Volume 31 Number 1
The influence of technique and type of sonotrode on debridement and patient experience using a low frequency ultrasound contact debridement device; a case series
Terry Swanson, Catherine Hirst, Scott Salzman, Nicoletta Frescos
Keywords debridement, wound bed preparation, Pain, low frequency ultrasound contact debridement, ultrasonic wound assisted debridement
For referencing Swanson T et al. The influence of technique and type of sonotrode on debridement and patient experience using a low frequency ultrasound contact debridement device; a case series. Wound Practice and Research 2023; 31(1):19-27.
DOI
https://doi.org/10.33235/wpr.31.1.19-27
Submitted 24 July 2022
Accepted 9 September 2022
Abstract
Wound bed preparation is essential for treatment of chronic and hard to heal wounds and clinicians have several options depending on skill and availability of equipment. There is growing evidence of benefit and usage of low frequency ultrasound contact debridement (LFUCD) across the globe but few studies that explore what the best sonotrode, technique using that sonotrode, intensity of ultrasound, or level of flow of irrigation have on the outcome of treatment or the patient experience. This observational case series study of 114 treatments of a convivence sample of 45 patients explored the use of three different sonotrodes / handpieces and five different techniques – mainly 100% ultrasound intensity and irrigation flow greater than 40% – to determine any variation in outcome or patient experience. The minimum data set (MDS) captured information regarding percent of tissue type and size of the wound before and after treatment, level of pain before, during and after treatment, technique(s), analgesia, length of treatment, aetiology and demographics. The results indicate LFUCD assisted in removing non-viable tissue and fibrin within minutes, while sparing granulation tissue, and was well tolerated regarding pain. This study did not find any significant difference in the type of techniques, but the type of sonotrode did.
Introduction
Wound bed preparation is a recognised paradigm and accepted essential practice for chronic and hard to heal wounds1–3. Wound debridement plays an important role in wound bed preparation; it consists of the removal of unhealthy or non-viable tissue and cellular debris to promote healing4.
Different methods of debridement are utilised depending on patient risk factors, clinician skill, and access to equipment and technologies. Common debridement techniques include autolytic, chemical, mechanical, biological, sharp, surgical, hydrosurgical and low frequency ultrasound debridement1,2,5,6.
A recent systematic review and meta-analysis regarding the use of low frequency ultrasound contact debridement (LFUCD) for diabetic foot ulcers showed higher healing rates and greater percentage of wound reduction, although non-statistical significance was observed7. Several authors have suggested that LFUCD is a safe and effective alternative when surgical debridement is contraindicated or not available7–9. However, there is limited and conflicting evidence in regard to wound bed preparation or debridement. A systematic review in 20136 suggested that although ultrasound-assisted debridement may promote healing there was little evidence for debridement, yet other studies found that LFUCD was effective in removing non-viable tissue without damaging healthy tissue7,10. Removal of fibrin was also seen as a positive in this study11. Further evidence suggests that ultrasound debridement appears most effective when used three times a week, and it has the potential to decrease exudate, slough and patient pain, disperse biofilm, and increase healing of various wound aetiologies8. There are also differences between non-contact and contact low frequency ultrasound in regard to debridement activity7,10,11. Madhok et al suggested that low frequency non-contact ultrasound was not as effective in debridement due to a lack of the cavitation effect, but did share benefits of increased protein synthesis6, promotion of growth factors11, antibacterial effects12,13, and enhance fibrinolysis14,15.
LFUCD for wound debridement has been used in Australia for over a decade; it has the major advantage of being an effective but relatively painless debridement method16. It provides a mechanical debridement alternative when surgical debridement is not an option16,17. The therapeutic effects of LFUCD include cavitation for debridement and rapid lysis of fibrin on the wound surface, bactericidal activity and upregulation of cellular activity10,11,16–19. The application of LFUCD has shown great potential, with sufficient evidence to indicate a safe and effective method when used for wound bed preparation9,20–23.
The LFUCD device consists of a generator, a sonotrode (handpiece) and an irrigation set (Figure 1). The ultrasound is delivered through the oscillating sonotrode tip which creates micro-gas bubbles in the fluid (cavitation) and fluid motion (acoustic streaming). When the bubbles collapse, emulsification of dead and non-viable tissues occurs, biofilm is disrupted, and cell membranes in healthy tissues are stimulated24.
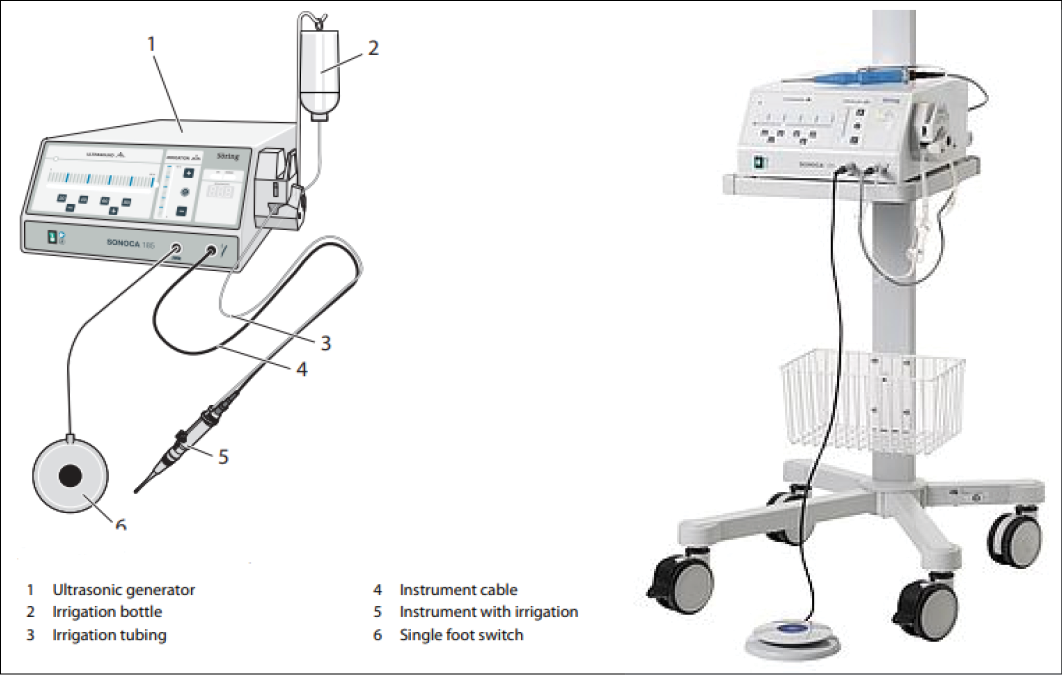
Figure 1. SONOCA 185 generator device (Söring)
https://www.soering.de/en/products/soering-ultrasonic-generators/sonoca-185/
There are three different sonotrode types that can be used to debride the wound (Figure 2); each has specific capabilities in removing debris, fibrin depositions or slough based on the shape of the sonotrode tip edge and debridement technique used. The double ball tip is used for cavity wounds, the hoof is used for undulated flat surfaced wounds with sloped edges, and the spatula is used for large, flat surfaced wounds.
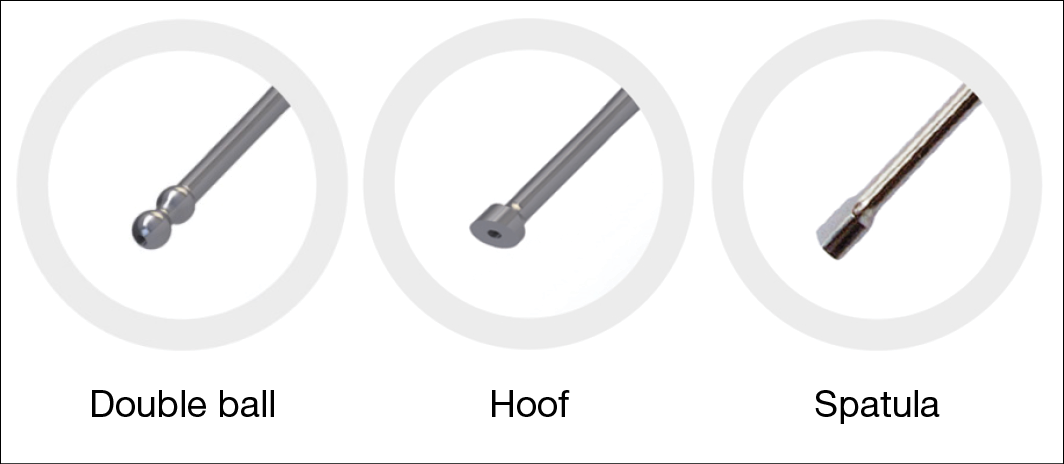
Figure 2. The three sonotrode types (tips)
The type of techniques using the sonotrode are: undermining; slicing; sliding; sliding with rotation and milling; and a non-contact option of moistening (Figure 3). The moistening technique (E) is a non-contact option for treating patients that have a highly sensitive wound to touch or testing sensitivity to the LFUCD; this is then titrated up to the other options or stays with moistening. The most aggressive techniques are slicing (B) or sliding with rotation and milling (E) as the sonotrode is used to contact the wound, and uses the technique and instrument to mechanically remove and lift unwanted tissue. Undermining (A) occurs using the double ball sonotrode for cavities.
The type of sonotrode and technique used, the level of flow of solution, and the intensity of the ultrasound amplitude all depend on the wound size, shape and wound bed contours and patient tolerance/preference.
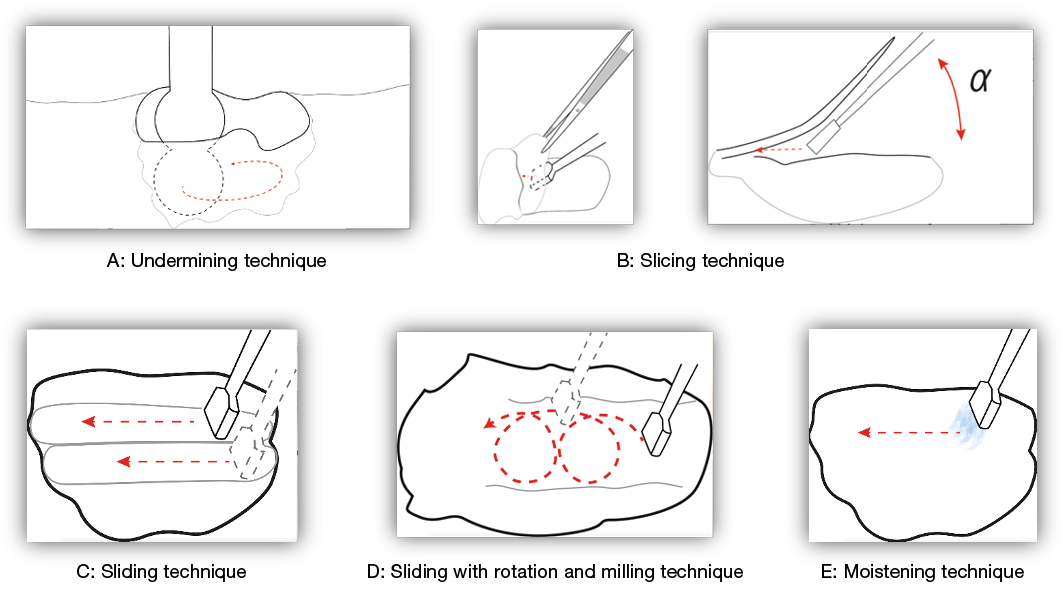
Figure 3. The debridement techniques using the different sonotrode types
Study rationale
To date there are no studies that have explored whether there are differences in the efficacy for wound debridement between the sonotrode types or if they influence the pain perspective of the patient, nor how the sonotrodes are used (type of technique) in the clinical environment. The user can also influence how the treatment is completed by titrating the flow of the solution, but again no research has explored this aspect of LFUCD. These variables in practice were therefore the impetus to initiate this study as information regarding technique and sonotrode type and how it influenced efficacy and the patient experience was unknown or limited.
The aim of this study was to evaluate the effectiveness of LFUCD for debridement and to explore if using three different types of sonotrodes or different techniques influenced the wound debridement outcome or the patient’s experience of pain.
Methods
An observational case series study was conducted in an outpatient wound care clinic in a community health centre attached to an acute hospital in regional Australia between August 2018 and June 2019. The study used convenience sampling to recruit participants who were either currently being treated in the wound clinic or being admitted into the service.
Patients were eligible if they were aged 18 years or over and had a wound that required single or sequential debridement for wound bed preparation. Patients were excluded if healing was not anticipated or if they had a medical condition that could contraindicate ultrasound treatment or had uncontrolled medical condition (e.g., diabetes, hypertension). Patients were also excluded if they were pregnant or breastfeeding, had a malignant wound, or self-reported AIDS or HIV.
Ethical approval for the study was obtained from the relevant institutional ethics committee HREC14-2017 and all participants gave written informed consent.
A nurse practitioner (NP) wound management with 30 years’ experience and a clinical nurse consultant (CNC) wound management with 10 years in the field with 8 years’ experience in use of the LFUCD device, who both worked in same wound clinic, conducted the treatment procedures and data collection. For consistency, additional training was provided on the assessment of the wound bed to determine the type of wound tissue and percentage of necrotic, slough, fibrin, granulation or epithelial tissue. The minimum data set (MDS)/case report form (CRF) was pre-trialled by the two healthcare professionals to determine agreement on identification of tissue types and percentages of wound tissue, ease of use, and if any further information should be included. Minor modifications were made at that time.
LFUCD sonotrode type, debridement technique and setting of device
In this study, the NP and CNC conducted the wound debridement using the SONOCA 185 generator device, (Söring GmbH, Germany). The selection of a specific sonotrode type and the technique used to debride followed the manufacturer’s recommendations25 and was based on initial clinical evaluation of the wound including wound depth, type of tissue and wound edges. The double ball tip was used for cavity wounds, the hoof was used for undulated flat surfaced wounds with sloped edges, and the spatula was used for large, flat surfaced wounds. The different techniques used were either undermining, slicing, sliding, sliding with rotation and milling, or moistening25.
Device settings such as ultrasound intensity (amplitude) and rate of saline flow (irrigation) were manually set. The practitioner selected the intensity in accordance with patients’ tolerance and acceptance and per clinical judgement. The baseline settings were intensity at 100% and flow greater than 40%.
Data was collected on the device setting which included the percentage of the intensity of the LFUCD and the irrigation flow of saline fluid, the type of sonotrode and debridement technique used, and the duration of debridement for each debridement session.
Wound characteristics
Participants’ wound characteristics were assessed before and after each wound debridement. Assessment of the wound characteristics included wound size measurements, wound bed tissue type, periwound appearance, exudate levels, presence of oedema and signs of infection.
To determine the overall estimated volume of the wound area, length (cm), width (cm) and depth (cm) were recorded and used to calculate wound volume in cm3. The appearance of the wound bed was described indicating the percentage of different types of tissue observed such as necrotic tissue, slough, fibrin and granulation. Necrotic was described for the purposes of this study as black or brown tissue, slough was described as yellow or cream coloured soft tissue, fibrinous tissue was described as filmy yellow, white or grey substance found on the surface of a wound, granulation tissue was described as red (varying in depth of colour) tissue, and epithelial tissue was described as pink healed tissue. We required differentiation of the tissue types so that classification would be consistent26–32.
Patient pain
Pain intensity was measured throughout consultation using the validated numerical rating scale (NRS)33,34. This involved asking the patient to identify the level of wound-related pain where 0 represents no pain and 10 represents excruciating pain. Pain levels were assessed at three key points: before commencing debridement; during the procedure; and after debridement. Patients were encouraged to self-medicate with oral over-the-counter analgesia such as paracetamol prior to any appointment and, if instructed, they were to apply a topical anaesthetic gel before travelling to the clinic for the LFUCD. If topical anaesthetic gel was required, a prescription and written instructions to self-apply was provided. If not applied prior to treatment, a topical anaesthetic gel was applied prior to debridement unless the patient had loss of protective sensation or had a previous treatment that was pain free.
Digital images
Wounds were photographed before and after each debridement procedure. The standard procedure for digital image of the wound was conducted and recorded as per the existing standard of the wound clinic procedure. The digital images were used to demonstrate the visual wound assessment information and provide confirmation between the photo and the recorded wound characteristics data.
Statistical analysis
Microsoft Excel™ and SPSS Ver 21.0 (SPSS Inc, Chicago, Ill., USA) was used for subsequent data management and analysis.
To test for differences between two groups, the t-test was used. For all paired sets of observations for each patient (before debridement and after debridement) the pre-debridement measure was deducted from the post-debridement measure to obtain an overall estimate of the magnitude of change in the wound. This new variable was then tested against zero. That is, the mean of this new measure was hypothesised to be zero, in that no change overall had occurred.
When assessing for differences between more than two groups, initially an ANOVA was conducted, followed up by a series of post hoc t-tests, with possible inherent family-wise error adjusted for using the Bonferroni correction. For all hypotheses tests conducted, alpha was initially set to 0.05. Where applicable, a one-tailed hypothesis test was conducted. Confidence interval estimates (95%) for the mean or the median were used.
Non-parametric statistical modelling analogues such as the Mann-Whitney U or Sign Rank test in lieu of the t-test, and the Kruskal-Wallis or Welsh’s test in lieu of the one-way ANOVA were used for those cases where relevant modelling assumptions could not be met.
Where able, ordinary least squares regression was used, with subsequent hypotheses tests to determine if contributions of independent variables were significantly non-zero.
Ten independent measures were hypothesised to be possible predictors of wound changes as a result of LFUCD. The measures were – presence of fibrin, granulation tissue, necrotic tissue or slough, reported pain, wound duration, wound dimensions (depth, length and width), and participants’ age.
Results
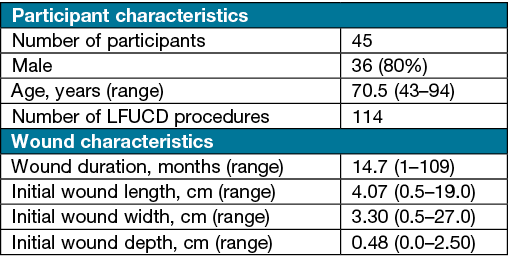
A total of 45 participants were recruited and consented to the treatment. The overall average age was 70.5 years (SD 12.1), the mean age for females was 69.9 years and for males was 70.8 years (Table 1).
In total, 114 debridement procedure data sets were analysed, with results showing various wound types, of which 73% were conducted on males. Treated wounds included venous leg ulcerations, pressure injuries, mixed arterial venous arterial ulcers, diabetic foot ulcers and other wound types (e.g., traumatic surgical wounds with delayed healing).
Table 1. Participant and wound characteristics
Debridement and wound characteristics
Averages of the differences in wound characteristics before and after debridement are presented in Table 2. Wound dimensions did not change significantly when observing the specific singular measurements of the wound pre- and post-debridement. The difference between width, length and depth were not statistically different; however, the volume of the wound showed significant differences before and after debridement t(114)=2.92 p<0.01. The increase in volume is assumed to be associated with the removal of non-viable wound tissue.
Table 2. Summary of average differences in wound characteristics before and after debridement
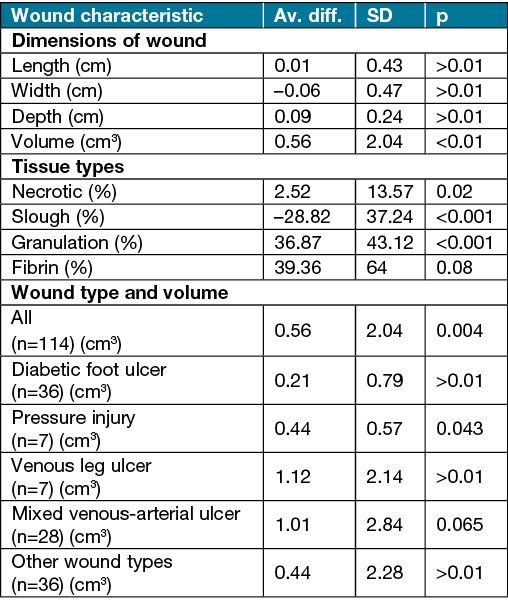
The results show that LFUCD was effective in removing non-viable tissue and increasing healthy granulation tissue. Differences in presence of tissue type before and after debridement show on average that there was a decrease in slough by 28.8%, necrotic tissue by 2.5%, fibrin by 6.5% and in increase in granulation tissue by 36.9%. In those wounds not presenting with necrotic tissue and slough before treatment, 80% reduction in fibrin after debridement was observed.
A multi-regression model was used to determine if any combination of wound variables (wound dimensions, tissue types, age of participants, duration of wound) would predict changes in wound tissue types post-debridement. The model showed a 56.3% variation in the change in fibrin deposition that occurs from the wound debridement procedure can be determined by determining the three measures of pre-debridement of necrotic, slough and granulation tissue. It was found that an increase in the presence of granulation, slough and necrotic tissue was likely to yield a decrease in fibrin after debridement. The overall change in the percentage of fibrin post-treatment appears to alter as a function of the presence of these three tissue types.
Debridement between sonotrode types, debridement technique and duration
Different types of sonotrodes were used for debriding different types of wounds, (spatula n=45; hoof n=47; double ball n=22). The double ball device was used for only 19% of treatments, whereas the spatula and hoof devices were used on 39% and 41% of participants respectively. Summaries of the various wound types and the different sonotrode types used are presented in Figure 4.
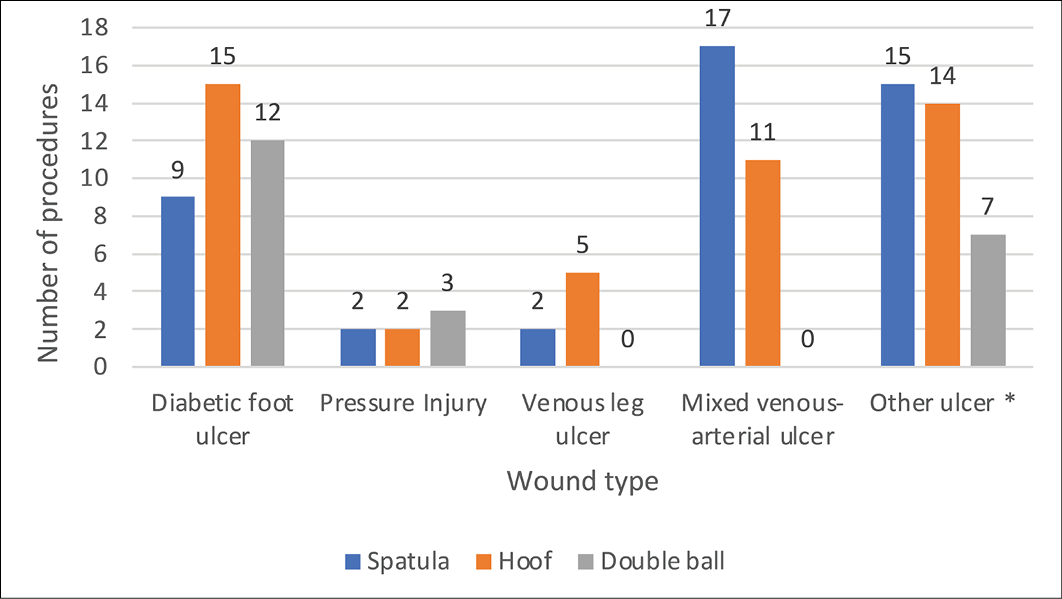
Figure 4. Sonotrode types used to debride different types of wounds
Differences in wound size (cm3) before and after debridement showed use of the spatula sonotrode type removed, on average, a significantly larger non-zero amount of unviable tissue, t(44)=1.961, p=0.028, compared to the hoof type of sonotrode (0.52cm3), and the double ball (0.31cm3). For all the three LFUCD sonotrode types used, 50% or less irrigation fluid was used, which did not alter debridement results.
The length and width of the wound showed significant differences between the type of sonotrode used, but there were no differences for depth. It appears that the double ball, the hoof, and the spatula debridement devices are used on wounds of increasing length respectively. Wound lengths, on average, were 1.5cm with the double ball, 3.1cm with the hoof and 4.2cm with the spatula (p=0.0096). Similarly, a significant difference was found between sonotrode types for use in width of wounds, p=0.000016. It appears that the double ball, the hoof, and the spatula debridement devices are used on wounds of increasing width (cm) respectively. Wound widths, on average, were 1.0cm (double ball), 2.0cm (hoof) and 3.0cm (spatula).
Overall, the duration of the debridement procedure was, on average, 5.28 minutes with a range from 1–17 minutes. In terms of duration of use between the three types, the mean length of time for the spatula was 6.22 minutes (SD 3.5), the hoof 5.19 minutes (SD 2.86) and the double ball 3.64 minutes (SD 2.06). The spatula was used for a significantly longer amount of time when compared to the double ball (Mann-Whitney U 234.5, p value 0.0005). A point estimate of this possible (median) difference was approximately 3 minutes longer.
The mean duration of debridement procedure in the wounds of female participants was 4.48 minutes, and 5.63 minutes in male participants. It appears that neither wound duration nor size of a wound related to the length of time spent debriding it. In all debridement procedures, maximum ultrasound intensity of 100% was used. It was found that, for all three sonotrodes used, irrigation ‘flow’ (40% or above) did not alter the size of the wound post-debridement.
Patient pain
The effects on participants’ experience of debridement in terms of reporting pain levels demonstrated that LFUCD increased wound related pain during the procedure. Using the NRS, pain reported during the debridement procedure was significantly higher, on average, than the pain levels reported either before, or after the procedure (Figure 5). On average (median), pain levels reported during the procedure were two pain unit levels higher than was reported either before (Mann-Whitney U 3232, z 6.486, p<0.0001), or after (Mann-Whitney U 3846, z 5.084, p<0.0001) the debridement procedure. Further, no significant differences were evident between the pain levels reported before and after the debridement procedure. That is, participants completed the debridement procedure with pain levels that were, on average, the same as those pain levels that they reported prior to the procedure beginning. Overall, females reported significantly higher pain compared to males before (p=0.0156), during (p=0.0005) and after (p=0.002) treatment (Figure 6).
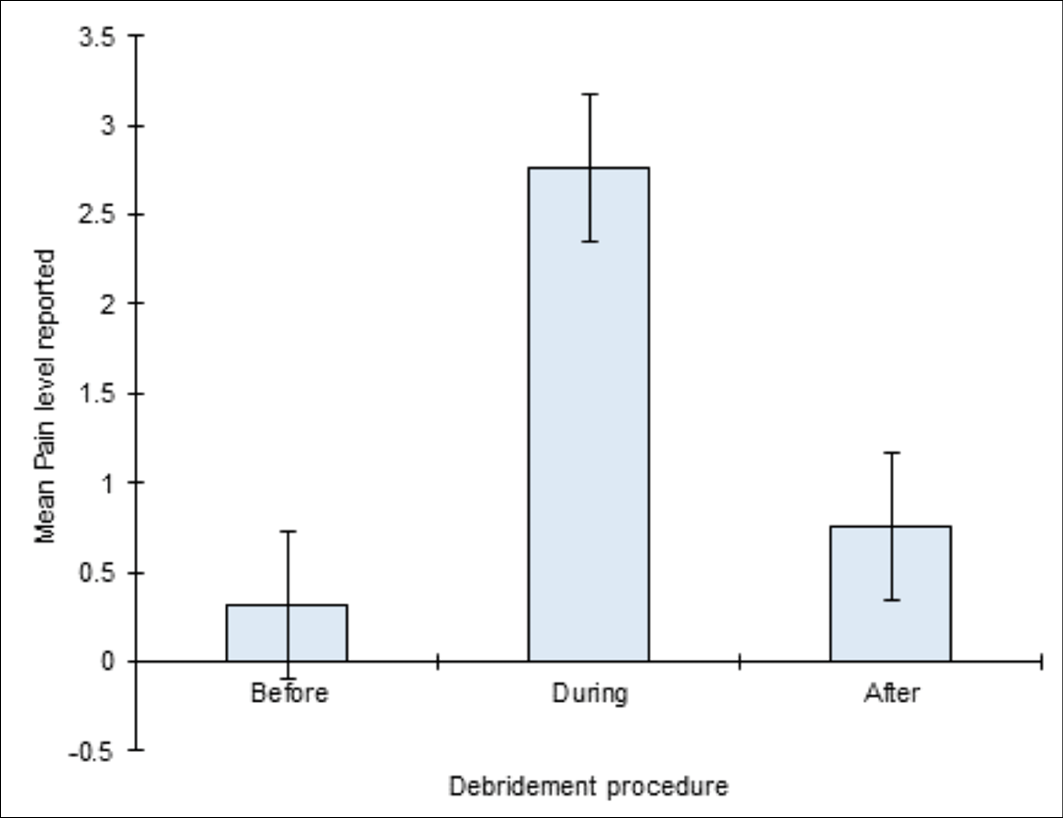
Figure 5. Mean of the pain scores reported before, during and after the debridement procedure using the NRS. Error bars represent a 95% confidence interval estimates for the mean.
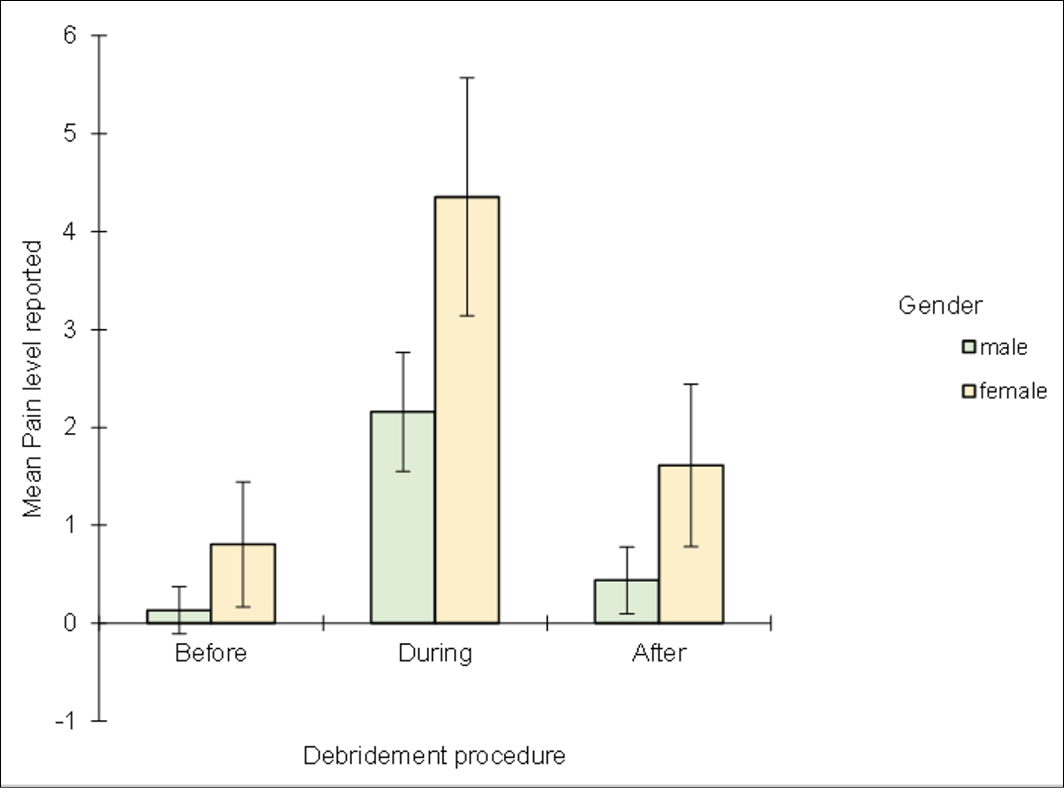
Figure 6. Mean of the pain scores reported before, during and after debridement between gender using the NRS. Error bars represent a 95% confidence interval estimates for the mean.
Pain levels reported during and after the procedure did not appear to be associated with a change in wound volume (cm3), wound tissue type, nor duration of time spent debriding the wound. The debridement procedure, although increasing the wound volume and thus removing a significant amount of non-viable tissue, did not significantly alter the participants’ perspective of their level of pain following the procedure. Pain experienced during and after the debridement procedure did not appear to be related to the sonotrode type used. Of the five debridement techniques used with the sonotrode (e.g., undermining, slicing, sliding, sliding with rotation and milling, and moistening), only the moistening technique showed significantly higher levels of pain during (p=0.010) and after the procedure (p=0.0311).
The length of time a participant had had a wound prior to this treatment had no meaningful relationship with the pain they experienced before, during or after treatment.
Discussion
The results of this study demonstrated decreased tissue of necrotic, slough and fibrin with LFUCD and was well tolerated regarding reported pain experience; this correlates well with previous studies8,20,22,35,36.
An explanation for the moistening technique to have reported higher levels of pain relates to why that technique is reserved for the more sensitive wounds or wounds that are in a high inflammatory state. The World Health Organization (WHO) pain ladder suggests that mild pain is reported at 1–3, moderate at 4–5 and severe at 6–10; other authors have also discussed the merits of NRS and its subjectivity37,38. Our normal pain assessment used the NRS as way to titrate the treatment to their immediate needs, and information on full disclosure was encouraged39.
Stanisic et al11 observed that 25Kz low frequency ultrasound rapidly and selectively solubilised fibrin selectively without harming granulation tissue, and our findings of 80% reduction of fibrin at the wound base is consistent in that observation.
In Stanisic’s study they also hypothesised that technique of slow back and forth movement (which is similar to the technique of sliding in this study) would allow for greater penetration of ultrasound energy. It also prevented an increase in thermal energy and the additional mechanical action along with the cavitation and acoustic streaming that makes it such an effective debridement modality, although our study did not find any difference in the type of technique implemented, yet the type of sonotrode did. Our study found that the spatula was used longer and removed more unwanted tissue. Given that the spatula sonotrode is recommended by the manufacturer for larger and flatter surfaces, this would be expected.
In this current study, having the choices in sonotrode types and debridement technique allowed the clinicians to best adapt the LFUCD sonotrode and technique to the wound contours, thereby effectively removing visible unhealthy tissue within a relatively short period of time, on average 5.3 minutes. Although the manufacturer provide guidelines, optimal settings, especially for LFUCD, have not been determined and, as such, there are inconsistencies in the literature which require further research40.
The best practice for hard to heal wounds includes: disruption of microbes through therapeutic cleansing using surfactant cleansers; mechanical or sharp debridement of the wound and wound edges, followed by the use of antimicrobials dressings to delay reformation of biofilm and slough; as well as appropriate moisture management and host management therapies8,41,42.
Results of our study confirm visible and immediate changes of the wound appearance as a result of LFUCD debridement. The effectiveness of LFUCD was measured as overall change in tissue post-treatment. Figure 7 is one example of a before and after treatment with LFUCD.
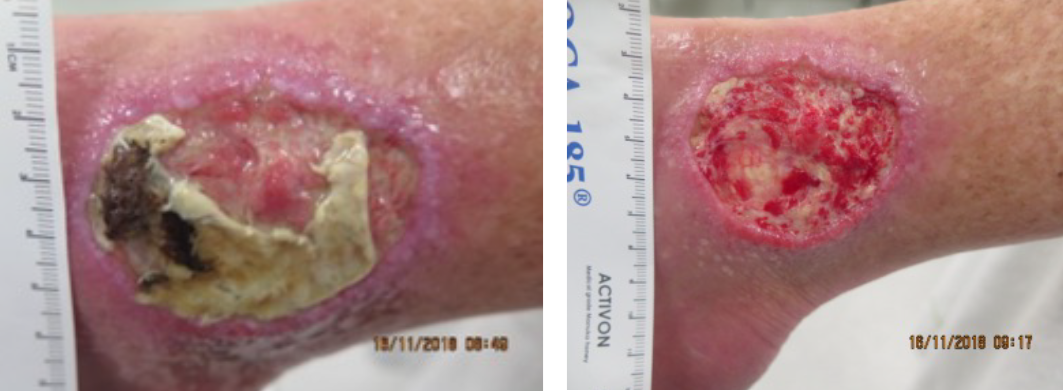
Figure 7. Venous leg ulcer right lateral ankle before and after using LFUCD with a spatula sonotrode
Wounds that are debrided regularly have a wound base properly prepared, thus maximising the effect of any adjunctive wound care modality such as modern wound dressings and/or use of skin grafts for definitive wound closure8,9,16. Results of our study suggest LFUCD can assist in preparing the wound bed for adjunctive wound care. These results strongly suggest that debridement with the LFUCD is assisted in removing non-viable tissue/unhealthy tissue and wound debris, while protecting healthy wound bed components such as granulation tissue which are required for wounds to progress towards healing.
Local anaesthetics were used to control participants’ pain during LFUCD; however, despite 100% ultrasound intensity being used for all LFUCD procedures, the participants reported an increase in pain levels during the procedure. This is consistent with other findings that indicated topical analgesia is required for almost all wounds being treated with LFUCD20,43. The specific aetiological basis underlying gender differences in pain is unknown; however, gender differences have been shown in pain perception, experiences and behaviour in people with chronic lower limb wounds44. Some studies have found that women report pain more frequently and experience painful stimuli more intensely than men; this has been explained as gender roles, whereby the expression of pain is more acceptable in women, which may imply that severe pain is under-reported in men45. Furthermore, psychosocial processes such as pain coping and early-life exposure to stress may also explain sex differences46.
Limitations
Several methodological shortcomings are noted in this observational case series. This study is a case series with a small sample size and did not involve a control group that received manual debridement of the wound. Wound volume was calculated by length x width x depth; this does not accurately reflect total volume when there is a variable depth, undermining or sinuses. A more formally validated wound assessment tool such as the Wollina Wound Score47,48 would have reduced subjectivity and potential bias49. However, as the intent was not to change but to evaluate practice efficacy with LFUCD, the standardised wound assessment tool and practices established as standard of care of this facility were continued. Efforts were made in the protocol and practical application of the MDS for consistency between the two wound specialists in determination of tissue type and percentages but some variation in inter-rater reliability or bias could have occurred.
The study was conducted on a sample of convenience of participants with wounds deemed appropriate for LFUCD and not wound aetiology, size or duration. Selection bias may have been influenced by participants wanting to undertake LFUCD to please the clinicians or to try a new form of therapy.
Conclusions
This study contributes to the growing body of literature on low frequency ultrasonic debridement for hard to heal wounds. It is one of the first studies to investigate the effectiveness of wound debridement using LFUCD using different sonotrode types and debridement techniques, and determining whether irrigation flow and ultrasonic intensity influenced the wound bed preparation.
The use of LFUCD demonstrated a decrease in non-viable and fibrinous tissue whilst increasing granulation within 5 minutes of treatment. Debridement techniques, the type of sonotrode used, irrigation flow and intensity do not significantly impact on the efficacy of debridement in this case series. Participants’ pain experience did increase during the debridement procedure but resumed to pre-treatment levels after treatment ceased, and pain levels were managed well with simple analgesics and topical anaesthetics.
This study suggests that LFUCD provides debridement for the management of wounds by reducing the amount of visible slough and fibrin while increasing post-treatment of visible granulation. Further longitudinal studies are needed to confirm the findings of this study and demonstrate the use of LFUCD as an adjunctive modality for improved wound bed preparation and wound healing.
Due to a lack of consistency in the literature or guidelines outside of the manufacturer’s recommendations regarding ideal settings for LFUCD, additional clinical research is needed to inform the clinician and provide best outcomes.
Acknowledgements
The authors would like to thank Dr Cecilia Welling (MMSI Consulting) for her expertise and assistance throughout all areas of this research, and for her guidance in completing this paper. Team members at SWH who assisted and supported the research include, Rene Clapham, Kait Brown, Simon Fogarty and Keryn Anderson. Thank you to Dr Keryln Carville and Dr Bill McGuiness for reviewing and providing advice.
Conflict of interest
Terry Swanson has provided consultation or participated in industry supported educational events by Söring GmbH. Scott Salzman has received funding from Söring GmbH for conducting statistical data evaluation.
Ethics statement
Ethical approval for the study was obtained from the relevant institutional ethics committee HREC14-2017 and all participants gave written informed consent.
Funding
Söring GmbH provided equipment and consumables to conduct the trial.
Author contribution
Terry Swanson was the lead and main author. Kate Hirst assisted with development of the study, implementation of the study as well as development and review of the manuscript. Scott Salzman was the statistician and developed the models for analysis, reviewed the manuscript and authored sections on statistics. Nikki Frescos was co-author and reviewer of the manuscript to improve academic standard.
Author(s)
Terry Swanson*1, Catherine Hirst1, Scott Salzman2, Nicoletta Frescos3
1South West Healthcare, Ryot Street, Warrnambool, VIC 3280, Australia
2Deakin Business School, Department of Information Systems and Business Analytics, Department of Economics, Deakin University, Warrnambool Campus, VIC 3280, Australia
3Adjunct Lecturer, St Vincent’s Hospital, Melbourne VIC 3065, Adjunct Lecturer, La Trobe University, VIC 3086
*Corresponding author Email swansonterry7@gmail.com
References
- Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vandscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1–S28.
- Sibbald RG, Elliott JA, Persaud-Jaimangal R, Goodman L, Armstrong DG, Harley C, Coelho S, Xi N, Evans R, Mayer DO, Zhao X. Wound bed preparation 2021. Adv Skin Wound Care 2021;34(4):183–95.
- Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012;9(s2):1–19.
- Kotronis G, Vas PRJ. Ultrasound devices to treat chronic wounds: the current level of evidence. Int J Low Extrem Wounds 2020;19(4):341–9.
- Murphy C. The UltraHeal Randomized Controlled Trial: investigating the effects of low frequency ultrasound debridement in a nurse-led vascular wound clinic. J Vasc Nurs 2016;34(2):68.
- Madhok BM, Vowden K, Vowden P. New techniques for wound debridement. Int Wound J 2013;10(3):247–51.
- Flores-Escobar S, Álvaro-Afonso FJ, García-Álvarez Y, López-Moral M, Lázaro-Martínez JL, García-Morales E. Ultrasound-Assisted Wound (UAW) debridement in the treatment of diabetic foot ulcer: a systematic review and meta-analysis. J Clin Med 2022;11(7):1911.
- Chang Y-JR, Perry J, Cross K. Low-frequency ultrasound debridement in chronic wound healing: a systematic review of current evidence. Plast Surg (Oakv) 2017;25(1):21–6.
- Herberger K, Franzke N, Blome C, Kirsten N, Augustin M. Efficacy, tolerability and patient benefit of ultrasound-assisted wound treatment versus surgical debridement: a randomized clinical study. Dermatology 2011;222(3):244–9.
- Lázaro-Martínez JL, Álvaro-Afonso FJ, García-Álvarez Y, Molines-Barroso RJ, García-Morales E, Sevillano-Fernández D. Ultrasound-assisted debridement of neuroischaemic diabetic foot ulcers, clinical and microbiological effects: a case series. J Wound Care 2018;27(5):278–86.
- Stanisic MM, Provo BJ, Larson DL, Kloth LC. Wound debridement with 25 kHz ultrasound. Adv Skin Wound Care 2005;18(9):484–90.
- Schoenbach SF, Song IC. Ultrasonic debridement: a new approach in the treatment of burn wounds. Plastic Reconstruct Surg (1963);1980;66(1):34–7.
- Scherba G, Weigel RM, O’Brien WD. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl Environ Microbiol 1991;57(7):2079–84.
- Suchkova V, Carstensen EL, Francis CW. Ultrasound enhancement of fibrinolysis at frequencies of 27 to 100 kHz. Ultrasound Med Biol 2002;28(3):377–82.
- Weichenthal M, Mohr P, Stegmann W, Breitbart EW. Low-frequency ultrasound treatment of chronic venous ulcers. Wound Repair Regen 1997;5(1):18–22.
- Korzendorfer H, Hettrick H. Biophysical technologies for management of wound bioburden. Adv Wound Care 2014;3(12):733–41.
- Voigt J, Wendelken M, Driver V, Alvarez OM. Low-frequency ultrasound (20–40kHz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta-analysis of eight randomized controlled trials. Int J Low Extrem Wounds 2011;10(4):190–9.
- Yarets Y. Effective biofilm removal and changes in bacterial biofilm building capacity after wound debridement with low-frequency ultrasound as part of wound bed preparation before skin grafting. Chronic Wound Care Manage Res 2017;4:55–64.
- Murphy CA, Houghton P, Brandys T, Rose G, Bryant D. The effect of 22.5 kHz low-frequency contact ultrasound debridement (LFCUD) on lower extremity wound healing for a vascular surgery population: a randomised controlled trial. Int Wound J 2018;15(3):460–72.
- Butcher G. Low frequency ultrasonic debridement: a new tool in our armoury? J Foot Ankle Res 2011;4(S1):P7-P.
- Shannon MK, Williams A, Bloomer M. Low-frequency ultrasound debridement (Sonoca-185) in acute wound management: a case study. Wound Pract Res 2012;20(4):200–5.
- Swanson T, Lázaro-Martínez JL, Braumann C, Kirchhoff J-B, Gächter B, van Acker K. Ultrasonic-assisted wound debridement: report from a closed panel meeting. J Wound Care 2020;29(2):128–35.
- Carville K, Howse L, Edmondson M, Petkovska G, Morey P, Maguire C, Tuvik A. Nurse practitioners and their use of low-frequency ultrasound debridement in the management of chronic wounds. Wound Pract Res 2018;26(3):122–6.
- Webster D, Harvey W, Dyson M, Pond J. The role of ultrasound-induced cavitation in the ‘in vitro’ stimulation of collagen synthesis in human fibroblasts. Ultrasonic 1980;18(1):33–7.
- Söring. SONOCA 185 instruction for use; Sept 2015. Available from: https://wwwbioclinicalservicescomau/soring--3/sonoca-ultrasonic-generators/sonoca-185-instructions-for-use-sept-20152015.
- Murphy C, Atkin L, Vega de Ceniga M, Weir D, Swanson T, Walker A, Mrozikiewicz-Rakowska B, Ciprandi G, Lazaro-Martinez JL, Cernohorska J. Embedding wound hygiene into a proactive wound healing strategy. J Wound Care 2022;31(Sup4a):S1-S19.
- Nichols E. Describing a wound from presentation to healing. Wound Essent 2015;10(1):56–61.
- Young T. Accurate assessment of different wound tissue types. Wound Essent 2015;10(1):51–4.
- White W, Asimus M. Assessment and management of non-viable tissue. In: Swanson T, Asimus M, McGuiness B, editors. Wound management for the advanced practitioner. 1st ed. Melbourne, Australia: IP Communications; 2014. p. 170–203.
- Angel D. Slough: what does it mean and how can it be managed. Wound Pract Res 2019;27(4):164–7.
- Dowsett C, Swanson T, Karlsmark T. A focus on the Triangle of Wound Assessment – addressing the gap challenge and identifying suspected biofilm in clinical practice. Wound Int 2019;10(3):34–9.
- World Union of World Healing Societies (WUWHS). Position document: advances in wound care: the Triangle of Wound Assessment. Florence Congress; 2016. p. 1–32.
- Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain® 2011;152(10):2399–404.
- Gloth FM, Scheve AA, Stober CV, Chow S, Prosser J. The Functional Pain Scale: reliability, validity, and responsiveness in an elderly population. J Am Med Dir Assoc 2001;2(3):110–4.
- Michailidis L, Bergin SM, Haines TP, Williams CM. Healing rates in diabetes-related foot ulcers using low frequency ultrasonic debridement versus non-surgical sharps debridement: a randomised controlled trial. BMC Res Notes 2018;11(1):1–5.
- Crone S, Garde C, Bjarnsholt T, Alhede M. A novel in vitro wound biofilm model used to evaluate low-frequency ultrasonic-assisted wound debridement. J Wound Care 2015;24(2):64, 69, 72.
- Frescos N. Pain assessment tools for chronic lower limb wounds: a scoping review. Wound Pract Res 2019;27(1):27–35.
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni AM, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011;41(6):1073–93.
- Boring BL, Walsh KT, Nanavaty N, Ng BW, Mathur VA. How and why patient concerns influence pain reporting: a qualitative analysis of personal accounts and perceptions of others’ use of numerical pain scales. Frontier Psychol 2021;12:663890-.
- Kataoka Y, Kunimitsu M, Nakagami G, Koudounas S, Weller CD, Sanada H. Effectiveness of ultrasonic debridement on reduction of bacteria and biofilm in patients with chronic wounds: a scoping review. Int Wound J 2021;18(2):176–86.
- Percival SL, Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care 2015;24(11):498–510.
- Murphy C, Atkin L, Swanson T, Tachi M, Tan YK, de Ceniga MV, Weir D, Wocott R, Cemnohorska J, Ciprandi G, Dissemond J, James GA, Lazaro-Martinez JL, Mrozikiewicz-Radowska R, Wilson P. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: wound hygiene. J Wound Care 2020;29(Sup3b):S1-s26.
- Braschi F, Bartoli F, Bruni C, Fiori G, Fantauzzo C, Paganelli L, De Paulis A, Rasero L, Matucci-Cerinic M. Lidocaine controls pain and allows safe wound bed preparation and debridement of digital ulcers in systemic sclerosis: a retrospective study. Clin Rheumatol 2016;36(1):209–12.
- Frescos N. Assessment of persistent pain in chronic lower limb wounds: Thesis (PhD). La Trobe University; 2019.
- Bartley EJ, Fillingim RB, Colvin L, Rowbotham DJ. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111(1):52–8.
- Pieretti S, Di Giannuario A, Di Giovannandrea R, Marzoli F, Piccaro G, Minosi P, Aloisi AM. Gender differences in pain and its relief. Ann Ist Super Sanita 2016;52(2):184–9.
- Wollina U, Heinig B, Naumann G, Scheibe A, Schmidt W-D, Neugebauer R. Effects of low-frequency ultrasound on microcirculation in venous leg ulcers. Indian J Dermatol 2011;56(2):174–9.
- Schmidt WD, Fassler D, Liebold K, Wollina U. Contact-free spectroscopy of leg ulcers: principle, technique, and calculation of spectroscopic wound scores. J Invest Dermatol 2001;116(4):531–5.
- Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatol Ther 2006;19(6):383–90.



