Volume 31 Number 1
Evaluation of osteomyelitis in diabetic foot ulcers with exposed bone
Handan Derebaşınlıoğlu, Onur Aksoy
Keywords diabetic foot ulcers, osteomyelitis
For referencing Derebaşınlıoğlu H & Aksoy O. Evaluation of osteomyelitis in diabetic foot ulcers with exposed bone. Wound Practice and Research 2023; 31(1):28-39.
DOI
https://doi.org/10.33235/wpr.31.1.28-39
Submitted 5 November 2022
Accepted 16 January 2023
Abstract
Purpose Exposed bone and periosteal damage promote the adherence of pathogens to bone matrix components. Damage to the periosteum adversely affects bone perfusion and creates a more suitable environment for pathogens. The purpose of this study was to determine the prevalence of osteomyelitis among patients with diabetic foot ulcers with bone exposure and to identify the role of simple serological markers in the diagnosis of osteomyelitis in this patient group.
Methods Patients who underwent amputation and debridement due to diabetes-related foot wound were included in the study. The pathology results were evaluated according to presence of osteomyelitis. C‑reactive protein (CRP), erythrocyte sedimentation rate (ESR), complete blood count (CBC), platelet/lymphocyte ratio, neutrophil/lymphocyte ratio (NLR) and microbial growth were analysed.
Results Patients with ESR of 79mm/h or lower had a 3.046-fold higher risk of osteomyelitis. The risk of osteomyelitis was 2.901-fold higher at lymphocyte percentages of 12.3% or higher. Patients with a neutrophil percentage of 78% or lower had a 3.010-fold higher risk of osteomyelitis. Patients with NLR of 6.02 or lower had a 2.901-fold risk of osteomyelitis. When ESR was evaluated with neutrophil percentage, lymphocyte percentage and NLR, the sensitivity was calculated as 86.76% for osteomyelitis.
Conclusion We believe that the immune response caused by bone exposure to the environment is different than the immune response caused by soft tissue infection in the classical diabetic foot. The combined evaluation of multiple diagnostic parameters increases the sensitivity of osteomyelitis diagnosis.
Introduction
Diabetic osteomyelitis generally occurs as a result of an open chronic wound of the foot becoming infected and extending to the bone. Approximately 50–60% of severe foot infections are complicated by osteomyelitis1,2. Furthermore, the prevalence of osteomyelitis can be as high as 66% in existing infected foot wounds3.
Chronic wounds carry high risk for osteomyelitis. Weight distribution on the feet is disrupted due to orthopaedic deformities, leading to non-healing wounds and further increasing the risk of osteomyelitis4–7. Ulcers larger than 2cm2 are reported to have 56% sensitivity and 92% specificity for the diagnosis of osteomyelitis. The incidence of underlying osteomyelitis in deep ulcers (>3mm) compared to superficial ulcers is 33% versus 82%1,8.
Three independent risk factors have been identified for the development of diabetic osteomyelitis – wounds that penetrate to the bone or joint, history of previous lower limb wound, and recurrent or multiple wounds. Among these factors, the relative risk of osteomyelitis was calculated as 23.1 for wounds penetrating to the bone or joint9. Exposed bone and periosteal damage promote the adherence of pathogens to bone matrix components. Damage to the periosteum adversely affects bone perfusion and creates a more suitable environment for pathogens.
The probe-to-bone test, which determines the relationship between the bone and the external environment, is another criterion used in the diagnosis of osteomyelitis. It has been reported that this test has 66–87% sensitivity and 85–91% specificity in the diagnosis of osteomyelitis3,10,11. However, although the probe-to-bone test is the most useful clinical examination, its reliability may be affected by factors such as the performing clinician’s technique and experience, the ulcer’s location, and its aetiology12–14.
According to the International Working Group on the Diabetic Foot (IWGDF) Infection Guideline 201915, in a person with diabetes and suspected osteomyelitis of the foot, advanced diagnostic imaging of the foot is not recommended if simple and accessible methods such as plain X-ray and clinical and laboratory findings are compatible with osteomyelitis; advanced imaging methods are recommended only in doubtful cases15. However, characteristic changes may not occur until approximately 2 weeks after the development of infection, and this should be taken into account to avoid a premature diagnosis11.
Although advanced imaging modalities such as MRI, radiolabelled white blood cell (WBC) single-photon emission computed tomography/computed tomography (SPECT/CT), and 18F‑FDG positron emission tomography/CT (PET/CT) can also be used in the diagnosis of osteomyelitis, the gold standard test for osteomyelitis is histopathological or microbiological studies of a bone biopsy or aspiration of pus from the bone. However, biopsy is an invasive procedure that often requires anaesthesia, and obtaining results can take days16.
Many recent studies have investigated the use of serological markers such as C‑reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), platelet/lymphocyte ratio, and neutrophil/lymphocyte ratio (NLR) in the diagnosis and treatment follow-up of osteomyelitis17–19. These parameters can be measured from patient blood and serum samples at a much lower cost compared to other methods or can be easily calculated from measured parameters.
Most previous studies using these parameters have focused on the relationship between diabetic foot infection and osteomyelitis. The aim of this study at Sivas Cumhuriyet University Plastic Reconstructive and Aesthetic Surgery Clinic was to determine the role of NLR, platelet/lymphocyte ratio, CRP and ESR as biomarkers in the diagnosis of osteomyelitis in patients with bone exposure associated with a diabetes-related ulcer.
Methods
Patients who underwent surgery for diabetes-related foot wounds were retrospectively screened from the electronic records system. The pathology results of amputation and bone biopsy materials obtained from patients who had a diabetic foot ulcer with exposed bone or positive probe-to-bone test between 1 January 2006 and 30 April 2020 were evaluated.
Patients who underwent amputation and debridement for a diabetes-related foot wound under operating room conditions were included in the study. Bone biopsy was performed in patients with bone exposure by using Rounger forceps to obtain bone fragments depending on the size of the bone being examined. Amputation materials were sent for histopathological examination after the surgical procedure. Tissue biopsy for microbiological examination was obtained under sterile conditions during the operation. Tissue biopsy cultures were performed to assess bacterial growth in the wounds. However, the tissue cultures contained soft tissue and/or bone because the bone and/or soft tissue were sent together. Therefore, tissue separation could not be made in cultures and only histopathological analysis was used for the diagnosis of osteomyelitis.
Based on the histopathologic results, the patients were divided into the osteomyelitis group and non-osteomyelitis group. Patients without osteomyelitis were also classified based on the presence of pathologic changes in the bone marrow such as bone marrow inflammation and/or oedema.
Analysis of CRP level, ESR, complete blood count (CBC) values, and microbial growth in wound site cultures were done prior to amputation and biopsy. From the CBC results, WBC, platelet, neutrophil, lymphocyte, monocyte and basophil counts and percentages were used to calculate platelet/lymphocyte ratio and NLR. Parameters that showed a statistically significant difference between the groups in initial comparisons were further analysed to determine cut-off, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR) and negative LR.
Patients who did not have histopathological evaluation of bone tissue, whose histopathological evaluation could not be performed due to insufficient material, or whose tissue culture, CBC, ESR or CRP results could not be obtained were excluded from the study. Other exclusion criteria were having undergone amputation and debridement because of ischaemic conditions or Buerger disease and history of haematological disease.
Statistical analyses of the data were performed using NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA) software. Descriptive statistical methods (mean, standard deviation, median, frequency, percentage, minimum, maximum) were used to summarise the data. Normal distribution of quantitative data was tested using the Shapiro–Wilk test and graphical methods. For pairwise comparisons of numerical data, the Student’s t-test was used for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. Pearson’s chi-square test was used for comparisons of qualitative data. Receiver operating characteristic (ROC) curve analysis and diagnostic screening tests were used to determine optimal cut-off points for the differentiation of patients with and without osteomyelitis; p value <0.05 was considered statistically significant.
The measures of diagnostic accuracy used were:
- Sensitivity: Ability of the test to identify patients who have osteomyelitis.
- Specificity: Ability of the test to identify patients who do not have osteomyelitis.
- PPV: Probability that a patient with a positive result is truly positive (osteomyelitis).
- NPV: Probability that a patient with a negative result is truly negative (non-osteomyelitis).
- Positive LR: sensitivity / (1 – specificity)
- Negative LR: (1 – sensitivity) / specificity
Results
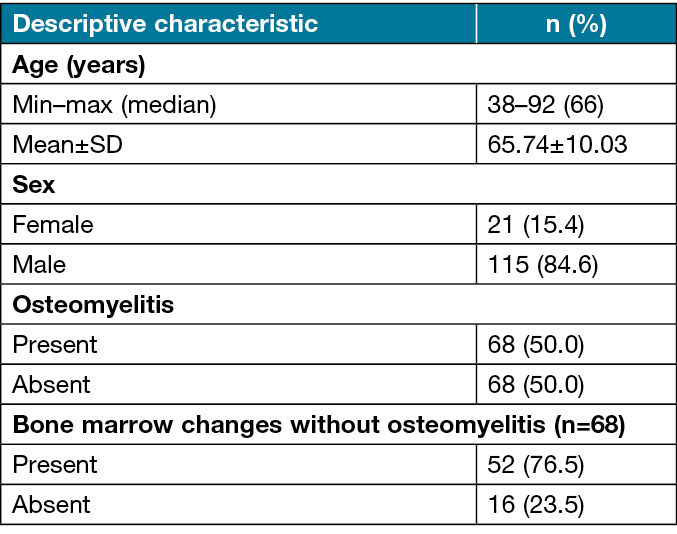
Patients who were operated for a diabetes-related foot wound at Sivas Cumhuriyet University Plastic Reconstructive and Aesthetic Surgery Clinic between 1 January 2006 and 30 April 2020 were identified from hospital records. Of these, data for all analysed parameters were obtained for 136 patients (15.4% [n=21] women; 84.6% men [n=115]) with exposed bone or positive probe-to-bone test. The patients ranged in age from 38–92 years, with a mean age of 65.74±10.03 years. The patients’ descriptive characteristics are shown in Table 1.
Table 1. Distribution of the patients’ descriptive characteristics
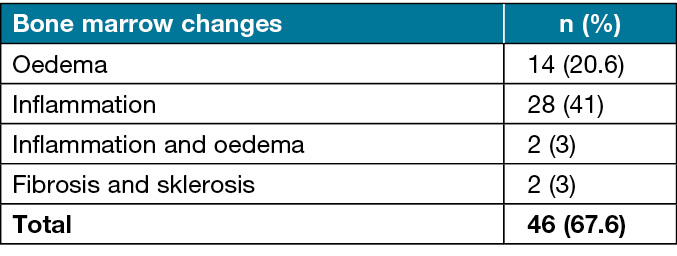
Osteomyelitis was present in 50.0% (n=68) of the patients and absent in 50.0% (n=68). Bone marrow changes were observed in 67.6% (n=46) of patients without osteomyelitis (Figure 1). Bone marrow changes detected in the patients are shown in Table 2.
Figure 1. Distribution of osteomyelitis status
Table 2. Distribution of bone marrow changes
Osteomyelitis was reported as acute in 53% (n=36) and chronic in 47% (n=32) of patients in the osteomyelitis group. Diabetic microangiopathy was detected on histopathological examination in 44.1% (n=60) of the patients.
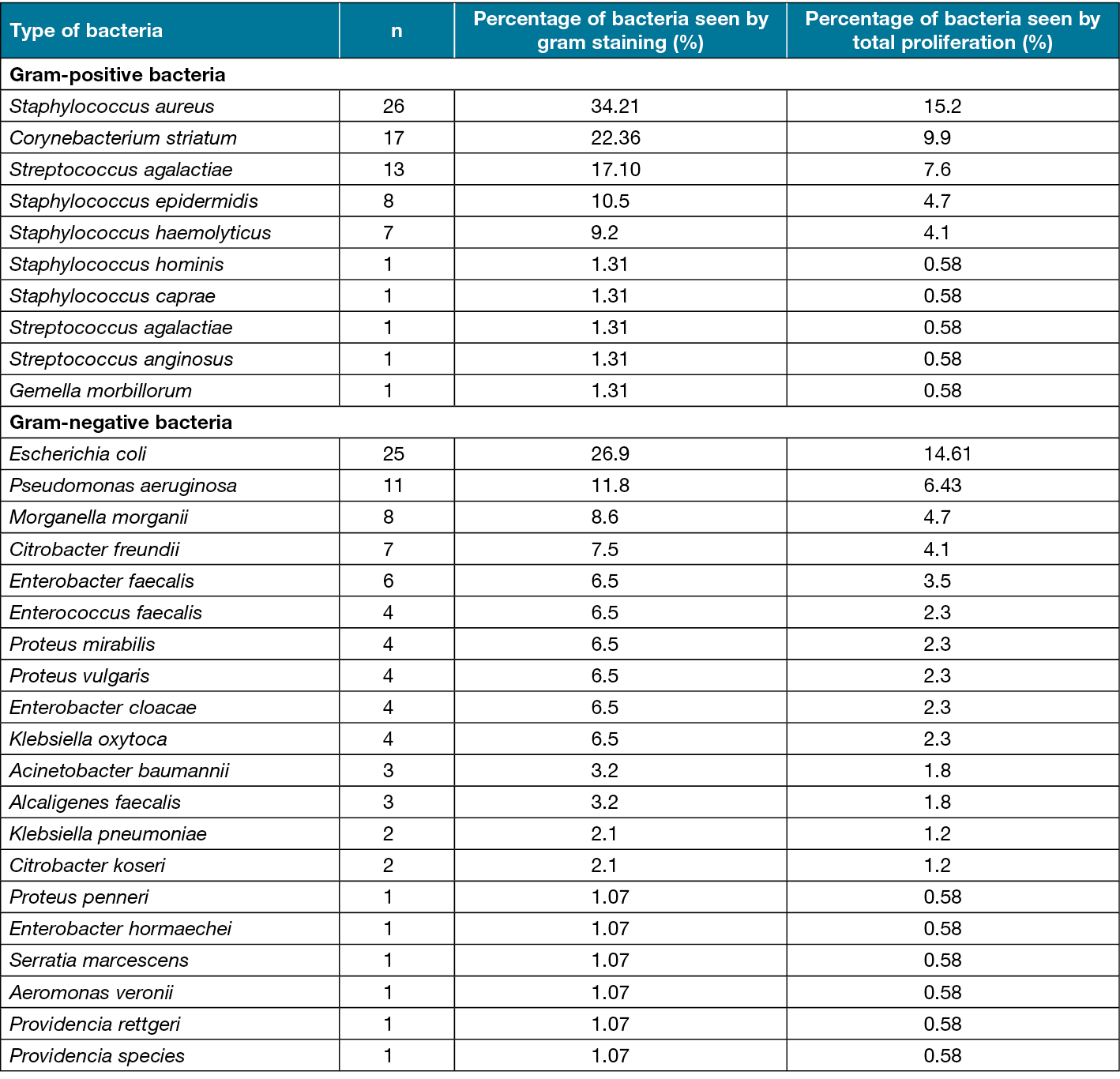
Biopsy culture was negative for 27 patients (19.9%). By group, cultures were negative in 13 patients in the non-osteomyelitis group (19.1%) and 14 patients in the osteomyelitis group (20.5%). Biopsy cultures were positive in the remaining 109 patients (80.1%), 55 of whom were in the non-osteomyelitis group (80.9%) and 54 of whom were in the osteomyelitis group (79.5%). The positive cultures yielded a single organism in 49 patients (36%), two organisms in 47 patients (34.5%), three organisms in six patients (4.5%), and four organisms in one patient (0.7%). In total, 171 microbial agents were isolated, including 93 (54.3%) gram-negative bacteria, 76 (44.4%) gram-positive bacteria, and fungi in two (1.1%) cultures. The isolated agents are presented in Table 3. The most common agent was Staphylococcus aureus (15.2%), followed by Escherichia coli (14.6%) (Table 3).
Table 3. Bacteria cultured from tissue biopsy specimens (bone and/or soft tissue) collected intraoperatively
Overall, the mean CRP level was 127.02±101.49mg/L, the mean ESR was 72.07±32.55mm/h, the mean WBC count was 11.81±5.04 x 109, the mean platelet count was 312.67±120.77 x109, the mean lymphocyte count was 1704.26±884.93 x109, the mean neutrophil percentage was 75.92±9.32%, the mean lymphocyte percentage was 16.08±7.96%, the mean NLR was 6.70±5.38, and the mean platelet/lymphocyte ratio was 214.02±116.68 (Table 4).
Table 4. Patients’ laboratory results
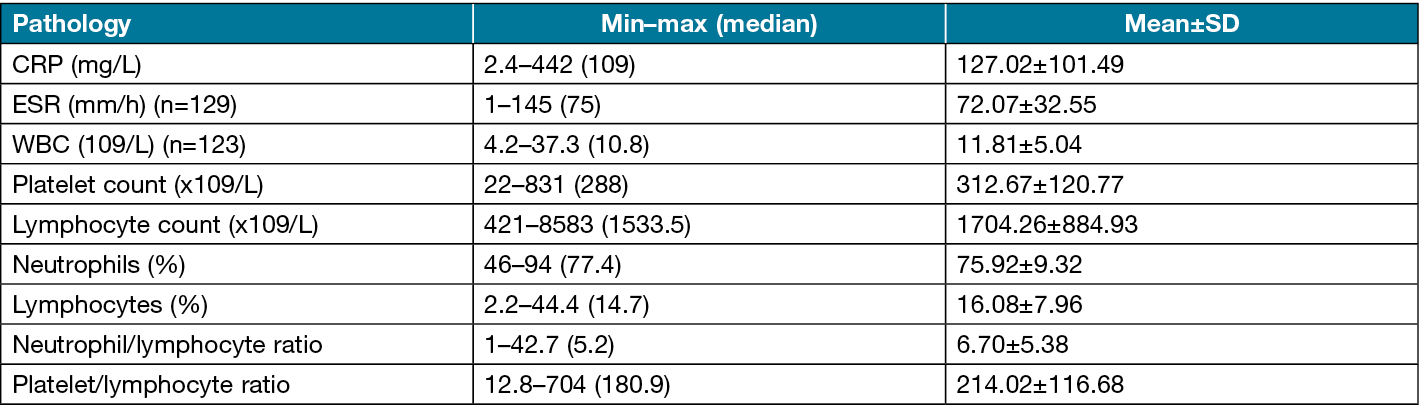
In comparison of patients with and without osteomyelitis, there was no difference in sex distribution, but the mean age was significantly lower in the osteomyelitis group (p=0.017). The CRP level and WBC count showed no significant difference between the groups (p>0.05). The ESR was significantly lower in the osteomyelitis group compared to the non-osteomyelitis group (p=0.010) (Figure 2a). The neutrophil percentage was also significantly lower in the osteomyelitis group compared to the non-osteomyelitis group (p=0.021) (Figure 2b). In contrast, the lymphocyte percentage was significantly higher in the osteomyelitis group (p=0.024) (Figure 2c). The NLR was also significantly lower in the osteomyelitis group compared to the non-osteomyelitis group (p=0.025) (Figure 2d). Comparisons of patient characteristics and biomarkers between the groups are shown in Table 5.
Table 5. Comparisons of patient characteristics and biomarkers based on presence of osteomyelitis
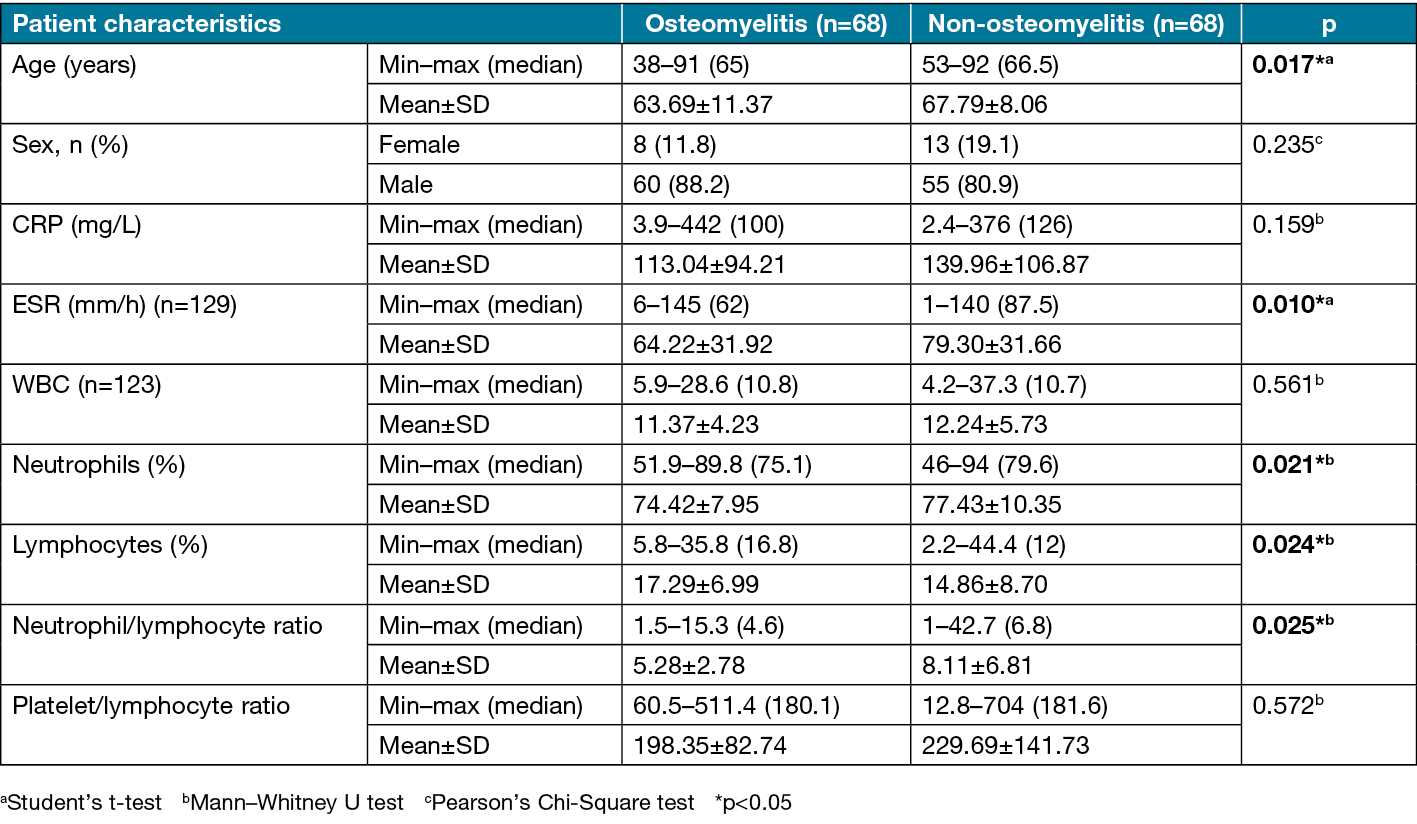
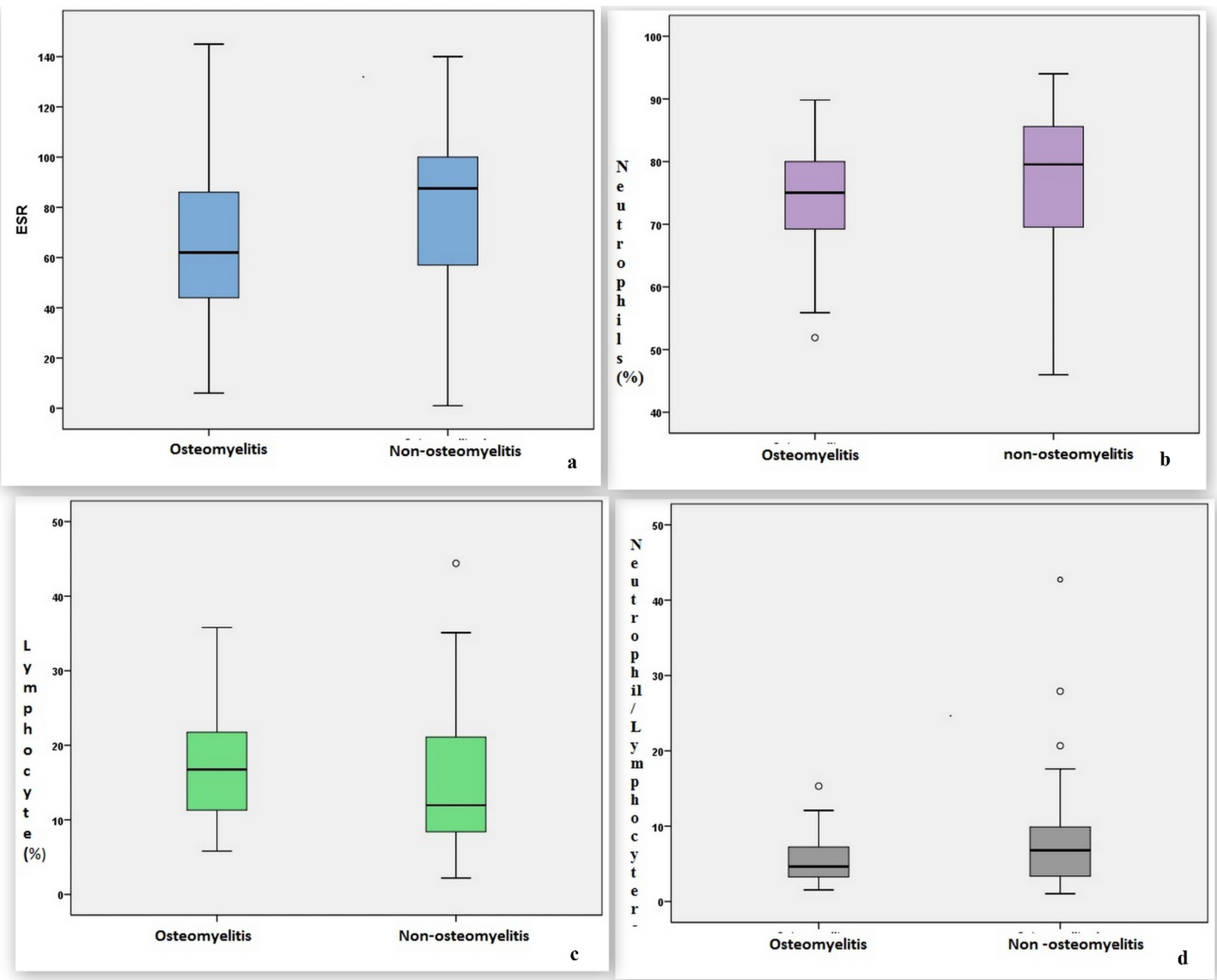
Figure 2. Distributions of a) ESR, b) neutrophil percentage, c) lymphocyte percentage and d) neutrophil/lymphocyte ratio in patients with and without osteomyelitis
Determination of cut-off values
ROC curve analysis and diagnostic screening tests were used to determine cut-off values for the parameters that differed significantly between the groups (ESR, neutrophil percentage, lymphocyte percentage and NLR).
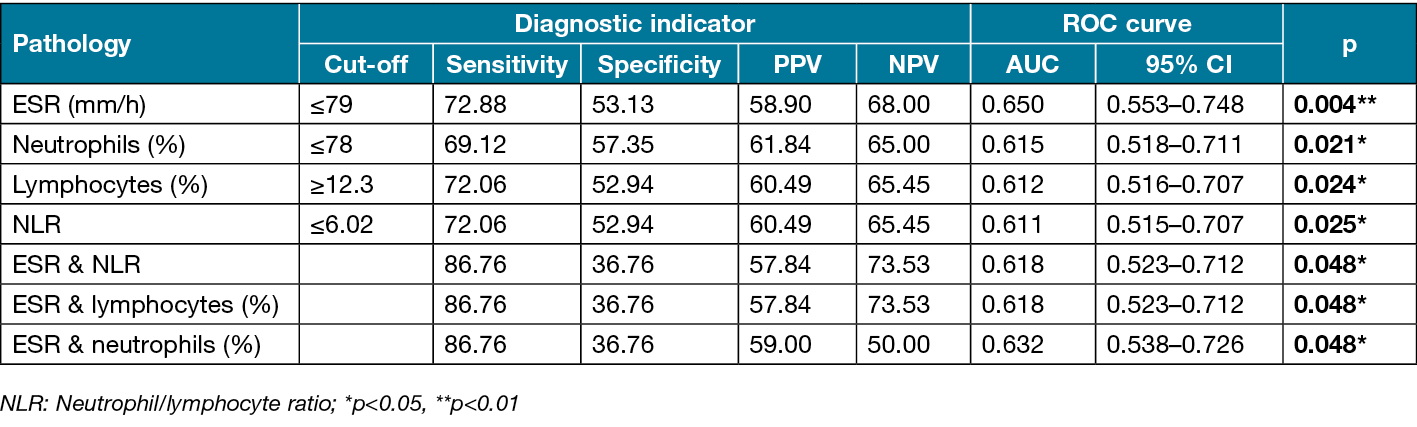
The cut-off point for ESR was identified as 79mm/h. An ESR of 79mm/h or lower had sensitivity of 72.88%, specificity of 53.13%, PPV of 58.90%, NPV of 68.00%, and accuracy of 62.60% in the diagnosis of osteomyelitis (positive LR=1.55, negative LR=0.5). Area under the ROC curve (AUC) was determined as 65.0% with standard error of 5.0% (Figure 3a). In addition, a statistically significant difference was detected between the groups based on the cut-off point obtained for ESR (≤79mm/h) (p=0.003). Patients with ESR of 79mm/h or lower had 3.046-fold higher odds of osteomyelitis (odds ratio [OR]: 3.046, 95% confidence interval [CI]: 1.431–6.482).
In ROC curve analysis of neutrophil percentage, the optimal cut-off point was 78%. At this cut-off value, neutrophil percentage had sensitivity of 69.12%, specificity of 57.35%, PPV of 61.84%, NPV of 65.00%, and accuracy of 63.24% (positive LR=1.62, negative LR=0.53). The ROC AUC was 61.5% with standard error of 4.9% (Figure 3b). There was a statistically significant difference between the groups based on the neutrophil percentage cut-off point (≤78%) (p=0.002). Patients with neutrophil percentage of 78% or lower had 3.010-fold higher odds of osteomyelitis (OR: 3.010, 95% CI: 1.489–6.085).
For lymphocyte percentage, a cut-off point of 12.3% was identified. At this cut-off value, neutrophil percentage had sensitivity of 72.06%, specificity of 52.94%, PPV of 60.49%, NPV of 65.45%, and accuracy of 62.50% (positive LR=1.55, negative LR=0.5). The ROC AUC was 61.2% with standard error of 4.9% (Figure 3c). A statistically significant difference was detected between the groups based on the lymphocyte percentage cut-off point (≥12.3%) (p=0.003). The odds of having osteomyelitis were 2.901-fold higher at lymphocyte percentages of 12.3% or higher (OR: 2.901, 95% CI: 1.423–5.915).
The cut-off point for NLR was identified as 6.02, which had sensitivity of 72.06%, specificity of 52.94%, PPV of 60.49%, NPV of 65.45%, and accuracy of 62.50% (positive LR=1.53, negative LR=0.52). The ROC AUC was 61.1% with standard error of 4.9% (Figure 3d). There was a statistically significant difference between the groups based on the NLR cut-off point (≥6.02) (p=0.003). Patients with NLR of 6.02 or lower had a 2.901-fold risk of osteomyelitis (OR: 2.901, 95% CI: 1.423–5.915).
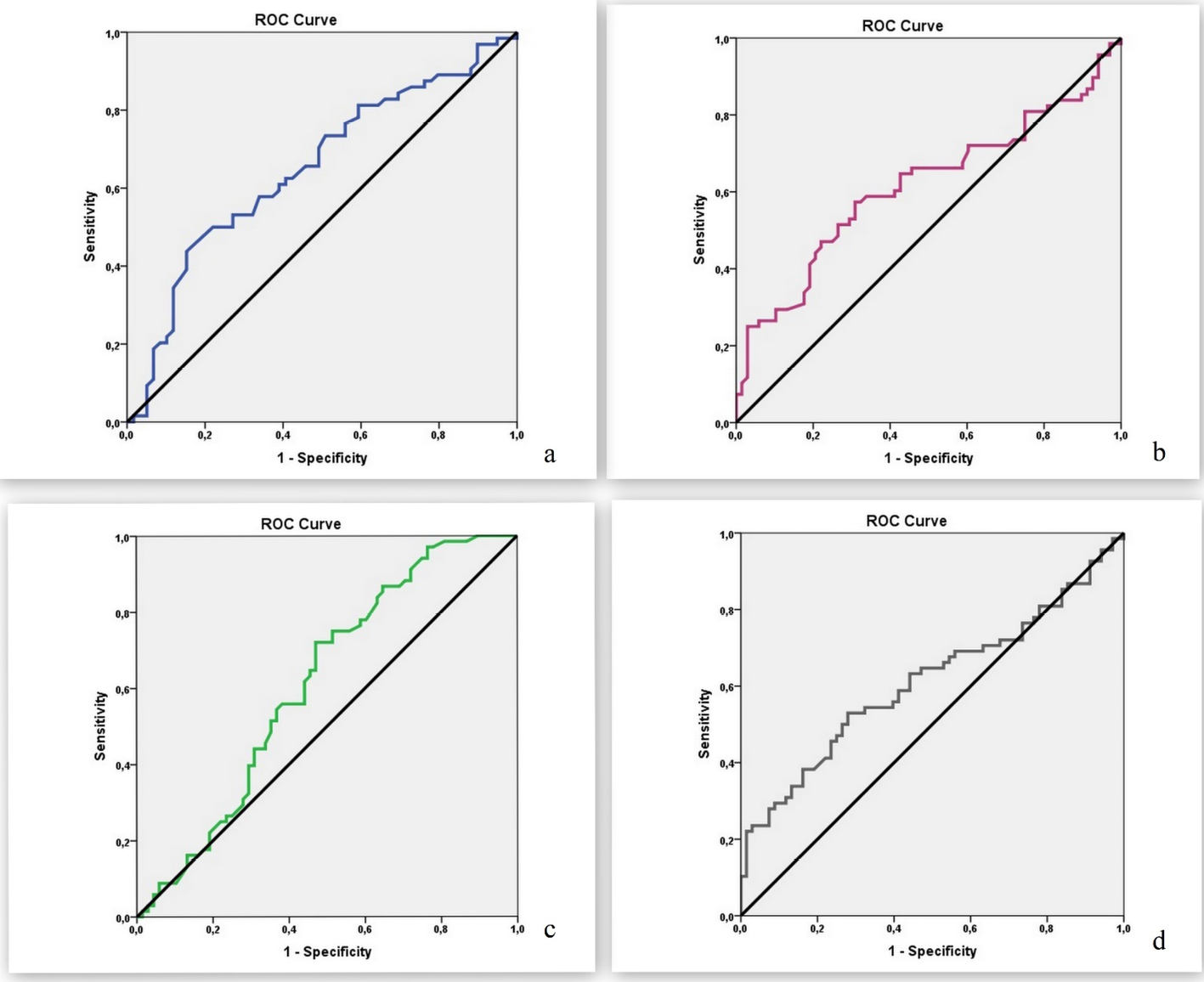
Figure 3. ROC curves for a) ESR, b) neutrophil percentage, c) lymphocyte percentage and d) neutrophil/lymphocyte ratio
Analysis of the diagnostic accuracy of biomarker combinations showed that ESR (≤79mm/h) and NLR (≥6.02) considered together had sensitivity of 86.76%, specificity of 36.76%, PPV of 57.84%, NPV of 73.53%, and accuracy of 61.76% (positive LR=1.37, negative LR=0.36). The ROC AUC was 61.8% with a standard error of 5.3%.
The combination of ESR (≤79mm/h) and lymphocyte percentage (≥12.3%) had sensitivity of 86.76%, specificity of 36.76%, PPV of 57.84%, NPV of 73.53%, and accuracy of 61.76% (positive LR=1.37, negative LR=0.36). The ROC AUC was 61.8% with standard error of 5.3%.
ESR (≤79mm/h) and neutrophil percentage (≤78%) together had sensitivity of 86.76%, specificity of 36.76%, PPV of 59%, NPV of 50%, and accuracy of 57.63% (positive LR=1.37, negative LR=0.36). The ROC AUC was 61.8% with standard error of 5.3%. The indicators of diagnostic accuracy for the analysed biomarkers and their combinations are shown in Table 6.
Table 6. Diagnostic indicators and ROC curve results for biomarkers and their combinations
Discussion
Osteomyelitis is associated with longer treatment duration and increased risk of amputation and mortality. A diagnosis of osteomyelitis is relevant to the duration of antibiotherapy and requires repeated debridement. The prevalence of foot ulcers among diabetic patients is approximately 4–10%, with up to 25% of people with diabetes developing a foot ulcer during their lifetime14. Infection is a problem in more than half of diabetic foot ulcers. Osteomyelitis develops in 20% of moderate infections and 50–60% of severe infections20. Although osteomyelitis can be diagnosed using methods such as MRI and labelled WBC scintigraphy, the gold standard is pathologic examination.
Bone infections in diabetic osteomyelitis are histopathologically categorised as acute, chronic, chronic acute and fibrotic. The frequency of these types of osteomyelitis varies in different series. Cecilia-Matilla and Tardáguila-García et al reported chronic osteomyelitis as the most common type21,22, while Aragón-Sánchez et al reported that acute osteomyelitis was most common23, as in our study. Fibrosis and sclerosis were detected on pathological examination of the bone marrow in three patients in our study. In one of the cases, these changes were reported together with acute osteomyelitis, while in the others they were reported as bone marrow changes in the absence of osteomyelitis.
The diagnostic sensitivity of histologic examination for the presence of osteomyelitis has been reported to be as high as 95%, with a diagnostic specificity of 99%8. The negative microbiological culture rate in histologically proven osteomyelitis is stated to be 40–60%24,25. In another study, the rate of positive bone culture was 87.5% in patients with positive probe-to-bone test and was higher in patients with positive histopathology compared to those with negative results26.
White et al reported that microbiological culture alone had 42% sensitivity in the diagnosis of osteomyelitis but that sensitivity increased to 84% when combined with histological examination24. Tardáguila-García et al reported that bone culture had diagnostic specificity of 70% and sensitivity of 40%22. They emphasised that histopathological evaluation was more accurate in diagnosing diabetic osteomyelitis than microbiology, especially in patients with chronic diabetic osteomyelitis. These patients may be underdiagnosed because bone culture gives false negative results. Therefore, both histopathological evaluation and bone culture are recommended to confirm the presence of diabetic osteomyelitis22.
In our study, 20.5% of biopsies that were histopathologically diagnosed as osteomyelitis did not have microbiological growth in culture. However, we attribute this to the fact that cultures were performed using samples that included both bone and soft tissue. We also observed a 79.5% positive culture rate in patients without osteomyelitis, indicating that these percentages are more related to soft tissue infection than histopathological misdiagnosis. Because cultures were not done exclusively with bone, the differential diagnosis of osteomyelitis was based on histopathological findings in this study. Another reason for negative cultures may be a history of antibiotic use, which was unknown in our patients. However, it has been reported that the rate of positive bone culture did not differ between patients who received antibiotic therapy and those who did not26.
Bone exposure may occur in diabetes-related foot wounds because of tissue loss, neurotrophic ulcer, or fistula formation. If a sterile device inserted into the wound contacts bone (i.e., the probe-to-bone test), it is considered exposed bone. The prevalence of osteomyelitis in these wounds was reported to be 66% and this method had 87% sensitivity and 83% specificity in the identification of patients with osteomyeltis27.
It has been reported that 31.3% of the patients with osteomyelitis had exposed bone under the lesion and 94.6% had a positive probe-to-bone test23. In another study, the prevalence of osteomyelitis was found to be 53%28. Aragón-Sánchez et al reported this rate as 72.4%26. In our series, the prevalence of osteomyelitis was 50%, while 67.6% of the patients without osteomyelitis exhibited bone marrow changes such as inflammation and oedema, which are used as criteria in the diagnosis of diabetic osteomyelitis20. These changes in the bone marrow may be indicative of nascent but not yet fully developed osteomyelitis.
Grayson et al emphasised that foot ulcers with palpable bone also have a high prevalence of osteomyelitis. In fact, they stated that special x-ray and radionuclide tests are not necessary to diagnose osteomyelitis in these cases3. However, they noted that although a positive probe-to-bone test result supports the diagnosis of diabetic foot osteomyelitis, the reliability of this method is not high and that MRI or bone biopsy is required in suspicious cases29. It should be noted that the probe-to-bone test is especially valuable in neurotrophic ulcers30.
Aragón-Sánchez et al reported that they could safely diagnose diabetic foot osteomyelitis in the presence of positive probe-to-bone test or plain x-ray, and especially when both are positive26. In another study, Álvaro-Afonso et al emphasised the importance of evaluating the probe-to-bone test and simple radiography together for the diagnosis of diabetic foot osteomyelitis13. The reliability of the probe-to-bone test depends on the clinician’s experience and location of the wound31,32.
There have been numerous studies on serological markers such as CRP and ESR in the diagnosis and follow-up of osteomyelitis associated with diabetes-related foot wounds, and some of these studies have reported cut-off values33–36. The results of meta-analyses indicate that ESR is the parameter with the most evidence for the diagnosis of diabetic osteomyelitis11,37,38.
Lymphocyte, ESR and CRP elevation may also be useful in identifying and diagnosing patients who are likely to develop complications. Serial blood tests can be an easy and useful tool for early detection and prevention of complications38. In several studies, an ESR of 70mm/h or higher was accepted as the critical value for osteomyelitis17,39. In a diabetic osteomyelitis series with a positive probe-to-bone test rate of 76.6%, an ESR cut-off value of 43mm/h or higher was reported to have 82.9% sensitivity and 70.5% specificity. The same study reported that when using the probe-to-bone test alone to diagnose diabetic foot osteomyelitis, the sensitivity was 76.6% and the specificity 93.2%, while both ESR >43mm/h and positive probe-to-bone test together had 98% specificity in the diagnosis of osteomyelitis11. In our study, the cut-off value for ESR was 79mm/h, and the risk of osteomyelitis was about three times higher below this threshold.
Although additional morbidities of the patients could not be determined in this retrospective series, ESR may decrease in conditions such as haemoglobinopathy, extreme leukocytosis, and the use of steroids or non-steroidal anti-inflammatory drugs. Rabjohn et al investigated the effects of comorbidities that influence ESR in patients with diabetic foot osteomyelitis and reported that ESR values were not significantly affected by comorbidities in diabetic osteomyelitis40. Therefore, we do not believe comorbidities contributed to low ESR values in our patients.
In general, the accepted cut-off value for ESR in diabetic osteomyelitis is 70mm/h17,39. The much lower value reported in the abovementioned study may be related to the high rate of bone exposure and may be specific to their patient group. Damage to the bone tissue and periosteum as a result of exposure of bone to the external environment may cause changes in the inflammatory response. Therefore, the difference in findings between studies may be related to the inflammatory changes caused by bone exposure.
There are varying results in the literature on the role of CRP in the diagnosis of osteomyelitis. While some studies have reported that CRP is the best marker in the diagnosis of osteomyelitis, some other publications have reported that it is a poor marker41–45.
Serum CRP levels have consistently been found to be significantly higher in infected diabetic foot ulcers than in non-infected diabetic foot ulcers, with levels increasing significantly in association with infection severity46–48. In our patient group, we found that CRP and WBC count had no statistical significance in the diagnosis of osteomyelitis. This may indicate that infection was of similar severity in both groups.
Although ESR is a simple marker with high sensitivity and specificity in the diagnosis of diabetic foot osteomyelitis37,43–48, CRP was found to be superior to ESR for evaluating early treatment response30. It has also been emphasised that both neutrophil count and CRP values are higher in patients with soft tissue infection without osteomyelitis compared to osteomyelitis patients44.
CRP, ESR and NLR were reported to have no diagnostic value in patients with diabetic foot infection, whereas the less costly and more accessible marker WBC count was found to be more useful during follow-up for the evaluation of diabetes-related foot wound severity18. However, in another study, it was emphasised that leukocytosis is a poor marker of diabetic foot osteomyelitis because WBC count is normal in 50% of patients with osteomyelitis and high WBC count may be associated with soft tissue or systemic infection47. However, Eren et al stated that ESR, WBC and CRP values showed a positive correlation in the diagnosis of diabetic osteomyelitis42.
In our study, WBC count showed no statistically significant difference according to the diagnosis of osteomyelitis in diabetes-related foot wound patients with bone exposure and was not found to be a significant marker of osteomyelitis in our series.
There are many studies with conflicting results regarding the effect of diabetes mellitus on the immune system. Both in vivo and in vitro studies have demonstrated that chronic hyperglycaemia causes oxidative burst and anomalies in lymphocyte subpopulations and immunoglobulins49–56. In a study with diabetic ulcer patients and diabetic controls, no significant difference was detected between the two groups in terms of total leukocyte count. No difference was detected between the two groups in markers of innate immunity other than low absolute natural killer cell counts. However, decreases in the absolute numbers of nearly all subpopulations of both B and T lymphocytes and changes in humoral immunity have been observed in patients with an infected diabetic foot ulcer. The main findings differentiating patients with a chronic infected diabetic foot ulcer from diabetic controls without a diabetic ulcer are the significant decreases in percentages of lymphocyte and absolute lymphocyte count56. Moreover, no significant difference was detected in patients with a diabetic foot infection and chronic bacterial infection in terms of active phagocytic polymorphonuclear leukocyte count and initial phagocytic activity or the humoral component of non-specific immunity, and it was concluded that the non-specific immune response is slightly altered in patients with a diabetic foot infection and a chronic bacterial infection57.
In a study investigating the effect of osteomyelitis on immune response, there was no statistically significant difference between the osteomyelitis group and healthy control group in terms of lymphocyte count58. In a study on patients with a diabetic foot infection and healthy controls, it was found that levels of CD3+ T cells and inhibitory/cytotoxic CD8+ T cells were significantly higher, while there was no difference in terms of serum monocyte and lymphocyte counts, CD4+ helper T cells, or CD4+/CD8+ T cell ratio59.
In our study, diabetic osteomyelitis patients with positive probe-to-bone test results had higher lymphocyte percentage but lower neutrophil percentage compared to the group of patients with positive probe-to-bone test but without osteomyelitis, resulting in a lower NLR. Our patient population consisted entirely of patients with diabetes-related foot wounds, and although lymphocyte ratios and counts may be lower compared to patients without diabetes-related foot wounds, these findings indicate that diabetic patients with exposed bone respond to osteomyelitis with an increased lymphocyte percentage. In patients with a lymphocyte percentage of 12.3% or higher and those with a neutrophil percentage of 78% or lower, the risk of osteomyelitis was found to increase approximately threefold. However, our results contradict a previous report that increased NLR may be an indicator of osteomyelitis in patients with diabetes-related foot wounds19. Moreover, many studies have emphasised that high NLR is an independent risk factor for progression to amputation19,60,61. The physiological response of circulating leukocytes to stress manifests as an increase in neutrophil count and decrease in lymphocyte count. High NLR results in neutrophilic hyperactivity, which leads to endothelial damage and dysfunction62. NLR may be an indicator of increased risk of microvascular complications of diabetes63, and the relationship between high NLR and amputation risk may be associated with this vascular mechanism independent of osteomyelitis, which may be an additional factor that contributes to this relationship.
All of the patients in our series had exposed bones, suggesting that the immune response was caused by different factors such as bone desiccation and necrosis, which led to the differences in serological marker values. Moreover, while high platelet/lymphocyte ratio was previous found to be associated with osteomyelitis in diabetic foot infections64, no statistically significant difference in this ratio was observed between the groups with and without osteomyelitis in our study. As no other studies on this specific patient group could be found in the literature, we based our comparison on studies of general diabetes-related foot wounds.
Using a combination of diagnostic methods is recommended to increase the reliability of diagnoses1. In our study, we evaluated parameters that were significant in terms of osteomyelitis in pairs (ESR ≥79mm/h and NLR ≥6.02; ESR ≤79mm/h and lymphocyte percentage ≥12.3%; and ESR ≤79mm/h and neutrophil percentage ≤78%) and found that the sensitivity increased to 86.8% in patients where both were positive.
Looking at the microorganisms isolated in diabetic foot infections, generally aerobic gram-positive cocci are isolated65,66. However, deep or chronic wounds often have both aerobic gram-negative and obligatory anaerobic bacteria together67. In our series, gram-negative bacteria accounted for 54.3% and gram-positive bacteria for 44.4% of wound culture isolates. No microorganisms were observed in 19.9% of the cultures. Karthik et al reported in their study that 46% of bone culture samples were sterile and 58% yielded gram-negative organisms68.
Machado et al divided patients with diabetes-related foot wounds into two time periods, 2010–2011 and 2016–201769. They reported that, overall, bacterial cultures yielded 59.6% gram-positive pathogens and 40.4% gram-negative pathogens, with 18.5% of cultures being negative. Monomicrobial infections were seen in 50.0% of cases in the earlier group, while this rate fell to 35.5% in the later group. They noted an increase in the median number of pathogens isolated per sample over the years69.
In the present study, 36% of cultures yielded a single pathogen and 34.5% yielded two bacterial pathogens. Overall, polymicrobial infections were more common in our series. The higher rate of gram-negative bacteria in our series and the higher incidence of polymicrobial infections may be related to the deep infections associated with bone exposure to the external environment.
Aragón-Sánchez et al reported that S. aureus was the most common bacterium, detected in 47.5% of patients with osteomyelitis, and Staphylococcus epidermidis was the second most common, at 10%. A single organism was isolated in 64% of cultures23. Machado et al also reported S. aureus as the most common gram-positive agent in their series, while the most common gram-negative agent was Enterobacteriaceae69.
Karthik et al reported that the most common organisms detected in bone culture were Staphylococcus spp., followed by Pseudomonas, Klebsiella spp., E. coli,and Enterococcus spp., respectively68. In our study, S. aureus was isolated most frequently, at a rate of 15.2%. However, E. coli was the second most frequent isolate (14.6%). The fact that the second most common bacterium was gram-negative and was similar in frequency to S. aureus may be due to the fact that our case series included patients with deeper tissue infections.
In some studies examining the relationship between age and the appearance of osteomyelitis in diabetes-related foot wounds, a statistically significant relationship was not found11,43. However, another study showed that lower mean age was significantly associated with the presence of osteomyelitis, with patients aged over 70 representing 33.3% of the osteomyelitis group and 54.4% of the group without osteomyelitis8. This is consistent with our finding of a lower mean age in our osteomyelitis patients.
Conclusions
The patient group examined in this study consisted of those with contact between bone tissue and the external environment as a result of a diabetes-related foot ulcer. We believe that this patient group is distinct from other diabetes-related foot ulcer groups. The results of this study demonstrated differences in the epidemiological and laboratory findings and calculated risk values in this patient group compared to the group with general diabetes-related foot wounds and osteomyelitis. We believe that the immune response caused by the exposure of bone to the environment is different than the immune response caused by soft tissue infection in classical diabetic foot. Experimental and clinical studies are needed to elucidate the underlying histopathogenesis.
The major limitation of this study is that it was retrospective. Another limitation is not knowing the patients’ duration of bone exposure. Longer exposure may increase infection and necrosis and might alter inflammatory responses. In addition, the study included both patients with bone exposure and those with positive probe-to-bone test. The additional contact with the external environment and greater loss of soft tissue in wounds with bone exposure compared to those with positive probe-to-bone test will cause more necrosis due to fluid loss. Moreover, as mentioned earlier, bone biopsies were not cultured separately and the culture results are from bone and soft tissue together. However, considering the lack of a similar study, it may contribute to the literature.
Acknowledgements
We thank Jacqueline Renee Gutenkunst and Oguz Gönen for the English translation and editing, and Emire Bor and Sanem Nemnezi Karaca for statistical analyses.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
Approval was obtained from Cumhuriyet University Ethics Committee (decision no: 2020-05/24, date: 20 May 2020).
Funding
The authors received no funding for this study.
Author contribution
OA took part in data collection and/or processing processes. Other processes were carried out by HD.
Author(s)
Handan Derebaşınlıoğlu*1, Onur Aksoy2
1Cumhuriyet University Medical Faculty, Plastic Reconstructive and Aesthetic Surgery Department, 58140 Sivas, Turkey
2Istanbul Prof.Dr. Cemil Taşçioğlu City Hospital, 34384 Şişli, İstanbul, Turkey
*Corresponding author Email handanderebasinlioglu@gmail.com
References
- Lipsky BA. Medical treatment of diabetic foot infections. Clin Infect Dis 2004;39:S104–14. doi:10.1086/383271
- Lázaro-Martínez JL, Tardáguila-García A, García-Klepzig JL. Diagnostic and therapeutic update on diabetic foot osteomyelitis. Endocrinol Diabetes Nutr 2017;64:100–108.
- Grayson ML, Gibbons GW, Balogh K, et al. Probing to bone in infected pedal ulcers: a clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995 Mar 1;273(9):721–3. doi:10.1001/jama.1995.03520330051036
- Hartemann-Heurtier A, Senneville E. Diabetic foot osteomyelitis. Diabetes Metab 2008 Apr;34(2):87–95. doi:10.4239/wjd.v8.i4.135
- Lavery LA, Armstrong DG, Wunderlich RP, et al. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. doi:10.2337/dc05-2425
- Ha Van G, Siney H, Hartmann-Heurtier A, et al. Nonremovable, windowed, fiberglass cast boot in the treatment of diabetic plantar ulcers: efficacy, safety, and compliance. Diabetes Care 2003;26:2848–52. doi:10.2337/diacare.26.10.2848
- Walker SC, Helm PA, Pullium G. Total contact casting and chronic diabetic neuropathic foot ulcerations: healing rates by wound location. Arch Phys Med Rehabil 1987;68:217–21.
- Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis 1997;25:1318–1326. PMID:9431370.
- Lavery LA, Peters EJ, Armstrong DG, et al. Risk factors for developing osteomyelitis in patients with diabetes-related foot wounds. Diabetes Res Clin Pract 2009 Mar;83(3):347–52. doi:10.1016/j.diabres.2008.11.030
- Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care 2007;30:270–274. PMID:17259493. doi:10.2337/ dc06-1572
- Xu J, Cheng F, Li Y, Zhang J, Feng S, Wang P. Erythrocyte sedimentation rate combined with the probe-to-bone test for fast and early diagnosis of diabetic foot osteomyelitis. Int J Low Extrem Wounds 2021;20(3):227–231. doi:10.1177/1534734620923278
- Senneville E. Editorial commentary: probe-to-bone test for detecting diabetic foot osteomyelitis: rapid, safe, and accurate-but for which patients? Clin Infect Dis 2016;63:949–50.
- Álvaro-Afonso FJ, Lázaro-Martínez JL, Aragón-Sánchez, et al. Inter-observer reproducibility of diagnosis of diabetic foot osteomyelitis based on a combination of probe-to-bone test and simple radiography. Diabetes Res Clin Pract 2014;105:e3–5.
- Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–173. PMID:22619242. doi:10.1093/cid/cis346.
- Lipsky BA, Senneville É, Abbas ZG, et al. International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020 Mar;36 Suppl 1:e3280. doi:10.1002/dmrr.3280.
- Llewellyn A, Kraft J, Holton C, Harden M, Simmonds M. Imaging for detection of osteomyelitis in people with diabetic foot ulcers: a systematic review and meta-analysis. Eur J Radiol 2020;131:109215. doi:10.1016/j.ejrad.2020.109215
- Kaleta JL, Fleischli JW, Reilly CH. The diagnosis of osteomyelitis in diabetes using erythrocyte sedimentation rate: a pilot study. J Am Podiatr Med Assoc 2001;91:445–50. doi:10.7547/87507315-91-9-445
- Ong TE, Farran S, Salloum M, et al. The role of inflammatory markers: WBC, CRP, ESR and Neutrophilto-Lymphocyte Ratio (NLR) in the diagnosis and management of diabetic foot infections. Open Forum Infect Dis 2015;2:1526. doi:10.1093/ofid/ofv133.1079
- Yapıcı O, Berk H, Öztoprak N, et al. Can ratio of neutrophil-to lymphocyte count and erythrocyte sedimentation rate in diabetic foot infection predict osteomyelitis and/or amputation? Hematol Rep 2017;9(1):6981. doi:10.4081/hr.2017.6981.
- Meyr AJ, Singh S, Zhang X, Khilko N, Mukherjee A, Sheridan MJ, Khurana JS. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Sur Nov–Dec 2011;50(6):663–7. doi:10.1053/j.jfas.2011.08.005
- Cecilia-Matilla A, Lázaro-Martínez JL, Aragón-Sánchez J, et al. Histopathologic characteristics of bone infection complicating foot ulcers in diabetic patients. J Am Podiatr Med Assoc 2013;103:24–31.
- Tardáguila-García A, Sanz-Corbalán I, García-Morales E, García-Álvarez Y, Molines-Barroso RJ, Lázaro-Martínez JL. Diagnostic accuracy of bone culture versus biopsy in diabetic foot osteomyelitis. Adv Skin Wound Care 2021;34(4):204–208. doi:10.1097/01.ASW.0000734376.32571.20
- Aragón-Sánchez FJ, Cabrera-Galván JJ, Quintana-Marrero Y, et al. Outcomes of surgical treatment of diabetic foot osteomyelitis: a series of 185 patients with histopathological confirmation of bone involvement. Diabetologia 2008 Nov;51(11):1962–70. doi:10.1007/s00125-008-1131-8
- White LM, Schweitzer ME, Deely DM, et al. Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiol 1995 Dec;197(3):840–2. doi:10.1148/radiology.197.3.7480765
- Wu JS, Gorbachova T, Morrison WB, et al. Imaging-guided bone biopsy of osteomyelitis: are there factors associated with positive or negative cultures? AJR Am J Roentgenol 2007 Jun;188(6):1529–34. doi:10.2214/AJR.06.1286.
- Aragón-Sánchez J, Lipsky BA, Lázaro-Martínez JL. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med 2011;28(2):191–4.
- Lam K, van Asten SA, Nguyen T, La Fontaine J, Lavery LA. Diagnostic accuracy of probe to bone to detect osteomyelitis in the diabetic foot: systematic review. Clin Infect Dis 2016;63:944–948.
- Wrobel JS, Connolly JE. Making the diagnosis of osteomyelitis: the role of prevalence. J Am Podiatr Med Assoc 1998;88:337–343. doi:10.7547/87507315-88-7-337
- Mutluoglu M, Uzun G, Sildiroglu O, et al. Performance of the probe-to-bone test in a population suspected of having osteomyelitis of the foot in diabetes. J Am Podiatr Med Assoc 2012;102(5):369–73. doi:10.7547/1020369
- Morales Lozano RM, González Fernández ML, Martinez Hernández D, et al. Validating the probe-to-bone test and other tests for diagnosing chronic osteomyelitis in the diabetic foot. Diabetes Care 2010;33(10):2140–5. doi:10.2337/dc09-2309.
- García Morales E, Lázaro-Martínez JL, Aragón-Sánchez FJ, et al. Inter-observer reproducibility of probing to bone in the diagnosis of diabetic foot osteomyelitis. Diabetes Med 2011;28:1238–40.
- Álvaro-Afonso FJ, Lázaro-Martínez JL, Aragón-Sánchez FJ, et al. Does the location of the ulcer affect the interpretation of the probe-to-bone test in the diagnosis of osteomyelitis in diabetic foot ulcers? Diabet Med 2014;31(1):112–3.
- Soleimani Z, Amighi F, Vakili Z, Momen-Heravi M, Moravveji SA. Diagnostic value of procalcitonin, erythrocyte sedimentation rate (ESR), quantitative C-reactive protein (CRP) and clinical findings associated with osteomyelitis in patients with diabetic foot. Hum Antibodies 2021;29(2):115–121. doi:10.3233/HAB-21043
- Durmaz B, Yilmaz S, Derebasinlioglu H. The role of inflammatory markers in the diagnosis and follow-up of diabetic foot osteomyelitis. Ann Med Res 2020;27(4):1077–81. doi:10.5455/annalsmedres.2019.11.694
- Malabu UH, Al-Rubeaan KA, Al-Derewish M. Diabetic foot osteomyelitis: usefulness of erythrocyte sedimentation rate in its diagnosis. West Afr J Med 2007;26:113–6.
- Mutluoğlu M, Uzun G, İpcioğlu OM, et al. Can procalcitonin predict bone infection in people with diabetes with infected foot ulcers? A pilot study. Diabetes Res Clin Pract 2011;94:53–6. doi:10.1016/j.diabres.2011.05.023
- van Asten SAV, Geradus Peters EJ, Xi Y, Lavery LA. The role of biomarkers to diagnose diabetic foot osteomyelitis: a meta-analysis. Curr Diabetes Rev 2016;12(4):396–402. doi:10.2174/1573399811666150713104401. PMID:26166314.
- Tardáguila-García A, García Álvarez Y, García-Morales E, et al. Utility of blood parameters to detect complications during long-term follow-up in patients with diabetic foot osteomyelitis. J Clin Med 2020 Nov 22;9(11):3768. doi:10.3390/jcm9113768.PMID:33266483
- Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit 2009;15:307–12.
- Rabjohn L, Roberts K, Troiano M, et al. Diagnostic and prognostic value of erythrocyte sedimentation rate in contiguous osteomyelitis of the foot and ankle. J Foot Ankle Surg 2007 Jul-Aug;46(4):230–7. doi:10.1053/j.jfas.2007.03.004. PMID:17586434.
- Sharma H, Sharma S, Krishnan A, Yuan D, Vangaveti VN, Malabu UH, Haleagrahara N. The efficacy of inflammatory markers in diagnosing infected diabetic foot ulcers and diabetic foot osteomyelitis: systematic review and meta-analysis. PLoS One 2022 Apr 27;17(4):e0267412. doi:10.1371/journal.pone.0267412. PMID:35476639. PMCID:PMC9045669
- Eren MA, Güneş AE, Ceylan MR, et al. Pilot study of the diagnostic value of CRP:albumin ratio for osteomyelitis in patients with diabetic foot ulcers. J Wound Care 2022;31(Sup3):S25–28. doi:10.12968/jowc.2022.31.Sup3.S25
- Lavery LA, Ahn J, Ryan EC, et al. What are the optimal cutoff values for ESR and CRP to diagnose osteomyelitis in patients with diabetes-related foot infections? Clin Orthop Relat Res 2019 Jul;477(7):1594–1602. doi:10.1097/CORR.0000000000000718
- Eneroth M, Larsson J, Apelqvist J. Deep foot infections in patients with diabetes and foot ulcer: an entity with different characteristics, treatments, and prognosis. J Diabetes Complications 1999;13:254–63. doi:10.1016/S1056-8727(99)00065-3
- Moallemi SK, Niroomand M, Tadayon N, Forouzanfar MM, Fatemi A. Diagnostic value of erythrocyte sedimentation rate and C-reactive protein in detecting diabetic foot osteomyelitis: a cross-sectional study. Arch Acad Emerg Med 2020 Sep 8;8(1):e71. PMID:33134967. PMCID:PMC7587984.
- Uzun G, Solmazgul E, Curuksulu H, et al. Procalcitonin as a diagnostic aid in diabetic foot infections. Tohoku J Exp Med 2007;213:305–12.
- Park JH, Suh DH, Kim HJ, et al. Role of procalcitonin in infected diabetic foot ulcer. Diabetes Res Clin Pract 2017;128:51–7.
- Jeandrot A, Richard JL, Combescure C, et al. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia 2008;51:347–52.
- Armstrong DG, Lavery LA, Sariaya M, Ashry H. Leukocytosis is a poor indicator of acute osteomyelitis of the foot in diabetes mellitus. J Foot Ankle Surg 1996;35:280–3. doi:10.1016/s1067-2516(96)80075
- Serlenga E, Garofalo AR, De Pergola G, et al. Polymorphonuclear cell-mediated phagocytosis and superoxide anion release in insulin-dependent diabetes mellitus. Cytobios 1993;74(298–299):189–95.
- Wilson RM, Reeves WG. Neutrophil phagocytosis and killing in insulin-dependent diabetes. Clin Exp Immunol 1986;63(2):478–84.
- Daoud AK, Tayyar MA, Fouda IM et al. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol 2009;6(1):36–41. doi:10.1080/15476910802604564.
- Nielson CP, Hindson DA. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes 1989;38(8):1031–5. doi:10.2337/diab.38.8.1031.
- Salman F, Erten G, Unal M et al. Effect of acute maximal exercise on lymphocyte subgroups in type 1 diabetes. Acta Physiol Hung 2008;95(1):77–86. doi:10.1556/APhysiol.95.2008.1.5.
- Mohammed N, Tang L, Jahangiri A, et al. Elevated IgG levels against specific bacterial antigens in obese patients with diabetes and in mice with diet-induced obesity and glucose intolerance. Metabolism 2012;61(9):1211–4. doi:10.1016/j.metabol.2012.02.007.
- Fejfarová V, Jirkovská A, Dubský M, et al. An alteration of lymphocytes subpopulations and immunoglobulins levels in patients with diabetic foot ulcers. J Diabetes Res 2016;2016:2356870. doi:10.1155/2016/2356870.
- Jirkovská A, Fejfarová V, Hosová J, et al. Non-specific immune responses in patients with chronic diabetic foot syndrome and chronic bacterial infection. Vnitr Lek 2002 Feb;48(2):142–6.
- Beard LJ, Ferris L, Ferrante A. Immunoglobulin G subclasses and lymphocyte subpopulations and function in osteomyelitis and septic arthritis. Acta Paediatr Scand Jun–Jul 1990;79(6–7):599–604. doi:10.1111/j.1651-2227.1990.tb11523.x.
- Fejfarová V, Hosová J, Stríz I, et al. Analysis of the inflammation reaction and selected indicators of immunity in patients with an infected diabetic ulcer. Cas Lek Cesk 2002;141(15):483–6.
- Arıcan G, Kahraman HÇ, Özmeriç A, et al. Monitoring the prognosis of diabetic foot ulcers: predictive value of neutrophil-to-lymphocyte ratio and red blood cell distribution width. Int J Low Extrem Wounds 2020 Dec;19(4):369–376. doi:10.1177/1534734620904819
- Luo H, Yuan D, Yang H, et al. Posttreatment neutrophil-lymphocyte ratio independently predicts amputation in critical limb ischemia without operation. Clinics (Sao Paulo) 2015;70(4):273–7. doi:10.6061/clinics/2015(04)09.
- Ünlü M, Arslan Z. The relation between neutrophil–lymphocyte ratio and endothelial dysfunction. Angiology 2015;66(7):694. doi:10.1177/0003319715584879
- Sayiner ZA, Eraydın A, Atakur S, et al. The relationship between microvascular complications in type 2 diabetes with mean platelet volume, red blood cell distribution width, neutrophil lymphocyte ratio. Turkiye Klinikleri J Inter Med 2017;2:113–117 doi:10.5336/intermed.2017-57111
- Demirdal T, Sen P. The significance of neutrophil-lymphocyte ratio, platelet lymphocyte ratio and lymphocyte/monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res Clin Pract 2018;144:118–125. doi:10.1016/j.diabres.2018.08.009.
- Hatipoglu M, Mutluoglu M, Uzun G, Karabacak E, Turhan V, Lipsky BA. The microbiologic profile of diabetic foot infections in Turkey: a 20-year systematic review. Diabetic foot infections in Turkey. Eur J Clin Microbiol Infect Dis 2014;33(6):871e8. doi:10.1007/s10096-014-2047-5
- Nikoloudi M, Eleftheriadou I, Tentolouris A, et al. Diabetic foot infections: update on management. Curr Infect Dis Rep 2018 Aug 1;20(10):40. doi:10.1007/s11908-018-0645-6.
- Xie X, Bao Y, Ni L, Liu D, Niu S, Lin H, et al. Bacterial profile and antibiotic resistance in patients with diabetic foot ulcer in Guangzhou, Southern China: focus on the differences among different Wagner’s grades, IDSA/IWGDF grades, and ulcer types. Int J Endocrinol 2017;2017:8694903. doi:10.1155/2017/8694903
- Karthik S, Babu L, Joseph M, Bhatt A, Babu T. Microbiology of diabetic foot osteomyelitis: is it geographically variable? Foot 2022;52:101878. doi:10.1016/j.foot.2021.101878
- Machado C, Teixeira S, Fonseca L, et al. Evolutionary trends in bacteria isolated from moderate and severe diabetic foot infections in a Portuguese tertiary center. Diabetes Metab Syndr May-Jun 2020;14(3):205–209. doi:10.1016/j.dsx.2020.02.010



