Volume 30 Number 3
The use of gentian violet and methylene blue impregnated foam for treatment of chronic leg ulcers
Alexander Savage, Lisa J Ellis and Patricia Terrill
Keywords dressings, chronic leg wounds, antimicrobial
For referencing Savage A et al. The use of gentian violet and methylene blue impregnated foam for treatment of chronic leg ulcers. Wound Practice and Research 2022; 30(3):163-168
DOI
https://doi.org/10.33235/wpr.30.3.163-168
Submitted 23 February 2022
Accepted 27 April 2022
Abstract
Chronic leg ulcers are frustrating wounds to heal. Successful treatment relies on effective diagnosis and management of the underlying aetiology of the chronic leg ulcer as well as appropriate local wound treatment. Extensive knowledge of the range of dressing products is essential to be able to source the most appropriate dressing for your patient. We present here examples of our early positive experience with a newly introduced product to Australia and its use in difficult to heal wounds. The product is gentian violet and methylene blue (GVMB) impregnated into a polyvinyl alcohol foam (Hydrofera Blue CLASSIC®, AinsCorp Pty Ltd). It has many advantages including an antibacterial effect with negative pressure wicking of bacteria, exudate and debris, long wear time and pain free removal and it is cost effective. These case reports illustrate three cases where GVMB dressings have been used successfully in chronic leg ulcer management.
Introduction
Chronic leg and foot ulcers are a significant cause of morbidity in Australia with a point prevalence of 0.11% in one study1. In the elderly population, chronic leg ulceration is a common recurrent problem2. Numbers are likely to increase as population grows. Chronic leg ulcers provide a significant management dilemma for family physicians and specialists alike and place a strain on the Australian health system, costing an estimated A$3 billion per year, or 2% of Australian national healthcare expenditure3.
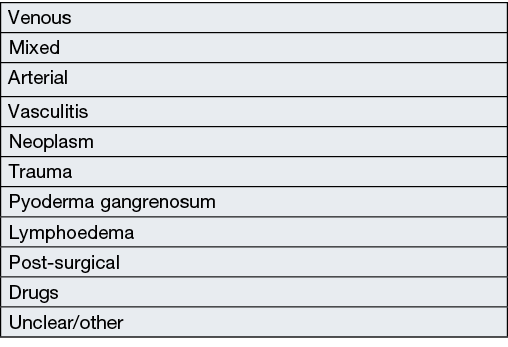
The common aetiologies of chronic leg ulcers are venous, arterial, mixed (both arterial and venous), trauma, vasulitis, and neoplasm, although other causes exist (Box 1)4,5. Effective treatment of chronic leg ulcers relies on management of comorbidities in combination with local wound treatment. Without appropriate diagnosis and treatment of the underlying aetiology, chronic leg ulcers will not heal with local treatment alone. Common comorbidities which compromise healing in leg ulcers include chronic venous insufficiency, diabetes mellitus, hypertension, obesity, dyslipidaemia and peripheral vascular disease4. These comorbidities lead to patterns of wound healing which may become stagnant or prone to regular break downs. Management of comorbidities can be poorly tolerated in some patients as they may be invasive (such as varicose or endovascular surgeries or surgical revascularisation of peripheral vascular disease), uncomfortable (such as with compression therapy), or difficult to manage in some patients (such as poorly controlled diabetes mellitus, weight loss or dyslipidaemia)6.
Box 1. Aetiology of chronic leg ulcers4,5
Local wound treatment has challenges too. An ideal wound dressing should reduce pain and discomfort, absorb wound exudate without drying out the wound, allow gaseous exchange, protect against physical, chemical and bacterial contamination, adapt to the prevalent wound healing phase, be easy and comfortable to apply and change, be economically viable and reduce bacterial load6.
The impact of microorganisms on chronic ulcers has been extensively studied. Wound infection is a continuum with stages ranging from contamination (where non-proliferating microbes are present within the wound but do not cause a host response), to colonisation (where microbes within the wound undergo limited proliferation without causing a host response), to local infection (where microbes are present in sufficient numbers or virulence to cause a local host response and impair healing), to spreading infection (where microbes invade the surrounding tissue such as muscle or fascia), and to systemic infection (where microbes from the wound affect the whole body, spreading via vascular or lymphatic channels)7,8. Topical antimicrobial dressings may be required in the management of chronic wounds when individuals are at increased risk of wound infection, when local infection is present, or for local treatment of spreading or systemic infection when combined with systemic antibiotics9,10.
A newly released product in Australia is Hydrofera Blue CLASSIC®. It is a combination of gentian violet and methylene blue (GVMB) impregnated into a polyvinyl alcohol foam (Box 2). Neither gentian violet (GV) nor methylene blue (MB) alone are new products, with GV discovered by French chemist Charles Lauth in 1861, and MB discovered by German chemist Heinrich Caro in 187611,12. GV has long been established as the basis of the Gram stain, with gram positive bacteria staining blue. It has had many roles as an antibacterial, antiseptic and anti-tumour agent, being used topically, intralesionally and intravenously11. It has good antimicrobial effect especially against gram positive bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), Candida and the parasite Trypanosoma cruzi (used in blood transfusions to prevent Chagas disease), with some but lesser effect against Gram-negative bacteria. MB has had many roles such as in the treatment of malaria, as an antidote to carbon monoxide and cyanide poisoning, and is used in sentinel lymph node biopsies, Alzheimer’s and bipolar disorder. These dyes are impregnated into a polyvinyl alcohol foam which, as suggested in a study by Heying in 2004, wicks bacteria laden exudate away from the wound via a negative pressure effect of somewhere between 17.8–71.2mmHg13. No more recent publications could be found regarding the negative pressure effect of GVMB dressing.
Box 2. Features of Hydrofera Blue CLASSIC®
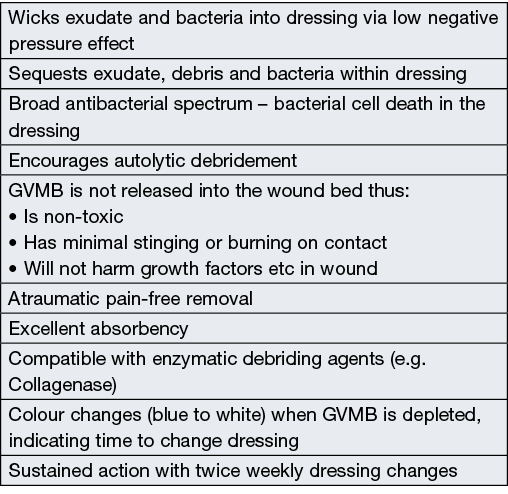
GVMB is not released into the wound bed but acts within the dressing. Bacteria are absorbed into the dressing and destroyed within 24 hours. The local antibacterial activity of GVMB is likely due to its ability to compromise the extracellular surface of bacterial membranes by altering their oxidative-reduction (redox) potential of oxidative metabolism, thus creating a hypoxic environment in the dressing, resulting in bacterial cell death14–18.
In a poster presented by Solano-Kiedaisch et al19 at the WUWHS Congress 2008, they showed that GV was more effective (lower minimal inhibitory concentration) than silver nitrate in inhibiting gram-positive bacteria (S. aureus and S. epidermidis), and Candida.albicans. Similar effectiveness was observed against Proteus mirabilis. GV was less effective than the silver nitrate against Gram-negative bacteria (E. coli and Klebsiella pneumoniae).
This study aimed to evaluate the effectiveness of GVMB dressings in the management of chronic leg ulcers which had failed to heal with alternative treatments. Three representative cases are reported. TGA approval was obtained for the use of GVMB in all patients.
Cases
Case A
Patient A was a 91-year-old male with bilateral below knee amputations for peripheral vascular disease. After using his left kneecap as a ‘bumper’ in his motorised wheelchair, he developed a chronic ulcer over the left patella with exposed bone. Following his presentation, Patient A underwent a flap repair of the wound. This flap repair failed, and the left patella was again exposed. The patient declined further surgery at that time and the ulcer was managed with a variety of standard moist wound healing dressings. 10 months following the initial surgery, there remained two wounds with exposed patella. He agreed to further minor surgery with a wound debridement and patella decortication and was managed with ultra-lightweight negative pressure wound therapy (PICO®) until the wounds granulated. Wound cultures were positive for Methicillin-sensitive S. aureus (MSSA).
Three weeks following debridement, the patient was started on GVMB dressing covered with a polyurethane film dressing. These dressings were changed weekly with saline hydration occurring twice per week (Figure 1). After 7 weeks with GVMB dressing, the wounds had healed. 1 week later there was secondary breakdown in the lateral area and the patient was recommenced on the GVMB dressing. Despite continuing to heal, the wound developed a secondary infection 9 weeks after the GVMB dressings were restarted. Cultures were positive for Serratia macerans and MSSA. The patient was prescribed antimicrobials and was started on topical Bactroban ointment. Three weeks later, GVMB dressings were recommenced and within 2 weeks the wound was completely healed.
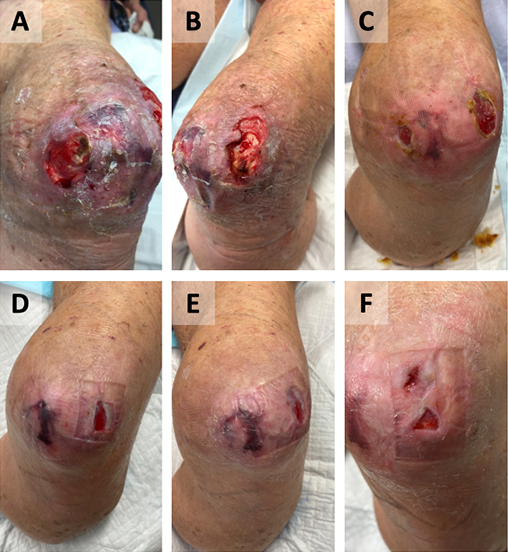
Figure 1. Case A
Wound appearance pre-debridement (A, B).
Wound post-debridement and PICO® (C).
Wound appearance post-commencement of GVMB dressings at 4 weeks (D), 6 weeks (E), and 3 months (F). The wound completely healed after 5 months.
Case B
Patient B was a 63-year-old male who presented with an ulcer on his left lateral malleolus after knocking his ankle in the shower. This was on a background of a compound ankle fracture in the 1970s which was treated with a tubed pedicle flap. Conservative management was attempted with Medihoney®, Flamazine®, Sorbact®, Bactroban ointment® and Iodosorb® with limited response after 15 months. The patient was commenced on GVMB dressings covered with low adherent dressing and a bandage. The dressing was hydrated with saline and reapplied twice weekly (Figure 2). Once again, the ulcer showed a steady reduction in size and was completely healed after 4 months.
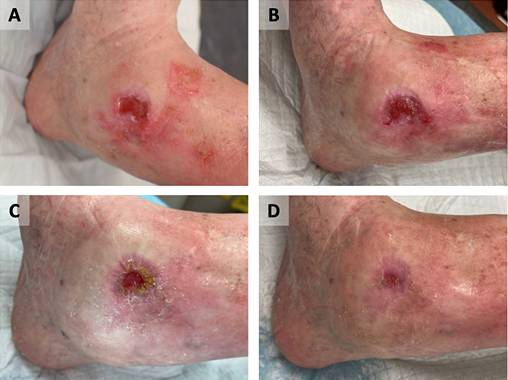
Figure 2. Case B
Wound appearance post-commencement of GVMB dressings at 3 weeks (A), 5 weeks (B), 9 weeks (C), and 11 weeks (D).
The wound completely healed after 4 months.
Case C
Patient C was a 38-year-old morbidly obese male with chronic bilateral leg wounds on a background of osteomalacia and multiple bilateral lower limb stress fractures as a complication of high dose steroids for asthma. He had severe bilateral peripheral oedema which, in combination with excoriation from eczema, saw him develop ulcers on his left lateral malleolus and right pretibial area. Cultures initially grew Stenotrophomonas maltophilia and MRSA. Ten months post-development of the ulcers he was taken to theatre for surgical debridement after which he was maintained as an inpatient for over a month. Despite trialling negative pressure wound therapy, silver dressings, Urgoclean® and Sorbact®, the ulcers continued to build up large amounts of slough with ongoing redness around the wounds. He was commenced on GVMB dressings 2 months after the debridement. The GVMB dressing was covered with absorbent pads and compression bandaging and was hydrated with saline and reapplied twice weekly. The wounds improved dramatically, with complete healing of the left lateral malleolus ulcer and nearly complete healing over the right pretibial area after 4 months (Figure 3 & 4). During the COVID‑19 crisis the patient elected not to attend the clinic for follow-up or allow district nursing visits and the wound over the right pretibial area worsened. Once lockdown restrictions were lifted he progressed again with adequate nursing care and GVMB dressings under compression.
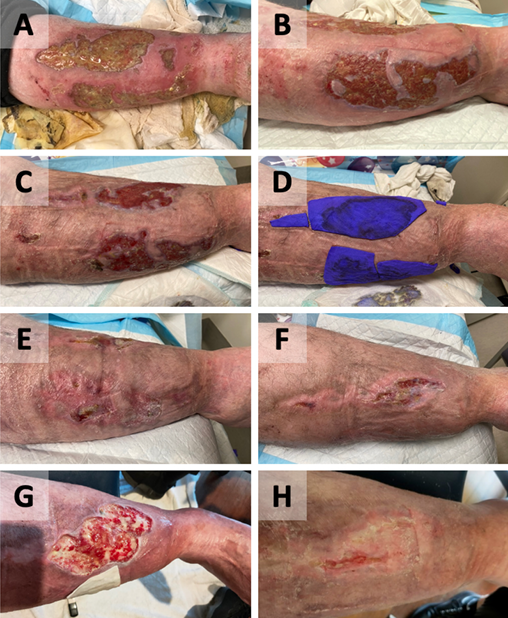
Figure 3. Case C – right pretibial area
Wound appearance post-commencement of GVMB dressings as 1 week (A), 1 month (B), 2 months (C), 2 months with dressing in situ (D), 3 months (E), and 4 months (F).
Recurrence with COVID‑19 isolation (G). Wound nearly completely healed 5 months post-recommencing adequate GVMB dressings under compression (H).
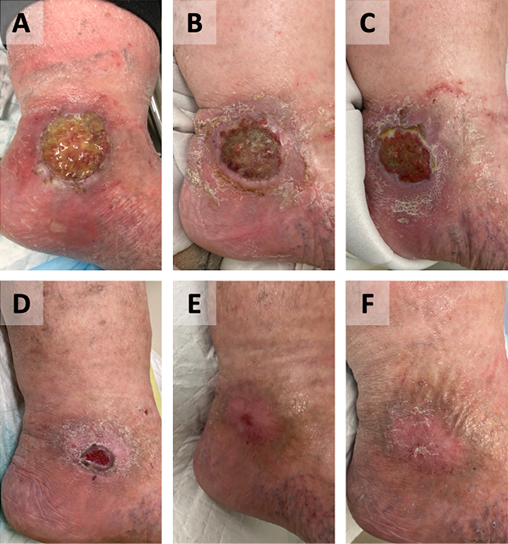
Figure 4. Case C – left lateral malleolus
Wound appearance pre-GVMB dressings (A). Wound appearance post-commencement of GVMB dressings at 1 week (B), 1 month (C), 2 months (D), 3 months (E) and 4 months (F).
Discussion
These case reports illustrate the successful use of GVMB dressings in chronic leg ulcer management. We have shown that GVMB dressings may be used as an alternative management strategy in chronic, non-healing ulcers. This was due to patients achieving complete or near complete healing with GVMB dressings despite numerous failed attempts with other dressings.
Many small case reports and posters have been presented on the use of GVMB dressings for acute and chronic wounds18,20–25, all showing beneficial effectiveness. However, few series have been reported and most have small case numbers with only one prospective randomised controlled trial (Table 1). Coutts et al26 reported 15 chronic foot/leg ulcers with bacterial load with 57% wounds improving over the 4 weeks of the observation window. Lullove27 reported its use in 53 patients over ovine-based Collagen Extracellular Matrix (CECM). He attributed the ulcer healing to the CECM and stated that other foam secondary dressings were suitable and so results in this study can not be attributed to the GVMB dressing per se. A study conducted by Woo et al28 which evaluated GVMB dressings for management of chronic wounds with local infection supported its antibacterial properties in the clinical setting. In this study, all 29 participants had decreased wound size, reduction in devitalised tissue and reduced infection after using GVMB dressings were applied for 4 weeks despite initial local wound infection.
Table 1. Review of other studies
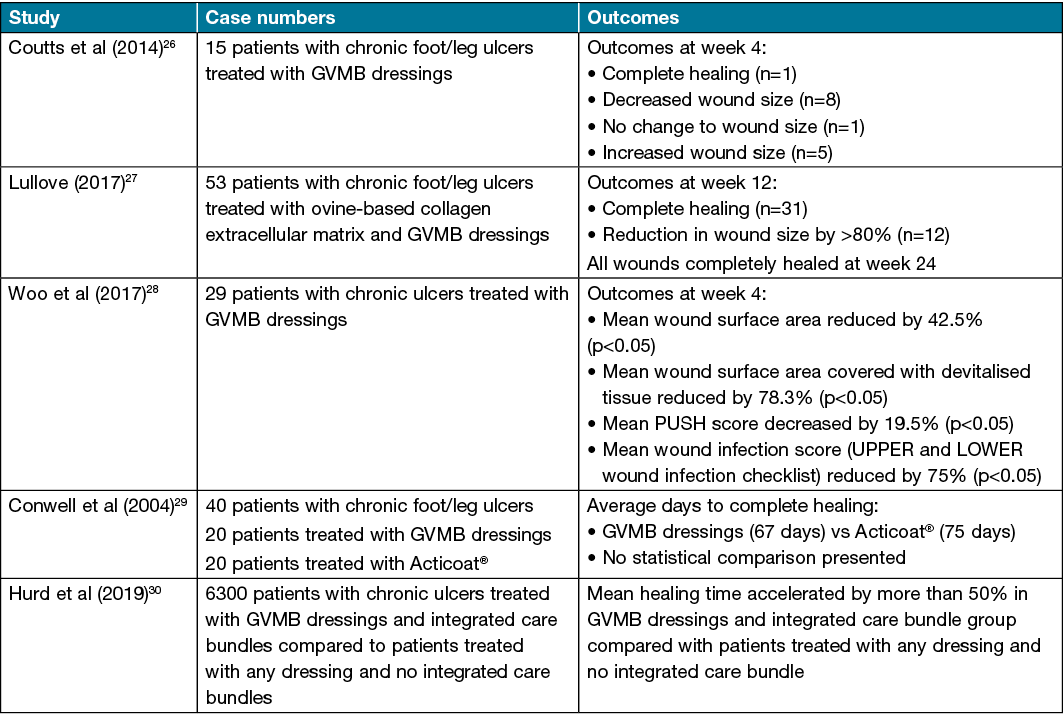
Conwell et al29 presented a poster reporting a prospective randomised controlled trial of 40 patients with lower leg wounds comparing GVMB dressing with Acticoat®. No statistics were presented but the GVMB dressing outperformed the Acticoat® in terms of reducing wound pain, flattening of wound edges, less maceration and more rapid healing. They showed considerable cost savings with the GVMB dressing over the silver containing dressing.
A Canadian study by Hurd30 demonstrated a significantly improved wound healing outcome for a population of 6300 patients who were managed with GVMB dressings and integrated care bundles when compared to patients who were not on integrated care bundles with any dressing product. Overall, there was a reduction in healing time for the GVMB dressings and integrated care bundles compared to the control group by over 50% and significantly fewer nursing attendances/dressing changes were required. How much can be attributed to the superior management of the patient and how much to the dressing product was not evident from this paper.
The limitations of this study and many of those reported are those that inherently affect any case reports and series. Interpretation of results may be limited by the lack of a control group, low case numbers, heterogeneity of participants and the single institution setting. Outcomes reported frequently reflect the senior author’s experiences. These limitations may affect the generalisability of these results. Further research is required in the form of multicentred randomised controlled trials in which GVMB dressings are compared with other dressings after a period of incomplete wound healing.
The secondary dressing over the GVMB dressing can be altered depending on the exudate level of the wound. Before applying, Hydrofera Blue CLASSIC® is hydrated with water or saline. If the ulcer has low exudate levels, it can be covered with a film dressing. If there are moderate to high exudate levels, a non-adherent dressing or absorbent pad may be used as a secondary dressing to absorb excess exudate. It may be used under compression. It is therefore very versatile in that it can be used for any wound exudate level and adjusted as exudate levels change by utilising alternative secondary dressings. It actively absorbs exudate, thereby reducing wound maceration and removing harmful bacteria that may lead to overt wound infection. It is recommended that the dressing be changed twice weekly, though we have left it on for up to 7 days in some cases (e.g., Case A). The dressing will signal when it needs changing as the blue colour changes to white as the supply of GVMB is exhausted.
There are numerous existing antimicrobial dressings; however, general superiority of one particular wound dressing over another has yet to be demonstrated10,31. One product will often work brilliantly in one patient’s wound but not in another. Ultimately, appropriate dressing choice comes down to cost, frequency of dressing change, who is doing the dressing, wound bed and exudate level, and then often trialling a dressing to see if it works! If there is no improvement in the wound after 2 weeks a change to an alternative product should be considered. GVMB dressing is certainly a useful antibacterial dressing product that you may consider adding to your selection process.
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethics statement
An ethics statement is not applicable. Fully informed patient consent was obtained.
Funding
Dressings were obtained under the TGA special access scheme. Dressings were donated by AinsCorp Pty Ltd to the patients, but this research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contribution
AS: Manuscript write-up and collection of data. LE: Manuscript write-up and collection of data. PT: Study supervisor.
Author(s)
Alexander Savage1*, Lisa J Ellis1,2 and Patricia Terrill2,3
1Peninsula Clinical School, Central Clinical School at Monash University, The Alfred Centre, 99 Commercial Road, Melbourne, VIC 3004, Australia
2Department of Plastic, Reconstructive and Hand Surgery, Peninsula Health, 2 Hastings Road, Frankston, VIC 3199, Australia
3Senior Lecturer, Department of Surgery, Monash University, Clayton, VIC 3168, Australia
*Corresponding author Email alexsavage12@outlook.com
References
- Baker SR, Stacey MC. Epidemiology of chronic leg ulcers in Australia. ANZ J Surg 1994;64(4):225–292.
- Nelzen O, Bergqvist D, Lindhagen A. Chronic leg ulcers: an underestimated problem in primary health care among elderly patients. J Epi Comm Health 1991;45:184–187.
- Graves N, Zheng H. The prevalence and incidence of chronic wounds: a literature review. Wound Pract Res 2014;22(1):4–12.
- Jockenhöfer F, Gollnick H, Herberger K, et al. Aetiology, comorbidities and cofactors of chronic leg ulcers: retrospective evaluation of 1000 patients from 10 specialised dermatological wound care centers in Germany. Int Wound J 2016;13(5):821–828.
- Körber A, Klode J, Al-Benna S, et al. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Dtsch Dermatol Ges 2011;9(2):116–121.
- Kahle B, Hermanns H-J, Gallenkemper G. Evidence-based treatment of chronic leg ulcers. Dtsch Arztebl Int 2011;108(14):231–237.
- Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012;9(Suppl 2):1–19.
- Wound Infection in Clinical Practice. Int Wound J 2008;5(s3):iii–11.
- Vowden P, Vowden K, Carvorsi J. Antimicrobial dressings made easy. Wounds Int 2011;2(1).
- Woo KY. The use of antimicrobial dressings in chronic wounds: NERDS and STONEES principles. Surg Technol Int 2010;20:73–82.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol 2013;22(12):775–780.
- Wiklund L, Sharma A, Patnaik R, et al. Chapter 7 – upregulation of hemeoxygenase enzymes HO-1 and HO-2 following ischemia-reperfusion injury in connection with experimental cardiac arrest and cardiopulmonary resuscitation: neuroprotective effects of methylene blue. In: Sharma HS, Sharma A, eds. Progress in brain research. Vol 265. Elsevier; 2021. pp. 317–375.
- Heying TL. Comments on the negative pressure created by the capillary flow properties of Hydrofera Blue™ wound dressings; 2004. Paper on file Hanstronics Medical Equipment Pty Ltd
- Hoffmann CE, Rahn O. The bactericidal and bacteriostatic action of crystal violet. J Bacteriol 1944;47(2):177–186.
- Ingraham MA. The bacteriostatic action of gentian violet and dependence on the oxidation-reduction potential. J Bacteriol 1933;26(6):573–598.
- Dubos R. The relation of the bacteriostatic action of certain dyes to oxidation-reduction processes. J Exp Med 1929;49(4):575–592.
- Premarked Notification 510(K) Hydrofera LLC. In: United States Food and Drug Administration; 2014.
- Boyar V. Healing with methylene blue/gentian violet. Wound Manage Prev 2019;65(9).
- Solano-Kiedaisch S, Jelf C, Ramsay S, Aust D, Roche E. Activity against wound pathogens and absorbency for silver dressings and a PVA sponge containing organic pigments. Paper presented at WUWHS Congress 2008.
- Alvarez J. The versatility of PVA foam dressing impregnated with methylene blue and gentian violet on acute and chronic wounds. Poster on File Hanstronics Medical Equipment Pty Ltd.
- Hill R. Optimizing the wound bed by removing devitalized tissue and using methylene blue and gentian violet (MBGV) antibacterial foam dressings: a case series. Presented at WOCN Society’s 49th Annual Conference 2017.
- Furtado S. A unique approach to heal a neonate’s open abdomen using gentian violet/ methylene blue PVA anti-bacterial foam. 2018. Presented at 2021 Wound Con Summer
- Miller MS. Use of a novel methylene blue/gentian violet open celled sponge with compression to treat venous insufficiency ulcers. 2006. Poster on File Hanstronics Medical Equipment Pty Ltd.
- Webb M. Using of a bacteriostatic PVA foam to inhibit growth of MRSA and VRE in deep soft tissue infected wounds in one long term care facility. 2011. Poster on File Hanstronics Medical Equipment Pty Ltd.
- Boyar V. Successful management of complex pediatric and neonatal wounds with methylene blue and gentian violet foam dressings. Wounds 2021;33(10):253–259.
- Coutts PM, Ryan J, Sibbald RG. Case series of lower-extremity chronic wounds managed with an antibacterial foam dressing bound with gentian violet and methylene blue. Adv Skin Wound Care 2014;27(3 Suppl 1):9–13.
- Lullove EJ. Use of ovine-based collagen extracellular matrix and gentian violet/methylene blue antibacterial foam dressings to help improve clinical outcomes in lower extremity wounds: a retrospective cohort study. Wounds 2017;29(4):107–114.
- Woo KY, Heil J. A prospective evaluation of methylene blue and gentian violet dressing for management of chronic wounds with local infection. Int Wound J 2017;14(6):1029–1035.
- Conwell P, Mikulski L, Tramontozzi M. A comparison of two antimicrobial dressings: a randomized prospective trial comparing PVA foam with two organic pigments to a silver based wound dressing. Symposium on Advanced Wound Care; 2004. Presented at 2021 Wound Con Spring.
- Hurd T. Improving the quality of chronic wound care using an advanced wound management program and gentian violet/methylene blue-impregnated antibacterial (GV/MB) dressings: a retrospective study. Surg Technol Int 2019;35:58–66.
- Nelson EA, Bradley MD. Dressings and topical agents for arterial leg ulcers. Cochrane Database System Rev 2007(2):CD001836.



