradable Temporizing Matrix (BTM), (PolyNovo Biomaterials Pty Ltd, Port Melbourne, VIC, Australia), a synthetic dermal substitute1, was successfully placed. In this case unplanned secondary intention epithelisation occurred on bilateral upper limbs and marks the largest areas of secondary healing on BTM in the literature2.
Integral to the healing of multiple and complex wounds, the clinical skills of a multidisciplinary team across acute and community service providers aimed at delivering a standard of care (SoC) that was flexible and goal- and outcome-based. The application of TIMERS (Tissue, Inflammation/Infection, Moisture, Edge, Regeneration/Repair and Social factors3) will be used to describe some of the challenging events experienced by the patient during wound healing over 12 months.
Wound management
Vasculitides
Vasculitis is inflammation of blood vessel walls that may result in wounds with ischaemic necrosis, and often presents with disturbed healing due to a deficit of intact or functional vessels4,5. Vasculitides can be categorised by the size of vessels affected and can affect vessels in multiple organs, commonly skin, kidney and lungs. Severe vasculitis can lead to bullae formation, ulceration and skin necrosis, and purpuric lesions are commonly seen with inflammatory disease more often in Sjogren’s syndrome and non-Hodgkin’s lymphoma6,7. The range and severity of vasculitis may vary greatly, and the specific cause is not fully understood but thought to be due to disturbances in the body’s immune system5.
Treatment is aimed at finding and treating any underlying inflammation7 and reducing circulating immune complex-mediated damage with high dose tapered oral corticosteroids and adjunct steroid sparing immunosuppressive agents such as cyclophosphamide. Oral corticosteroids provide significant benefit for steroid responsive disorders of dermatoses and are both dose- and time-dependent. Some cutaneous adverse effects include ecchymosis, skin thinning and atrophy, and impaired wound healing8. While essential in treating the primary pathology, steroids are detrimental to wound healing. Steroids can impede wound contraction, decrease tensile strength, increase blood glucose and suppress the inflammatory and proliferative phase9, and can present a challenge to wound specialists7.
Biodegradable Temporizing Matrix (BTM)
NovoSorb® Biodegradable Temporizing Matrix (BTM) is a bioabsorbable dermal matrix for the treatment of full thickness wounds involving the loss of dermal structure from trauma or surgical debridement10. This is achieved by facilitating dermal repair by providing temporary wound closure and scaffold for the generation of neodermis. It is composed of a porous polyurethane foam attached to a fenestrated transparent sealing membrane (also referred to as laminate) with the foam breaking down via hydrolysis1,11.
The application of BTM is a two-stage procedure. The first stage is undertaken in surgery where BTM is cut to the shape of the wound and applied with staples or sutures to the debrided wound bed. Integration time varies depending on both wound and patient specific factors. Integration occurs when the migration of blood vessels, cells and fibroblasts populate and produce collagen to form the vascularised neodermis. Capillary refill and a uniform pink colour are clinical signs of integration and usually appear after several weeks10,12.
The second stage involves removing the staples or sutures and the sealing membrane (known as delamination). Wound closure can be achieved with the application of a split skin graft (SSG)12. BTM helps replace the natural thickness of the dermis, minimises contractures and prevents tethering to underlying structures, and provides temporising of large surface wounds13. Wounds can be left to heal by secondary intention, without the application of a SSG following delamination; a few papers reported healing by secondary intention of small wounds on well patients. This complex case presents the largest area of secondary healing documented in the literature2,14.
Wound assessment
Wound assessment has been the traditional role of nurses – a greater appreciation of complexity of wound healing can benefit from a focussed and expanded assessment and interventions15. Wound assessments should provide the foundation for goals setting, planning and monitoring of the plan. In 2003, Schultz et al published wound bed preparation using the structured framework of Tissue, Infection/Inflammation, Moisture and wound Edge (TIME)16. This framework has been used widely internationally as part of a comprehensive approach for each patient17 but has been criticised as too wound focussed and not patient-centred18.
In 2019, two separate groups published additions to TIME with a focus on chronic and hard-to-heal wounds with a change from TIME to TIMERS, including the addition of (R) Repair/regeneration of tissue and (S) Social factors3. This new framework and guidance aimed to identify where advanced therapies and patient factors should be considered alongside the SoC. SoC refers to “the reasonable degree of care a person should provide to another person”19; standards for wound prevention and management are available in Australia20. The second group expanded TIME to include a Clinical Decision Support Tool (CDST)21.
TIMERS may also include advanced therapies cell and tissue engineering with all elements having supported recommendations with evidence to meet clinical goals3. Repair, regeneration and wound closure were enhanced through the application of BTM by providing a matrix to support cell infiltration.
The identification and management of patient specific factors such as wound aetiology and risk factors22 are supported when the same effective SoC is delivered over all health pathways. This occurs when the individual transitions between healthcare settings and professionals apply evidence-based treatment for each identified factor. The care pathway defines the “who, what and when” products and services are delivered incorporating local, national and international guidelines3. Social and patient-related factors reflect the importance of patient engagement with the multidisciplinary team and concordance with the agreed plan to minimise variation of the SoC3.
Case study
A 66-year-old woman was transferred to Launceston General Hospital (LGH) in northern Tasmania in March 2020 within 24 hours of presentation from her regional hospital with acute kidney injury, supraventricular tachycardia, community acquired pneumonia (CAP) and an undifferentiated vasculitis resulting in extensive cutaneous lesions on both her upper limbs and left lower limb. The patient was admitted to the intensive care unit and was diagnosed and treated for legionella CAP with targeted intravenous antibiotics and high dose and tapered oral corticosteroids. Prior to this presentation she was medically stable and living independently 90km away. She was a non-smoker and non-drinker, with multiple comorbidities of Non-Hodgkin lymphoma (2017), treated with radiation and chemotherapy and in complete remission, Sjogren’s Syndrome, leukocytoclastic vasculitis (2014), hypertension, hyperlipidaemia, aortic regurgitation and GORD. Usual medications included sotalol, esomeprazole and rosuvastatin.
The skin lesions on the upper arms and knee were described as a mixture of intact and deroofed haemoserous blisters. Histological tissue biopsies confirmed unspecified vasculitis with neutrophils, with the absence of necrosis, lymphoma, calciphylaxis, warfarin-induced changes and pyoderma gangrenosum. In keeping with the complexity of the diagnosis a multidisciplinary team from dermatology, rheumatology, wound, plastic and reconstructive surgery (PRS) and nutrition specialties were consulted3 and expanded to include renal, general medical, endocrinology, oncology, infectious diseases and cardiac specialities over the continuum of care. The patient’s condition stabilised, and she was transferred to a medical ward with additional medications which included frusemide, amlodipine, apixaban, paracetamol and metformin for steroid-induced hyperglycaemia.
Over a period of 2 weeks the lesions stabilised to dry necrotic tissue and declared their size (Figures 1 & 2). Goal-based wound plans included reducing the risk of infection, exudate absorption where present, and allowing the stabilisation of dry eschar/necrotic tissue. A mix of low adherent mesh, padding, light foam, conforming soft bandage, and light tubular bandaging over all the limbs. She was discharged home with wound care provided by community nursing and outpatient review by the LGH PRS team.
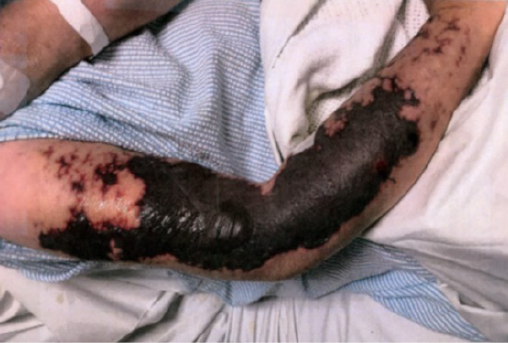
Figure 1. Right upper limb at 2 weeks after presentation
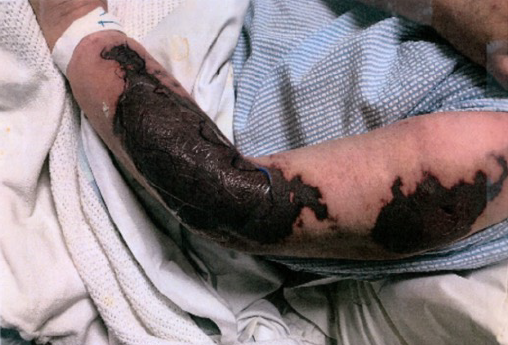
Figure 2. Left upper limb at 2 weeks after presentation
Towards the end of this admission the COVID-19 outbreak and subsequent closure of health facilities and community lockdown delayed follow-up at the outpatient department. This in turn delayed planned surgical debridement and application of BTM. The surgical plan was predicated on reducing corticosteroids to reduce risk of skin graft failure and the management of anticoagulation therapy prior to surgery. BTM was selected due to the depth and size of the vasculitic lesions and providing a wound cover in preparation for SSG. BTM would provide a healthy base for SSG and minimise the risks of additional wounds.
From the initial admission nutritional support was assessed and monitored, with the addition of two arginine powder sachets daily to supplement oral intake. The patient’s serum albumin fluctuated from 17g/L (normal range 35–52g/L) at first admission to one normal range of 40g/L. Thereafter the serum albumin remained below the normal range in the mid to high 20g/L.
At 3 months from initial presentation the patient was medically stable and the lesions were static, dry and not infected. The prednisolone dose was slowly reduced in the 2 months preceding surgery from 50mg to 12.5mg daily and apixaban was ceased 48 hours pre-surgery in preparation for the first stage of surgical debridement. Full thickness dermal necrosis from both arms and left lower limb was debrided under general anaesthetic. The wounds were dressed with a low adherent mesh and absorbent dressings; 2 weeks post-debridement, BTM was applied to the upper limbs and left lower leg in same-day surgery. Post-operative dressings included low-adherent mesh, nanocrystalline silver sheet and absorbent dressings with the frequency dependent on exudate.
One week after placement of BTM a pink flush was noted in the BTM on the arms (Figure 3). Over the next 4 months the patient experienced four admissions with a combination of recurrent CAP, acute kidney failure, anaemia of unknown origin and new tachy-bradycardia syndrome, and worsening cardiac failure thought to be related to immunosuppression and vasculitis.
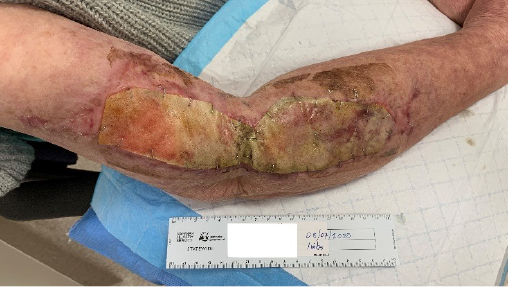
Figure 3. Right upper limb 1 week post-placement of BTM
The frequency of dressing changes (due to high exudate levels and some instability of the BTM on the lower limb) proved to be problematic at times, with the relatively high cost of nanocrystalline silver dressings in the community and increased frequency of dressings. A modified wound plan focussing on antimicrobial protection and wound hygiene was substituted with alternate solutions10 using either a topical antiseptic wash of aqueous chlorhexidine with cetrimide or polyhexamethylene biguanide daily as required to align clinic and community SoC.
Rolling and expressing exudate from the BTM was an essential element of managing the BTM during periods of delayed surgery due to acute illness. Guidance from the manufacturer reiterates the replacement of antimicrobial dressing once the period of therapeutic effect has expired23. BTM’s resistance to infection is well documented24 and during the long course of treatment and acute illness the patient had three courses of oral antibiotics to manage wound infection. Antibiotic stewardship was governed by the infectious diseases team and included intravenous piperacillin/tazobactam, azithromycin and oral medications of trimethoprim/sulphamethoxazole for CAP and amoxicillin/clavulanic acid for wound related infections. Pain was managed with oral paracetamol; the patient always declined stronger analgesia.
Frequent meticulous wound and skin care was required due to large volumes of exudate on the lower limb resulting in failure of the BTM to integrate, and was delaminated with minimal assistance in the clinic. At this time there was significant lower limb oedema related to a new cardiac event and a flare of vasculitis was complicated with a Staphylococcus aureus infection. Once the patient was physiologically stable, BTM was reapplied (Figure 4), with negative pressure wound therapy and compression therapy25 used to aid fluid management for several weeks postoperatively. During regular outpatient visits the patient was regularly assessed by the physiotherapist for mobility and was functioning independently. The upper arms were assessed for shoulder and elbow movement on a regular basis as well as wounds and scar progression.
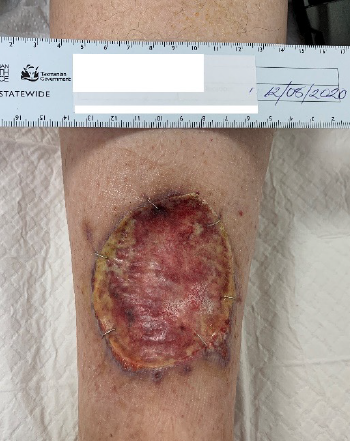
Figure 4. Left lower limb after re-application of BTM
During this period the integration of the BTM on the arms was stable despite periods of low physiological reserve and ongoing immunosuppression. Planned delamination of BTM on the upper limbs and SSG was deferred due to recurrent health issues and, 3 months after application, the arm wounds demonstrated signs of secondary healing and the BTM spontaneously began delamination (Figure 5). In the clinic the remaining membrane was easily and painlessly removed revealing granular tissue and contraction of the wounds. By 6 months the upper arms had complete epithelial cover.
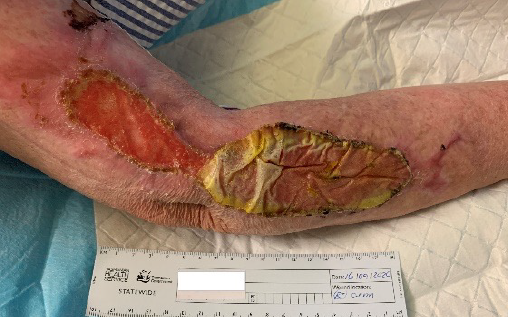
Figure 5. Right upper limb at 11 weeks with fully integrated BTM on the proximal wound and start of auto delamination of BTM
Goal-based wound plans were available following consultations on the Tasmanian Health Department digital medical record to provide a SoC, and regular sequential photographs documented the status of the wounds. The lower limb wound was delaminated (Figure 6) in the clinic and islands of granulation and epithelium were scattered over the wound surface. At the 28-week follow-up full epithelial cover was achieved (Figure 7). At 15 months (Figures 8 & 9) the wounds were contracting with full epithelial cover and scar tissue. The scars resembled hypertrophic scaring; however, remained thin and pliable and were well contoured with surrounding tissue. Full range of motion of both elbows was preserved and no itch or neuropathic pain was noted2.
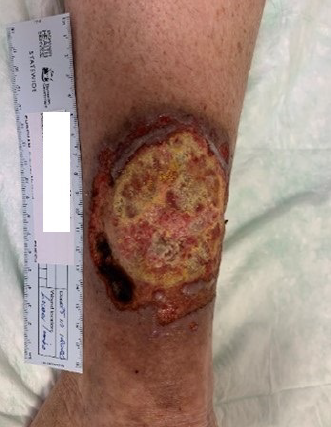
Figure 6. Left lower limb at 11 weeks showing auto delamination of BTM
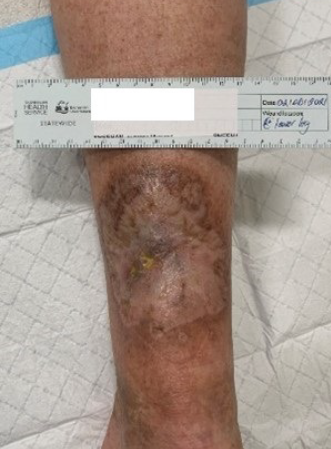
Figure 7. Left lower limb at 28-week follow-up with full epithelial cover
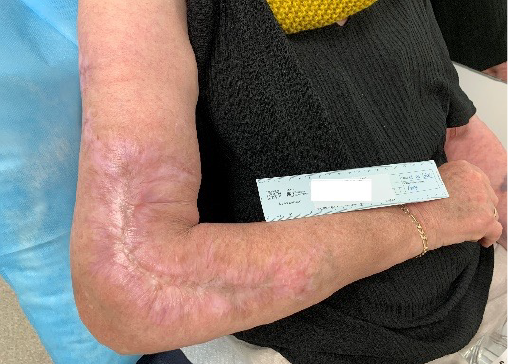
Figure 8. Right upper limb 1 year post BTM application
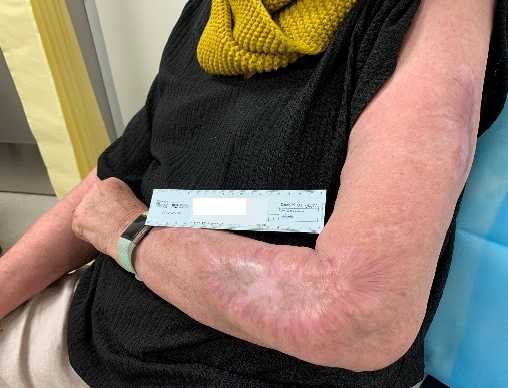
Figure 9. Left upper limb 1 year post BTM application
Summary
The application of BTM and surface epithelisation on a background of complex medical conditions and unexpected deviation from the usual plan still resulted in acceptable clinical outcomes for this patient. The application of the TIMERS wound assessment provides a disciplined, structured and systematic approach to developing wound care over a variety of care settings based on unique agreed plans and SoC.
The patient was supported by her partner and family through recurrent acute medical hospitalisations and deferred surgery. The multidisciplinary teams collaborated with the patient and her family to achieve a successful outcome through the long process of wound healing. At discharge from our clinic, wound care included scar management, skin protection and preservation, and advice to seek early medical care with any new vasculitic flare.
Addendum
At 20 months after initial presentation, the patient again presented with CAP and extensive cryoglobulinemic vasculitic lesions on all limbs. She was treated in the tertiary hospital and 1 week after discharge died from complications of non-wound related infection.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
The patient gave verbal consent for the case study and I confirmed consent to proceed with her partner after her death.
Funding
The authors received no funding for this study.
Author(s)
Clarissa A Young1*, Harriet K Semple2 and Gary M Kode
1Nurse Practitioner Wound Management, Launceston General Hospital, TAS, Australia
2Plastic and Reconstructive Surgery Registrar, Launceston General Hospital, TAS, Australia
3Plastic and Reconstructive Surgery, Launceston General Hospital, TAS, Australia
*Corresponding author Email Clarissa.young@ths.tas.gov.au
References
- Greenwood JE. The evolution of acute burn care: retiring the split skin graft. Ann R Coll Surg Engl 2017;99(6):432–8.
- Semple H, Young C, Kode G. Secondary healing over BTM in the unwell patient. ANZ J Surg 2022 (in press).
- Atkin L, Bućko Z, Montero EC. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care 2019;28(Suppl 3a):S1–50.
- Isoherranen K, O’Brien JJ, Barker J. Atypical wounds: best clinical practice and challenges. J Wound Care 2019;28(Suppl 6):S1–92.
- National Organization of Rare Disorders. Rare Disease Database – Vasculitis; 2008 [cited 2022 Apr 1]. Available from: https://rarediseases.org/rare-diseases/vasculitis/
- Davuluri S, Vaqar S. Cryoglobulinemic vasculitis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556045/
- Stanway S, Oakley A. Cutaneous vasculitis. DermNet NZ; 2016 [cited 2022 Feb 1]. Available from: https://dermnetnz.org/topics/cutaneous-vasculitis
- Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021 [cited 2022 Jan 20]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK531462/
- Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec 2012;4(4):84–91.
- Li H, Lim P, Stanley E, Lee G, Lin S, Neoh D, Liew J, Ng SK. Experience with NovoSorb® Biodegradable Temporizing Matrix in reconstruction of complex wounds. ANZ J Surg 2021;91(9):1744–50.
- Greenwood JE, Li A, Dearman BL, Moore TG. Evaluation of NovoSorb novel biodegradable polymer for the generation of a dermal matrix part 1: in-vitro studies. Wound Pract Res 2010;18(1):14–22.
- PolyNovo Biomaterials Pty Ltd. Clinical application guide for full thickness wounds MK-003; 2020. Available from: https://au.polynovo.com/
- Greenwood JE, Schmitt BJ, Wagstaff MJ. Experience with a synthetic bilayer Biodegradable Temporizing Matrix in significant burn injury. Burns Open 2018;1;2(1):17–34.
- Semple H, Young C, Kode G. Secondary healing over Biodegradable Temporizing Matrix in the unwell patient: a case presentation. ANZ J Surg 2021;91(Suppl 1):178.
- Flanagan M. Wound management. New York: Churchill Livingstone; 1997.
- Schultz GS, Sibbald RG, Falanga V. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28.
- Harries RL, Bosanquet DC, Harding KG. Wound bed preparation: TIME for an update. Int Wound J 2016;13(Suppl 3):8–14.
- Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years?. Int Wound J 2012;9(Suppl 2):1–9.
- Legal Dictionary; 2019 [cited 2022 Apr 2]. Available from: https://legaldictionary.net/standard-of-care/
- Wounds Australia. Standards for wound prevention and management. 3rd ed. Osborne Park: Cambridge Media; 2016.
- Moore Z, Dowsett C, Smith G. TIME CDST: an updated tool to address the current challenges in wound care. J Wound Care 2019;28(3):154–61.
- Dowsett C, Newton H. Wound bed preparation: TIME in practice. Wounds UK 2005;1(3):58–70.
- PolyNovo Biomaterials Pty Ltd. NovoSorb BTM Dressing Management Guide MK-021.
- Greenwood JE, Dearman BL. Comparison of a sealed, polymer foam biodegradable temporizing matrix against Integra® dermal regeneration template in a porcine wound model. J Burn Care Res 2012;33(1):163–73.
- Weller CD, Evans SM, Staples MP, Aldons P, McNeil JJ. Randomized clinical trial of three-layer tubular bandaging system for venous leg ulcers. Wound Rep Regen 2012;20(6):822–9.
- Baigrie D, Goyal A, Crane JS. Leukocytoclastic vasculitis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021 [cited 2022 Apr 5]. Available from: http://www.ncbi,nlm.gov/books/NBK482159



