Volume 31 Number 1
What is the evidence that there is antimicrobial resistance associated with the use of topical antimicrobial preparations?
Joanna Blackburn, Karen Ousey, Declan Patton, Zena Moore, Pinar Avsar
Keywords Wound care, wound healing, Infection, AMR, topical antimicrobials
For referencing Blackburn J et al. What is the evidence that there is antimicrobial resistance associated with the use of topical antimicrobial preparations? Wound Practice and Research 2023; 31(1):40-48.
DOI
https://doi.org/10.33235/wpr.31.1.40-48
Submitted 21 October 2022
Accepted 20 December 2022
Abstract
Aims This review aimed to examine the effect of using topical antimicrobial preparations on antimicrobial resistance (AMR) by critically evaluating the currently available evidence.
Method Using systematic review methodology, we considered original research studies employing a prospective design and written in English. The search was conducted in July 2022 using Ovid MEDLINE, Ovid EMBASE Medline and CINAHL databases. Data were extracted using a pre-designed extraction tool and all included studies were quality appraised using the Evidence Based Literature (EBL) appraisal checklist.
Results A total of 375 studies were identified, with 25 meeting the inclusion criteria. Studies were conducted between 1998 and 2021. Half of the studies included explored the use of silver in dressings as an antimicrobial. Two studies were performed in a hospital setting, one study employed an in vitro and in vivo design, with all remaining studies employing an in vitro approach.
Conclusion There was limited evidence of the effect of topical antimicrobial preparations on AMR, with most included studies exploring the effectiveness of topical antimicrobials on infection and wound healing. AMR remains an important issue for exploration and understanding to clearly determine whether topical antimicrobials contribute to AMR.
Introduction
Antimicrobial resistance (AMR) refers to a process in which microorganisms undertake genetic adaptations and become resistant to treatment as a consequence of an overexposure to antimicrobial medications1. AMR infections, often transmitted through poor sanitation and inadequate infection control, are a major global problem to population health1. Further, AMR infections are responsible for approximately 700,000 deaths each year2, with a trajectory to increase to an estimated 10 million deaths each year by 20503.
Managing the problem of AMR
Such is the magnitude of AMR that several national, international and global collaborative efforts have been established to manage and limit the future impact. The Tackling Antimicrobial Resistance 2019–2024 action plan4 has a national strategic objective to tackle AMR and is focused on reducing the need for antibiotics, optimising the use of antimicrobials, reducing the number of healthcare-associated Gram-negative bloodstream infections, and reducing the number of specific drug-resistant infections in people by 10% by 2025. The Global Action Plan on AMR (GAP)1 has a strategic approach on the appropriate use of antibiotics (including antibiotics and antifungals) in healthcare, and the ambition to reduce antimicrobial use in the UK by 15% by 2024 represents a key focus and challenge in healthcare4.
In Australia, the National Antimicrobial Resistance Strategy – 2020 and beyond presents a 20-year national vision for managing the problem of AMR5. A core focus of the strategy is encompassing a holistic approach incorporating how AMR can be managed in humans, animals, food and the environment. Indeed, Australia’s fourth report on antimicrobial use6 projects that AMR will be responsible for over 10,000 deaths in Australia between 2015 and 2050. Furthermore, over 40% of people in Australia were prescribed an antimicrobial in 2019, with over 80% of people with acute bronchitis or acute sinusitis being prescribed antimicrobials inappropriately6.
The problem of AMR can be demonstrated through evidence showing that a significant proportion of prescribed primary care antibiotics are unnecessary7, perhaps driven by uncertainties around appropriate use8. Despite a recent fall in antibiotic-resistant bloodstream infections between 2019–2020 (from 65,583 in 2019, to 55,384), the UK Health Security Agency9 report states that it is likely to be a reflection of behavioural societal changes, such as social isolation and increased hand hygiene as a consequence of the COVID‑19 pandemic, rather than a reduction in infections per se, and infections still remain higher than 6 years previous. The World Health Organization (WHO)10 further emphasise the financial burden associated with AMR and the urgent need to change antibiotic usage in order to prevent future treatments of infection and diseases being ineffective.
Topical antimicrobials
Antimicrobials include antibiotics, antiseptics and disinfectants and are substances that act to reduce or stop the development of microorganisms11. Topical antimicrobials are those substances that act directly on the skin to kill a microbe and are one of the most commonly prescribed antimicrobial treatments. Antimicrobials provide many advantages over other forms of antibiotic treatments, including ease of application, increased adherence to treatment, and reduced likelihood of side effects12. However, the evidence regarding the effectiveness in infection prevention of topical antimicrobial preparations has been inconclusive, and there is growing concern around AMR associated with topical preparations for infection prevention. For example, in a systematic review and meta-analysis of the available evidence on the prevention of uncomplicated wound infections by prophylactic topical antibiotics, Tong et al13 concluded that topical antibiotics were only slightly more effective in reducing wound infections after surgical procedures than antiseptics – defined by the International Wound Infection Institute (IWII)14 as “Non-selective agents that are applied topically in order to inhibit multiplication of or kill microorganisms. Prophylactic topical antibiotics may have a toxic effect on human cells. Development of resistance to antiseptics is uncommon”. The authors further suggest that the latter should be encouraged as an alternative to topical antibiotics in preventing infection.
Another systematic review and meta-analysis examining the efficacy of topical antibiotics in preventing postsurgical wound infections in a dermatology outpatient setting found no difference between infection rates when using either topical antibiotics, or petrolatum or paraffin15. In addition, Heal et al16 reported limited evidence that topical antibiotics prevent surgical site infection (SSI) compared to no antibiotic treatment, equating to 20 fewer SSIs per 1000 patients treated.
Evidence from comparative studies on topical antimicrobials have also reported mixed findings; in a study comparing the wound healing process when applying either a protectant Aquaphor Healing Ointment and Polysporin first-aid ointment after removal of Dermatosis papulosa nigra (DPN) lesions, Taylor et al17 found no difference in wound healing rates and suggested that topical antibiotics are not essential for effective wound healing of such wounds.
The evidence surrounding the effectiveness and impact of topical antimicrobial wound dressings is lacking, and several studies have focused on the existence of antimicrobial resistant bacteria in silver wound dressings. For example, Panáček et al18 found evidence that Gram-negative bacteria can become resistant to silver nanoparticles with repeated exposure. Hosny et al19 found the existence of silver-resistant bacteria in a sample of 150 clinical isolates from burns and wounds, suggesting that effective wound healing does not need to be reliant on topical antimicrobial preparations.
Conversely, a systematic review of the literature found no evidence for the presence of bacterial resistance of silver-based wound dressings20. Wang et al21 found no significant evidence to suggest that silver dressings promote wound healing and limited infection in chronic wounds any more than other types of wound dressings. Other evidence focusing on topical antimicrobials involving polyherbal formulations have demonstrated the positive impact on wound healing rates in healing diabetic wounds after repeated application and follow-up22. Mandrika et al23 also found that the anti-inflammatory plant extracts consisting of 13 herbal ingredients with copper sulfate fortified in oil were most active against clinical strains of multidrug resistant bacteria, and suggested the evidence provides support for the use of various herbs in the use of polyherbal formulations for non-healing wounds. In a randomised controlled trial (RCT) of the effectiveness of honey in treating venous leg ulceration (VLU), Jull et al24 found that at 12 weeks of being treated with either a honey-impregnated wound dressing (n=187) or standard care (n=181), there was no significant difference between groups.
Despite the problem of AMR, the currently available evidence pertaining the use of topical antimicrobial preparations on AMR is mixed. This systematic review with meta-analysis aimed to examine the effect of using topical antimicrobial preparations on AMR.
Methods
Criteria for considering studies for this review
This systematic review included original research studies employing a prospective design, written in English, which assessed the effect of using topical antimicrobial preparations on AMR. We excluded studies of a retrospective design, conference papers, opinion papers and qualitative methodology. There were no date of publication or study setting restrictions applied.
Outcomes
The primary outcome of interest was the incidence of AMR as a result of using topical antimicrobial preparations.
Electronic searches
The following electronic databases were searched to identify relevant literature:
- Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) (latest issue).
- Ovid MEDLINE (1946 to April 2022).
- Ovid MEDLINE (In-Process & Other Non-Indexed Citations) (latest issue).
- Ovid EMBASE (1974 to April 2022).
- EBSCO CINAHL Plus (1937 to search April 2022).
- PubMed.
- Scopus.
To identify further published, unpublished and ongoing studies, this systematic review:
- Scanned reference lists of all identified studies and reviews.
- Searched grey literature using OpenGrey (www.opengrey.eu).
- Searched research reports and dissertations.
The keywords used in the search included:
- Antimicrobial resistance OR
- AMR OR
- Topical antimicrobial OR
- Topical antimicrobial preparations OR
- Drug resistance
Study selection
The article titles were assessed by two authors (JB, PA) independently, and their abstracts (when available) were screened for their eligibility according to the criteria for considering studies for this review. The full-text version of potentially relevant studies was obtained and two authors independently screened these against the inclusion criteria. Where discrepancies were identified, a consensus between the two authors was reached through discussion.
Data extraction
Data from the retrieved articles were extracted and inserted into a data extraction table using the following headings – author, date of the study, setting and sample, intervention and results.
Data analysis and quality appraisal
Any meta-analysis was considered inappropriate due to the variation in study design and heterogeneity in the sample populations. Accordingly, the data were narratively summarised giving an overview of geographical location, study settings, sample sizes and results. This was followed by a structured narrative synthesis of each of the included studies based on the outcome measures. Each was then quality appraised using the Evidence Based Literature (EBL) appraisal checklist. This quality appraisal tool assesses the validity, applicability and appropriateness of each study based on four main steps of the research process: population; data collection; study design; results. According to this checklist, if the overall validity of the study (Yes/Total) is ≥75%, or (No + Unclear)/Total) is ≤25% then the study is considered valid.
Results
Overview of all included studies
Figure 1 depicts a PRISMA flow diagram of the results following the search and the subsequent removal of studies prior to synthesis25. Following reviews of a total of 375 hits, 342 were excluded. Following extraction of full texts, four of the remaining articles were rejected for not having relevant outcomes (Table 1). Finally, 25 articles were deemed to meet the inclusion criteria (Table 2).
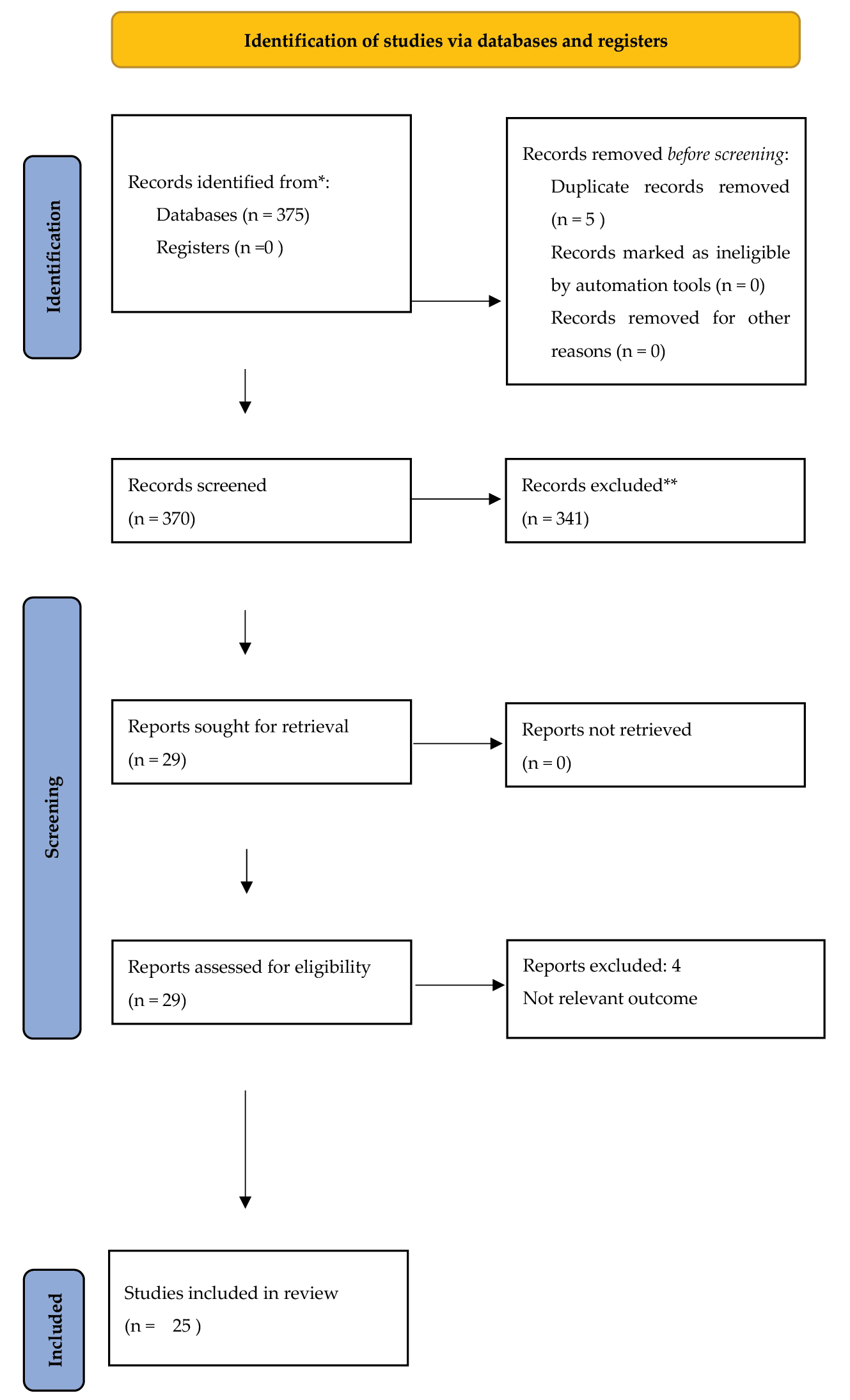
Figure 1. PRISMA 2020 flow diagram for study selection25
Table 1. Excluded studies with reasons for exclusion
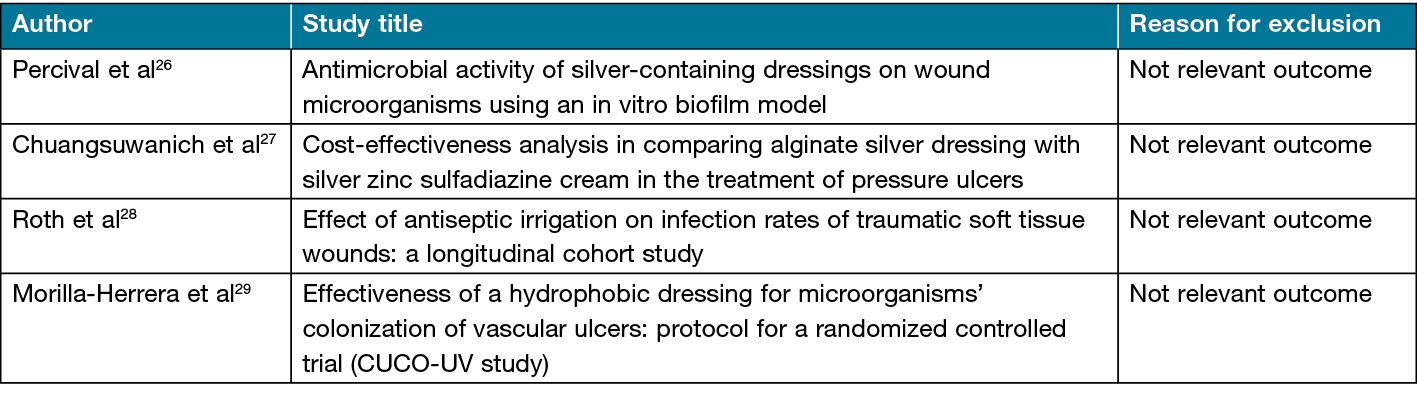
Table 2. Characteristics of included studies
Characteristics of studies
Geographical setting: The geographical location of the studies varied between the UK30–35,50,51, the USA36–44, Algeria45, Eygpt19, Slovakia46, Iran47, Sweden48, India23, the Czech Republic18 and Australia49.
Study settings: Two studies32,41 were performed in a hospital setting. All other studies were laboratory-based.
Participants and sample size: Two studies used human participants in their design. Jørgensen et al41 had a sample size of 129 patients and Michaels et al32 had a sample of 213 patients.
Study design: Two studies32,41 were performed in a hospital setting and utilised an RCT study design. One study employed an in vitro and in vivo design44, with all remaining studies employing an in vitro approach
Primary outcome
Most of the included studies (50%) explored the use of silver in dressings as an antimicrobial18,19,30,33,36,37,42–44,49,50,51. From the papers explored, the use of silver as an antimicrobial were all in vitro. One study used both in vivo and in vitro44.
One study found that there was not a correlate with the antibacterial activity (Parsons et al30). Exploration of the in vitro efficacy of previously identified silver-resistant clinical bacteria (Klebsiella pneumoniae and Enterobacter cloacae) against a variety of commercially available silver-based wound dressings was further investigated in one study. The authors found both silver-resistant strains were largely unaffected and exhibited phenotypic resistance, even when exposed to the high silver concentrations normally found in commercially available wound dressings. In another study, Castellano et al43 reported that all silver dressings and topical antimicrobials displayed antimicrobial activity, and silver-containing dressings with the highest concentrations of silver exhibited the strongest bacterial inhibitive properties. In vitro tissue contact and antimicrobial activity was shown with a silver-containing Hydrofiber® dressing (HF-Ag) over a 48-hour contact period in the Bowler et al50 study. In contrast, silver-containing foam dressings tested demonstrated areas of non-conformability which were associated with reduced antimicrobial activity. These in vitro studies confirm that both dressing conformability and silver availability to bacteria at the wound surface are critical to the optimum functioning of silver-containing dressings30,43,50.
Loh et al33 explored the prevalence of silver-resistance (sil) genes in methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococci (MR-CNS) isolated from wounds and nasal cavities of humans and animals, and also to determine the susceptibility of sil-positive and sil-negative MRSA isolates to a silver-containing Hydrofiber (SCH) wound dressing on planktonic silE-positive and silE-negative MRSA. Results confirmed that the SCH dressing was effective in killing all MRSA strains with and without the silE gene. In the Wright et al42 study, silver was demonstrated to be effective at killing the antibiotic-resistant strains tested. The silver-coated dressing was particularly rapid at killing the tested bacteria and was effective against a broader range of bacteria.
Two of the included studies explored the use of iodine. Malone et al49 reported that the ability of cadexomer iodine to reduce the microbial load of chronic non-healing diabetic foot ulcers (DFUs) was complicated by biofilm. Stoffel et al36 directly compared commercial products containing the commonly used topical antimicrobial agents iodine, silver, polyhexamethylene biguanide, octenidine, hypochlorous acid, benzalkonium chloride, and a surfactant-based topical containing poloxamer 188. The authors reported that the iodine and benzalkonium chloride-containing products were overall the most effective in vitro and were then selected for in vivo evaluation in an infected immunocompromised murine model. One paper explored the use of honey as an antimicrobial but did not explore the risk of AMR45. Two studies reported that there was no evidence to suggest topical antimicrobial is more effective than an antiseptic in infection prevention42,47.
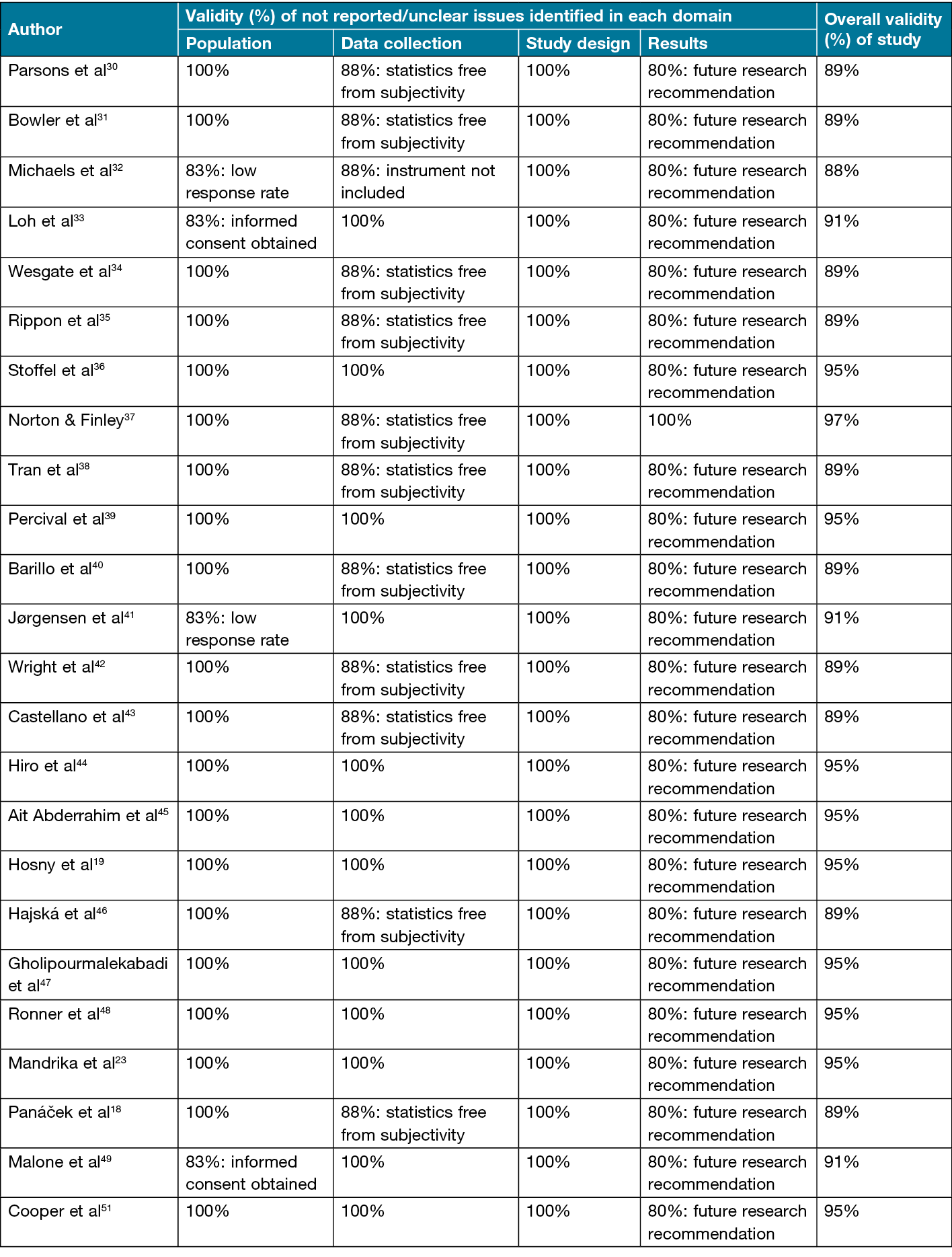
Quality appraisal of studies
The EBL appraisal checklist was used to assess the methodological quality of the included studies in this systematic review by focusing on the four main domains: population, data collection, study designs and results. The assessment of these domains is summarised in Table 3, where validity figures can be found as well as any not reported, or unclear issues identified in each domain52. The mean validity score for all studies was 92% (SD±0.02%). The minimum score was 89% whilst the highest overall validity was 95%. As can be seen in Table 3, all of the studies scored ≥75%, indicating that these studies were considered valid.
Table 3. Analysis of EBL appraisal checklist domains
Discussion
This review has synthesised the findings of 25 studies exploring the use of topical antimicrobial preparations against AMR. The majority of studies focused on the effectiveness of such preparations in wound healing and infection prevention; there was limited evidence that the use of topical antimicrobials increase the risk of AMR. Half of the studies included explored the use of silver as an antimicrobial and examined comparisons between commercially available silver wound dressings or the presence of antimicrobial activity. The majority of studies utilised an in vitro design, with only two studies being performed in a hospital setting using an RCT study design with human participants; these had inconclusive findings. These two studies exploring the effectiveness of antimicrobial silver dressings in VLU30,40 were inconclusive in their findings that antimicrobials were more effective than standard wound dressings.
The study by Wright et al42 specifically explored the effectiveness of topical silver applications in eliminating antimicrobial resistant bacteria and found that all the products investigated were effective in reducing bacteria. However, it was also reported that antiseptics were effective in infection prevention, suggesting that topical antimicrobials may not be the most cost effective or beneficial method of infection prevention in wound healing. Gholipourmalekabadi et al47 reported similar findings, also suggesting that the use of topical antiseptics in chronic wound care should be considered before antibiotics to limit their overuse and the risk of future resistance.
The study by Michaels et al32 explored the use of silver-donating versus non-silver low-adherence dressings in the treatment of VLU in a sample of 213 patients using an RCT design and found no significant group differences between patients randomised to receive a silver donating wound dressing (n=107) or a non-adherent wound dressing (n=106). However, the study suffered from several methodological flaws that limit the validity of the research findings, including several patients being lost to follow-up, patients not receiving the correct allocated study group dressing, and some patients receiving different products to those originally included in the study protocol. Despite these limitations, Michaels et al32 stated how increased cost associated with antimicrobial wound dressings and the lack of an obvious benefit in wound healing means there is limited benefit to their use. In contrast, Jørgensen et al41 explored the effect of a silver-release foam dressing and a non-silver dressing for wound healing of VLU in a sample of 129 patients and found that patients receiving the silver-release foam dressing (n=65) healed significantly better than those who did not.
Other studies included in this review explored the bacterial properties of honey, iodine and plant extracts in effective wound healing36,45,49 but specifically focused on the effectiveness of these topical antimicrobials in wound healing rather than if their use results in an increased risk of AMR. The limited evidence that there is AMR around the use of topical antimicrobials is an important finding, particularly given the emphasis on tackling AMR and its potential impact1.
Discussion of the methodological quality of the included studies
All studies presented with methodological issues in terms of the EBL appraisal checklist. In the population domain, the main areas of concern that arose in all studies were a lack of informed consent and poor response rate. In the data collection domain, the main aspects of concern were failure to use regularly collected statistics and to include the instrument. In the study design domain, all studies clearly describe this domain’s elements. Finally, in the results domain, the main areas of concern related to future research recommendation. Despite these failings, the review has identified all studies as valid.
Limitations
A number of important limitations need to be considered. Firstly, only studies published in English were used to search for evidence. Secondly, the broad methodological heterogeneity of the studies prevented the comparison between studies. This heterogeneity meant that meta-analysis could not be completed for all of the outcomes of interest. Furthermore, six studies had funding/conflict of interest29,32–34,38,47 whilst ten studies did not report whether they have funding42 or a conflict of interest28,31,36,37,39,41,42,46,48,51.
Conclusions
This review found limited evidence to suggest that topical antimicrobial preparations are associated with an increased risk of AMR. However, methodological differences between the studies and a focus on the effectiveness of topical antimicrobials in killing bacteria means that there was limited focus on cause and effect. AMR remains an important issue and, with the potential threat of AMR, understanding if, and how, topical antimicrobials may contribute to the problem of AMR is an essential area for exploration.
Key messages
- There is limited evidence that the use of topical antimicrobials increases the risk of AMR.
- This finding could be attributable to a focus on the effectiveness of topical antimicrobial preparations in wound healing and infection prevention.
- The majority of evidence surrounding the use of topical antimicrobials explores the use of silver in dressings as an antimicrobial.
- AMR and topical antimicrobials remains an important area of exploration.
Author contribution
All authors made significant contributions to the manuscript. All authors approved the final version prior to submission.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author(s)
Joanna Blackburn*1, Karen Ousey1,2, Declan Patton3,4, Zena Moore3–12, Pinar Avsar3,4
1Institute of Skin Integrity and Infection Prevention, School of Human and Health Sciences, University of Huddersfield, Huddersfield, UK
2Clinical Professor, Queensland University of Technology, QLD, Australia
3Skin Wounds and Trauma Research Centre, RCSI University of Medicine and Health Sciences, Dublin, Ireland
4School of Nursing and Midwifery, RCSI University of Medicine and Health Sciences, Dublin, Ireland
5Fakeeh College of Health Sciences, Jeddah, Saudi Arabia
6School of Nursing and Midwifery, Griffith University, QLD, Australia
7Faculty of Science, Medicine and Health, University of Wollongong, NSW, Australia
8Faculty of Medicine, Nursing and Health Sciences, Monash University, VIC, Australia
9Department of Public Health, Faculty of Medicine and Health Sciences, Ghent University, Belgium
10Lida Institute, Shanghai, China
11University of Wales, Cardiff, UK
12National Health and Medical Research Council Centre of Research Excellence in Wiser Wound Care, Menzies Health Institute Queensland, QLD, Australia
*Corresponding author email J.Blackburn3@hud.ac.uk
References
- World Health Organization (WHO). Antimicrobial resistance; 2018 [cited 2022 July 7]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- O’Neill J. Review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations; 2014 [cited 2022 July 13]. Available from: https://wellcomecollection.org/works/rdpck35v
- O’Neill J. Tackling drug-resistant infections globally: final report and recommendations: the review on antimicrobial resistance; 2016 [cited 2022 July 13]. Available from: https://wellcomecollection.org/works/thvwsuba/items
- Department of Health and Social Care (DoHaSC). UK 5-year action plan for antimicrobial resistance 2019 to 2024; 2019 [cited 2022 July 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- Australian Government – Antimicrobial Resistance. Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond; 2020 [cited 2022 December 16]. Available from: https://www.amr.gov.au/resources/australias-national-antimicrobial-resistance-strategy-2020-and-beyond
- Australian Commission on Safety and Quality in Health Care & Antimicrobial Use and Resistance in Australia (AURA). AURA 2021 highlights – fourth Australian report on antimicrobial use and resistance in human health; 2021 [cited 2022 December 16]. Available from: https://www.safetyandquality.gov.au/sites/default/files/2021-08/aura_2021_-_highlights_resource_-_august_17.pdf
- Smith DRM, Dolk FCK, Pouwels KB, et al. Defining the appropriateness and inappropriateness of antibiotic prescribing in primary care. J Antimicrob Chemo 2018;73:ii11-ii18. doi:10.1093/jac/dkx503.
- Tarrant C, Krockow EM, Nakkawita W, et al. Moral and contextual dimensions of “inappropriate” antibiotic prescribing in secondary care: a three-country interview study. Front Sociol 2020;5(7);1–9. doi:10.3389/fsoc.2020.00007.
- Gov.UK. UK Health Security Agency (UKHSA); 2021 [cited 2022 July 13]. Available from: https://www.gov.uk/government/organisations/uk-health-security-agency
- World Health Organization (WHO). Antimicrobial resistance surveillance in Europe 2022; 2021 [cited 2022 July 11]. Available from: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data
- Rozman U, Pušnik M, Kmetec S, et al. Reduced susceptibility and increased resistance of bacteria against disinfectants: a systematic review. Microorganisms 2021;9:1–2. doi:10.3390/microorganisms9122550.
- Ray P, Singh S, Gupta S. Topical antimicrobial therapy: current status and challenges. Indian J Med Microbiol 2019;37:299–308. doi:10.4103/ijmm.IJMM_19_443.
- Tong QJ, Hammer KD, Johnson EM, et al. A systematic review and meta-analysis on the use of prophylactic topical antibiotics for the prevention of uncomplicated wound infections. Infect Drug Resist 2018;11:417–425. doi:10.2147/idr.S151293.
- Wounds International. IWII: wound infection in clinical practice; 2016 [cited 2022 July 13]. Available from: https://www.woundsinternational.com/resources/details/iwii-wound-infection-clinical-practice
- Saco M, Howe N, Nathoo R, et al. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: a systematic review and meta-analysis. J Dermatolog Treat 2015;26:151–158. doi:10.3109/09546634.2014.906547.
- Heal CF, Banks JL, Lepper P, et al. Meta-analysis of randomized and quasi-randomized clinical trials of topical antibiotics after primary closure for the prevention of surgical-site infection. Br J Surg 2017;104:1123–1130. doi:10.1002/bjs.10588.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol 2011;64:S30–35. doi:10.1016/j.jaad.2010.11.009.
- Panáček A, Kvítek L, Smékalová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol 2018;13:65–71. doi:10.1038/s41565-017-0013-y.
- Hosny AEM, Rasmy SA, Aboul-Magd DS, et al. The increasing threat of silver-resistance in clinical isolates from wounds and burns. Infect Drug Resist 2019;12:1985–2001. doi:10.2147/idr.S209881.
- Dissemond J, Böttrich JG, Braunwarth H, et al. Evidence for silver in wound care – meta-analysis of clinical studies from 2000–2015. J Dtsch Dermatol Ges 2017;15:524–535. doi:10.1111/ddg.13233.
- Wang J, Smith J, Babidge W, et al. Silver dressings versus other dressings for chronic wounds in a community care setting. J Wound Care 2007;16:352–356. doi:10.12968/jowc.2007.16.8.27857.
- Kulkarni SB, Venkatesh S, Kruthi SR. Saline dressing versus povidone iodine dressing in chronic diabetic foot ulcer healing: a prospective comparative study. Int Surg J 2019;6(5):1524–1527. doi:10.18203/2349-2902.isj20191551.
- Mandrika I, Kumar S, Zandersone B, et al. Antibacterial and anti-inflammatory potential of polyherbal formulation used in chronic wound healing. Evid Based Complement Alt Med 2021;2021:9991454. doi:10.1155/2021/9991454.
- Jull A, Walker N, Parag V, et al. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br J Surg 2008;95:175–182. doi:10.1002/bjs.6059.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71.
- Percival SL, Bowler PG, Dolman J. Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int Wound J 2007;4:186–191. doi:10.1111/j.1742-481X.2007.00296.x.
- Chuangsuwanich A, Chortrakarnkij P, Kangwanpoom J. Cost-effectiveness analysis in comparing alginate silver dressing with silver zinc sulfadiazine cream in the treatment of pressure ulcers. Arch Plast Surg 2013;40:589–596. doi:10.5999/aps.2013.40.5.589.
- Roth B, Neuenschwander R, Brill F, et al. Effect of antiseptic irrigation on infection rates of traumatic soft tissue wounds: a longitudinal cohort study. J Wound Care 2017;26:79–87. doi:10.12968/jowc.2017.26.3.79.
- Morilla-Herrera JC, Morales-Asencio JM, Gómez-González AJ, et al. Effectiveness of a hydrophobic dressing for microorganisms’ colonization of vascular ulcers: protocol for a randomized controlled trial (CUCO-UV study). J Adv Nurs 2020;76:2191–2197. doi:10.1111/jan.14412.
- Parsons D, Bowler PG, Myles V, Jones S. Silver antimicrobial dressings in wound management: a comparison of antibacterial, physical and chemical characteristics. Wounds 2005;17:222–232.
- Bowler P, Jones S, Towers V, Booth R, Parsons D, Walker M. Dressing conformability and silver-containing wound dressings. Wounds UK 2010;6:14–20.
- Michaels JA, Campbell B, King B, et al. Randomized controlled trial and cost-effectiveness analysis of silver-donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br J Surg 2009;96:1147–1156. doi:10.1002/bjs.6786.
- Loh JV, Percival SL, Woods EJ, et al. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J 2009;6:32–38. doi:10.1111/j.1742-481X.2008.00563.x.
- Wesgate R, Grasha P, Maillard JY. Use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. Am J Infect Control 2016;44:458–464. doi:10.1016/j.ajic.2015.11.009.
- Rippon MG, Rogers AA, Westgate S. Treating drug-resistant wound pathogens with non-medicated dressings: an in vitro study. J Wound Care 2019;28:629–638. doi:10.12968/jowc.2019.28.9.629.
- Stoffel JJ, Kohler Riedi PL, Hadj Romdhane B. A multimodel regime for evaluating effectiveness of antimicrobial wound care products in microbial biofilms. Wound Repair Regen 2020;28:438–447. doi:10.1111/wrr.12806.
- Norton R, Finley PJ. Clinically isolated bacteria resistance to silver-based wound dressings. J Wound Care 2021;30:238–247. doi:10.12968/jowc.2021.30.3.238.
- Tran PL, Huynh E, Hamood AN, et al. The ability of quaternary ammonium groups attached to a urethane bandage to inhibit bacterial attachment and biofilm formation in a mouse wound model. Int Wound J 2017;14:79–84. doi:10.1111/iwj.12554.
- Percival SL, Thomas J, Linton S, et al. The antimicrobial efficacy of silver on antibiotic-resistant bacteria isolated from burn wounds. Int Wound J 2012;9:488–493. doi:10.1111/j.1742-481X.2011.00903.x.
- Barillo DJ, Barillo AR, Korn S, et al. The antimicrobial spectrum of Xeroform®. Burns 2017;43:1189–1194. doi:10.1016/j.burns.2016.10.023.
- Jørgensen B, Price P, Andersen KE, et al. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J 2005;2:64–73. doi:10.1111/j.1742-4801.2005.00084.x.
- Wright JB, Lam K, Burrell RE. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control 1998;26:572–577. doi:10.1053/ic.1998.v26.a93527.
- Castellano JJ, Shafii SM, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J 2007;4:114–122. doi:10.1111/j.1742-481X.2007.00316.x.
- Hiro ME, Pierpont YN, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings on in vitro and in vivo processes of wound healing. Eplasty 2012;12:409–419.
- Ait Abderrahim L, Taïbi K, Nawel AA, et al. Euphorbia honey and garlic: biological activity and burn wound recovery. Burns 2019 Nov;45(7):1695–1706. doi:10.1016/j.burns.2019.05.002.
- Hajská M, Slobodníková L, Hupková H, et al. In vitro efficacy of various topical antimicrobial agents in different time periods from contamination to application against 6 multidrug-resistant bacterial strains isolated from burn patients. Burns 2014;40:713–718. doi:10.1016/j.burns.2013.09.003.
- Gholipourmalekabadi M, Sameni M, Hashemi A, et al. Silver- and fluoride-containing mesoporous bioactive glasses versus commonly used antibiotics: activity against multidrug-resistant bacterial strains isolated from patients with burns. Burns 2016;42:131–140. doi:10.1016/j.burns.2015.09.010.
- Ronner AC, Curtin J, Karami N, et al. Adhesion of methicillin-resistant Staphylococcus aureus to DACC-coated dressings. J Wound Care 2014;23:484, 486–488. doi:10.12968/jowc.2014.23.10.484.
- Malone M, Johani K, Jensen SO, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non-healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother 2017;72:2093–2101. doi:10.1093/jac/dkx099.
- Bowler PG, Welsby S, Towers V, et al. Multidrug-resistant organisms, wounds and topical antimicrobial protection. Int Wound J 2012;9:387–396. doi:10.1111/j.1742-481X.2012.00991.x.
- Cooper RA, Jenkins L, Henriques AF, et al. Absence of bacterial resistance to medical-grade manuka honey. Eur J Clin Microbiol Infect Dis 2010;29:1237–1241. doi:10.1007/s10096-010-0992-1.
- Glynn L. A critical appraisal tool for library and information research. Library Hi Tech 2006;24:387–399. doi:10.1108/07378830610692154.



