Volume 31 Number 4
The impact of venous leg ulcers on quality of life
Joanna Smith, Keryln Carville, Cate Maguire, Karen Smith, Toby Richards
Keywords quality of life, venous leg ulcers, frailty, varicose veins
For referencing Smith J et al. The impact of venous leg ulcers on quality of life. Wound Practice and Research 2023; 31(4):164-173.
DOI
10.33235/wpr.31.4.164-173
Submitted 9 August 2023
Accepted 19 September 2023
Abstract
Aim To determine the quality of life (QoL) of clients with venous leg ulcers (VLU) treated in the community and investigate if age-related frailty or disability posed an additional impact on QoL.
Methods Clients with VLUs receiving wound care on 7 November 2019 from a community nursing service in Perth, Western Australia were invited to participate. Data was collected from an electronic management system on client demographics, comorbidities, key assessments and wound outcomes. A nurse-administered survey was used to collect data on clients’ current health status and VLU health experience, frailty via the FiND (Frail Non-Disabled tool), and QoL via the Wound-QoL tool.
Results There were 262 clients with VLUs who were invited to participate; 253 were considered eligible and 244 eligible clients completed the survey (96.4%). Common client comorbidities included obesity (48.9%), heart disease (34.5%), diabetes (24.2%), history of deep vein thrombosis (DVT) (19.7%) and varicose veins (44.7%), and 30% had their ulcer for ≥12 months. All but five clients (98%) reported the VLU had impacted their QoL. A total of 54% were classified as ‘disabled’ and 23.7% as ‘frail’. Independent predictors of poor QoL included a previous hospital admission, obesity and FiND classification of frail or disabled. Increasing age was protective of poor QoL.
Conclusion Almost all VLU clients reported an impact on their QoL. They also demonstrated high levels of frailty and had significant comorbidities. Interventions to improve QoL for these clients must be considered.
Introduction
Silverchain is a large not-for-profit Australian community health and aged care organisation and wound management comprises the largest component of clinical care delivered. Venous leg ulcers (VLU) are amongst the most common chronic wounds treated by the organisation1. VLUs are associated with chronic venous insufficiency (CVI), varicose veins and/or a history of deep vein thrombosis (DVT)2,3. They are more commonly associated with older age4 and individuals with comorbidities of obesity, immobility and rheumatoid disease5,6. Estimates of VLU prevalence vary from 0.6–4% amongst people aged over 60 years5,6.
The impact of VLUs on individuals is substantial, with compromise in individuals’ activities of daily living, comfort, alterations in cosmesis and body image, reduced mobility and disability, which subsequently impacts on quality of life (QoL)4–11. The World Health Organization defines QoL as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns”12. An individual’s perceptions of QoL are subjective and influenced by their health status and their ability or inability to fulfil their activities of daily living independently.
There are a considerable number of health-related quality of life (HRQoL) tools and several specifically for assessment of individuals with VLUs13. Although these tools have many common components, their utility for researchers and clinicians is dependent upon factors such as the number of responses required and the timespan over which QoL is to be evaluated14–20. Although not specifically designed for individuals with VLUs, the Wound-QoL tool is designed to ascertain QoL impacts associated with the presence of a chronic wound within the immediate context and the preceding 7 days15. The Wound-QoL for measurement of QoL in chronic wounds has been reported to be internally consistent, valid and responsive15. The ability to evaluate QoL in the immediate context was of particular interest to the researchers who perceived this information to be most relevant for contemporary clinical decision making and care planning. In addition, a generalised Wound-QoL tool was anticipated to facilitate future QoL assessment and comparisons amongst clients with other chronic wound types.
In 2019, in Western Australia (WA), Silverchain staff managed 1,164 clients with 2,537 VLUs. The mean age of these clients was 75 years. Overall, 82% of clients with VLUs were discharged from the service with wounds healed or relegated to self-care (almost healed) after a mean of 97 days (SD=112)21. However, the QoL of VLU clients was not routinely collected. This study aimed to determine the QoL of patients with VLUs treated in the community and, considering the age of VLU clients, it also aimed to investigate if age-related frailty or disability posed an additional impact on clients’ QoL.
Methods
Sample and setting
Clients were invited to participate in this study if they were receiving treatment of a VLU from the organisation in Perth, WA in November 2019 and met the inclusion criteria as follows:
Inclusion
- Clients identified in the electronic patient management system as being current patients in Perth, WA on 7 November 2019.
- Clients with a definitive diagnosis of a VLU.
- Able to understand and read English (or have someone who could assist with this).
Exclusion
- Clients with evidence of arterial disease (ankle-brachial pressure index <0.8).
- Clients with a cognitive disability that impacted on their ability to interpret the questions as determined by nurse assessment.
The organisation utilises a purpose-built, in-house electronic management system (EMS) which incorporates a digital wound module that enables the collection of wound assessment and management data at point of care by nurses on smartphones or tablets. Data from all clients meeting the study inclusion criteria was extracted from the EMS on 7 November 2019.
Assessment tools
The Wound-QoL comprises 17 questions attributed to three subscales – everyday life, body and psyche15. Answers to each item are coded with numbers (0=‘not at all’ to 4=‘very much’). A Wound-QoL global score on overall disease-specific QoL is computed by averaging all items. The Wound-QoL global score ranks a maximum score of 6822. The higher the score, the greater the impact of the VLU on participant’s QoL.
The Frail Non-Disabled (FiND) tool is designed to identify non-mobile, disabled elderly individuals23. It is suitable for self-completion, and is designed to differentiate frailty from disability. The tool has two questions related to physical disability (the ability to walk 400m, and the ability to climb a flight of stairs) and three other conditions generally considered components of the frailty syndrome – weight loss, exhaustion and sedentary behaviour. Mobility disability is defined as ‘a lot of difficulties’ or ‘inability’ to walk 400m and/or climb a flight of stairs. People who report one or more of the frailty criteria in the absence of mobility disability are classed as frail.
Survey
A digital survey platform (Microsoft Forms) was used to create the survey. Part 1 was designed to encourage the participants to reflect upon their current VLU experience with treating nurses. The questions included:
- Participant’s health status as related to the VLU.
- Participant’s weight and height.
- Presence of clinical risk factors for VLU.
Part 2 was completed by the participant and included:
- The FiND questionnaire23
- The Wound-QoL tool15
- Three additional questions about the impact of a VLU on the participant’s showering, wearing of shoes or clothing, and feeling attractive. These questions used the same rating scale as the Wound-QoL tool and were worded as follows. In the last 7 days:
- My wound treatment makes it difficult for me to shower or bathe.
- My wound treatment makes it difficult for me to wear the clothes and shoes I want.
- My wound makes me feel unattractive.
- A rating scale (1 = ‘no impact’ to 5 = ‘high impact’) to determine the client’s perception of the impact of the VLU on their QoL compared to their QoL prior to their VLU. This question was worded as follows: Compared to before you had an ulcer, how much has your quality of life been impacted?
Training
Information about the project methodology and education on the data collection tool was provided to 182 registered nurses (RNs) based at all service centres in the Perth metropolitan area. Each RN was provided with a working list of their clients. During the client’s usual treatment visit, the RN discussed the survey with their client (and carer if present) and provided each client with an information sheet. If they agreed to participate, consent was recorded digitally by RNs prior to survey participation.
Data analysis
Client demographic characteristics and current wound management data were collected from the EMS and linked to the survey responses. Data was analysed using STATA®1524. Scores from both the FiND survey questions23 and the Wound-QoL questions15 were calculated as per tool protocols22,23. The three additional questions added to the Wound-QoL were not included in the tool calculations and analysed separately. Body mass index (BMI) was calculated using height and weight variables, and descriptive statistics (mean, standard deviation and percentages) were calculated as appropriate. Differences between groups as classified by the FiND were identified using analysis of variance (ANOVA) for continuous variables and chi squared analysis for categorial variables.
Multivariable logistic regression was used to analyse the relationship between the Wound-QoL global score and the clients’ demographic and clinical details. The Wound-QoL score was transformed into a binary variable with scores of greater than the 75th percentile 21 being classified as high impact on QoL. This multivariable logistic regression approach included all demographic and clinical factors listed below due to their clinical relevance to VLU development and healing. A backward elimination strategy for this logistic regression was also used to determine the impact of removal of non-significant variables to the model. McKlelvey and Zavoina’s R2 was used to determine the impact of removal of variables on fit.
The demographic and clinical factors considered in the multivariable logistic regression were:
- Gender
- Age (years)
- Presence or absence of ischaemic heart disease, diabetes and/or chronic obstructive pulmonary disease
- Obesity (BMI ≥30) (yes/no)
- DVT in the past (yes/no)
- Varicose veins in the past (yes/no)
- Current varicose veins (yes/no)
- Current VLU treated for infection (yes/no)
- Time of existing VLU (1 year or less/greater than 1 year)
- Hospitalisation for existing VLU (yes/no)
- Current compression therapy (yes/no)
- FiND classification
Similarly, multivariable logistic regression was used to analyse the relationship between the clients’ perception of the impact of the VLU on their QoL compared to their QoL prior to their VLU adjusting for demographic and clinical details. The rating scale was transformed to a binary variable with those rating 5 (large impact) compared to other scores. This multivariable logistic regression approach used the same demographic and clinical factors as described above and used the same backward elimination strategy as described.
Results
On 7 November 2019, 309 clients were identified as having a current VLU on the EMS. A total of 47 of those clients either healed prior to the survey period, were hospitalised or were unavailable for a nursing visit during the data collection period. Overall, 262 clients were approached to participate in the survey. Of those, nine were not considered eligible as their attending RN did not consider them to be independently able to take part due to their cognitive deficits or their ability to understand English. Additionally, nine clients declined to participate, giving a total of 244 (96.4% of eligible) recruited clients (Figure 1).
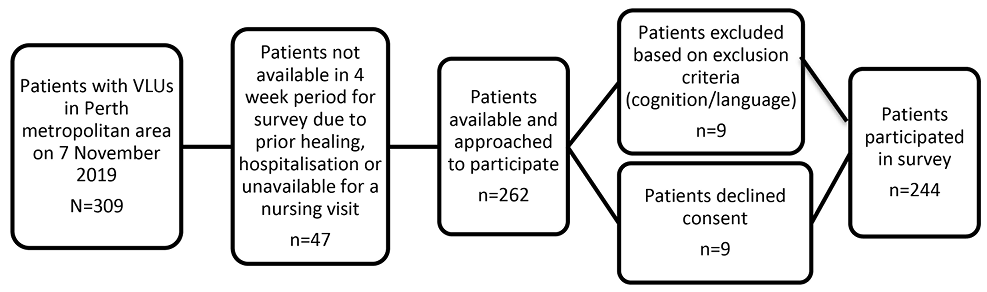
Figure 1. Survey participation flowchart
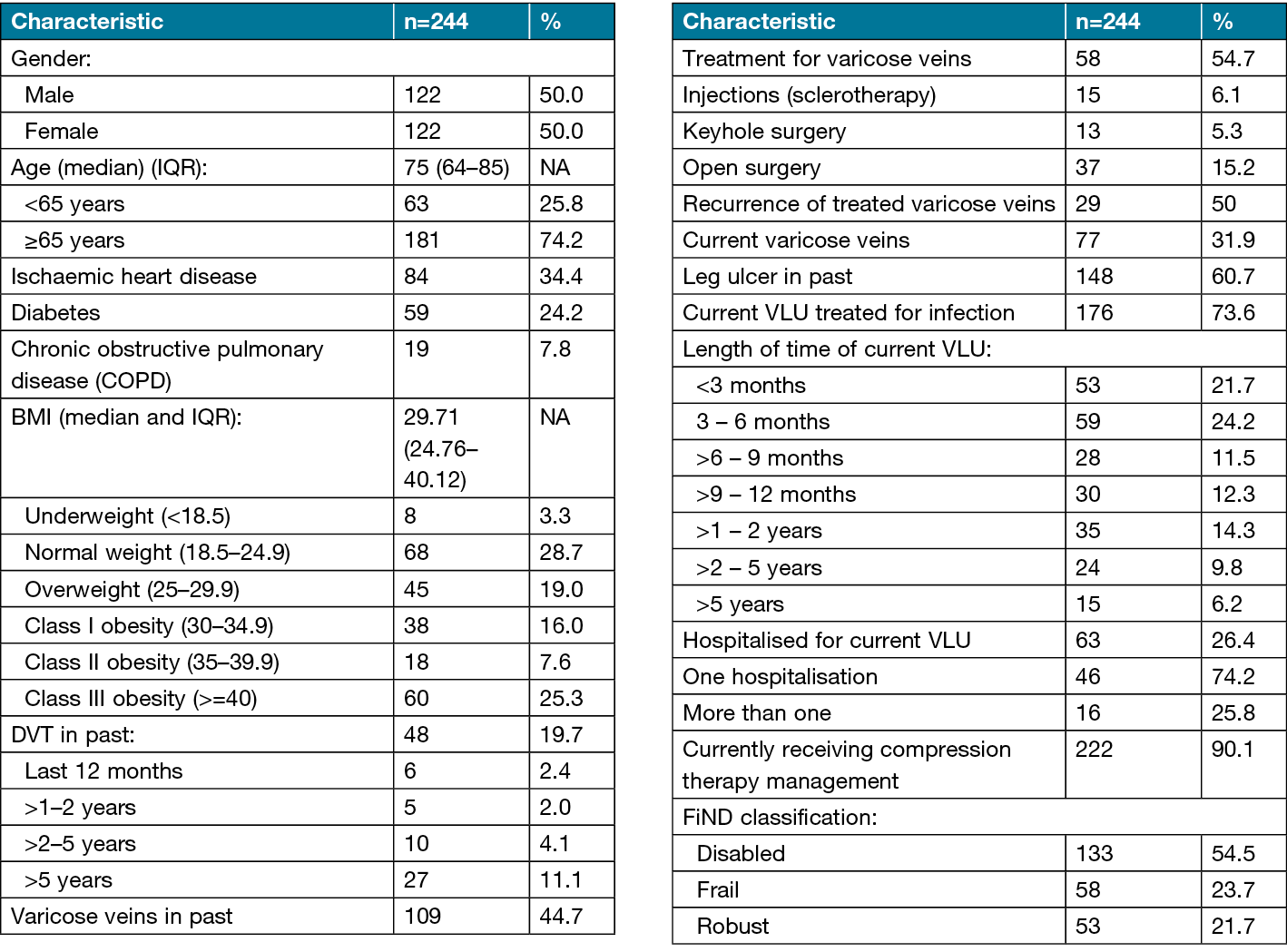
Half of the respondents were female (50%), and the mean age was 72.9 years, with 74.2% aged 65 or over. Obesity was common, with almost half (48.9%) presenting with Class I, II or III obesity based on BMI25. Other common comorbidities were ischaemic heart disease (34.5%) and diabetes (24.2%). Respondents reported significant vascular histories including DVT (19.7%) and varicose veins (44.7%). A total of 31% reported current varicose veins. Healing times were protracted for many of the VLUs, with over 30% of clients with ulcers taking more than 12 months to heal, indicating long admissions to community care1. According to the FiND classification, more than half (54.5%) of participants were classified as ‘disabled’, 23.7% as ‘frail’ and 21.7% as ‘robust’ (Table 1).
Table 1. Participant demographic characteristics
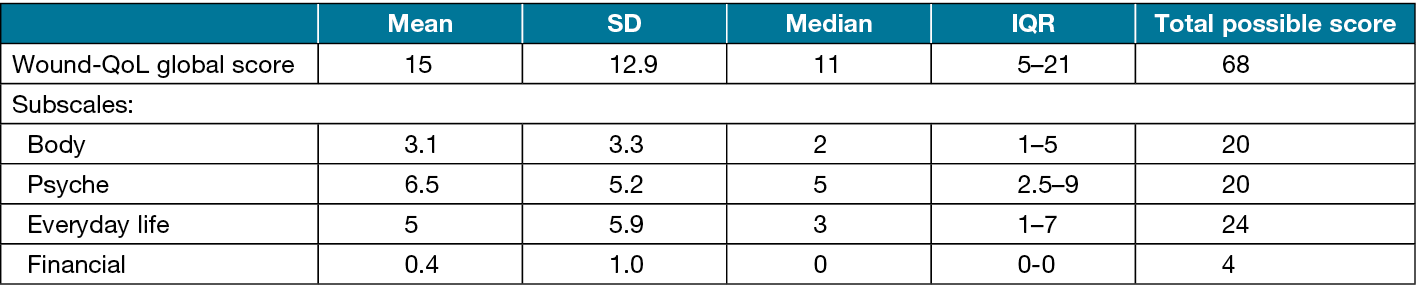
All but five participants (98.0%) reported that the VLU had impacted their QoL. Two (0.8%) individuals reported Wound-QoL global scores of more than 60, indicating that the wound had a significant impact on all aspects of their QoL. Subscale analysis showed scores for the psyche subscale had the largest impact, with a mean score of 6.5 out of a possible 20 (Table 2).
Table 2. Wound-QoL15 scores
Participants’ frustration over the time taken for the VLU to heal was high, with 37% stating it had ‘quite a lot’ or ‘very much’ effected their QoL. Recreational activities were the most impacted (21.3%) area of everyday life. Two of the questions added to the survey elicited high responses – the ‘Wound treatment makes it difficult for me to shower or bathe’ (36.6%) and the ‘Wound treatment makes it difficult for me to wear the clothes and shoes I want’ (36.3%) (Figure 2).
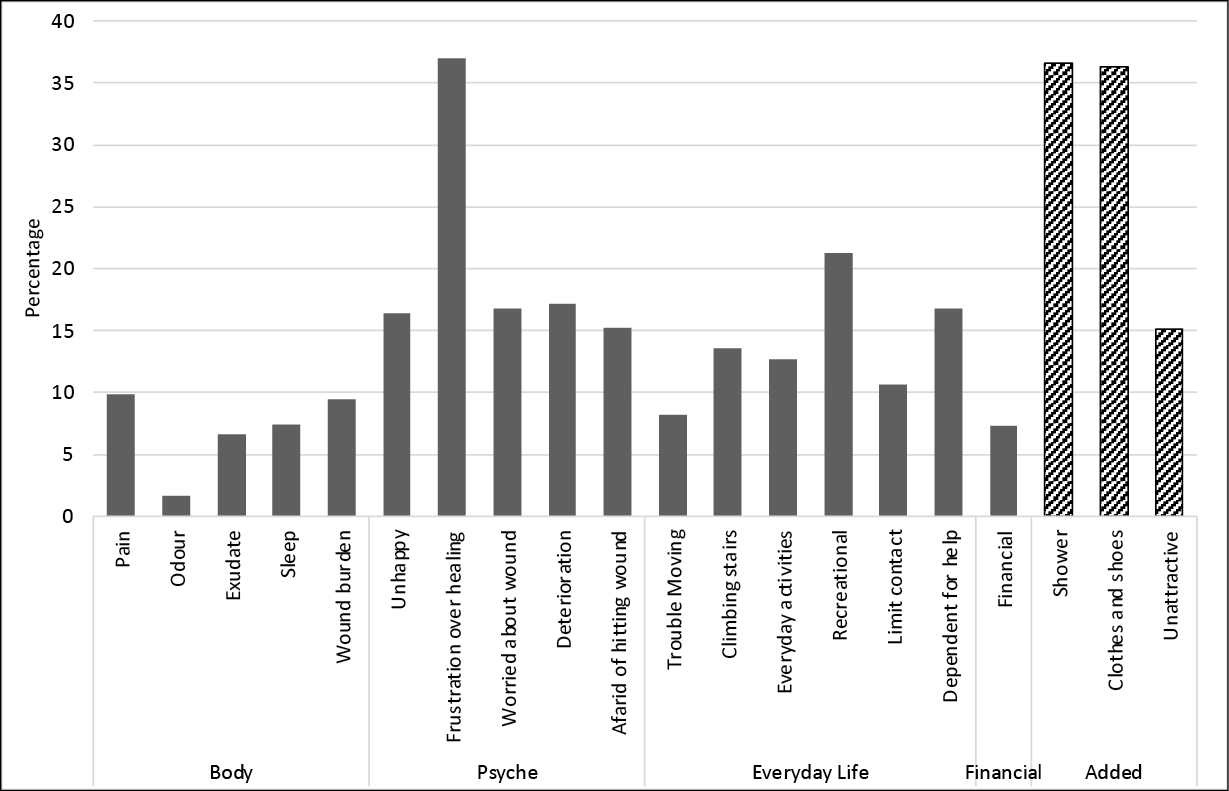
Figure 2. Wound-QoL scores with a response of ‘quite a lot’ or ‘very much’, plus additional survey questions
The mean scores for the Wound-QoL global and subscale scores15 were calculated for each of the FiND23 classifications, robust, frail and disabled. There was a significant difference between each of the FiND23 categories and the Wound-QoL global score15 and all subscales. Participants who were classified as disabled had a consistently worse Wound-QoL scores for each subscale (p<0.05), indicating a greater QoL impact of the VLU compared to those classified as frail or robust (Figure 3).
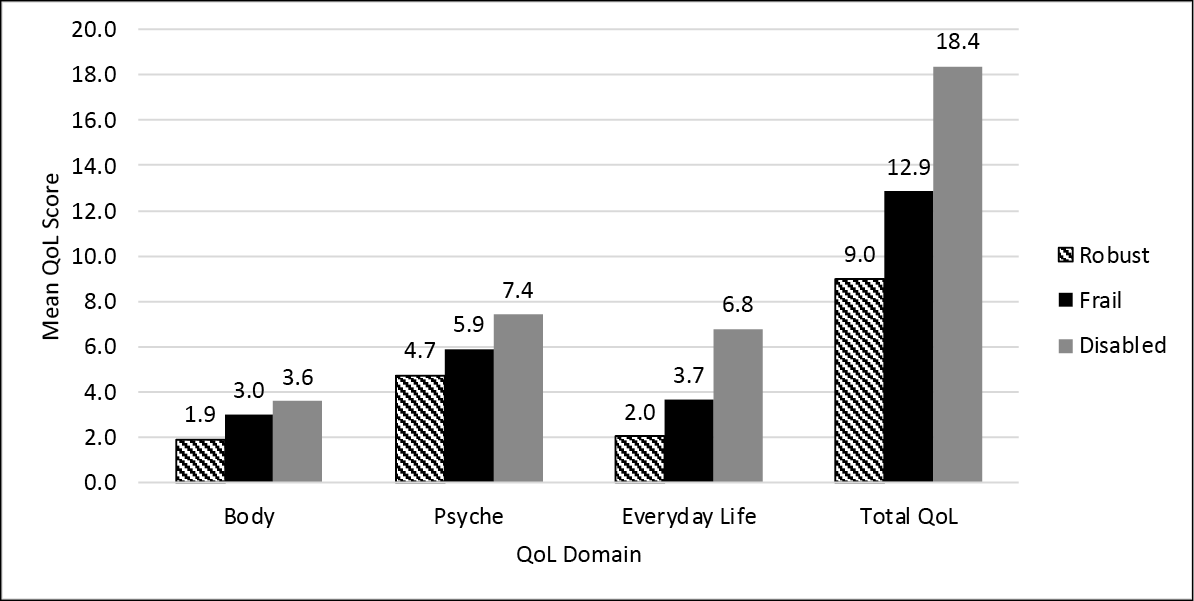
Figure 3. Wound-QoL domains by FiND23 classification
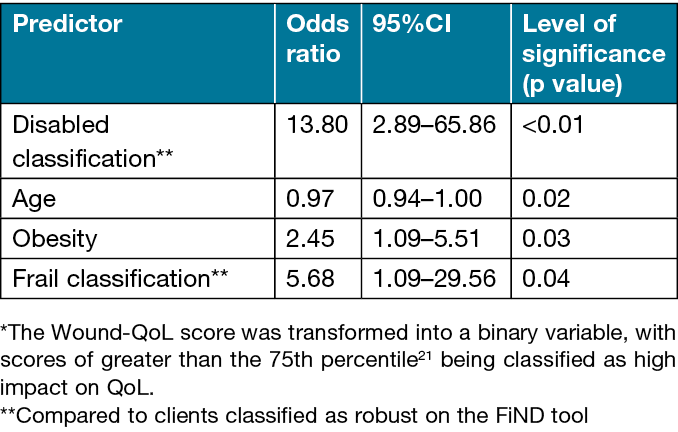
Multivariable logistic regression showed that, holding all other predictor variables constant, the odds of a high Wound-QoL global score (>21) being reported increased with: having a previous hospital admission compared to no admission; being obese compared to not obese; and being classified as frail or disabled compared to being classified as robust. In addition, a 1-year increase in age decreased the odds of a high impact Wound-QoL score. The final model included disabled classification, age and obesity. The removal of non-significant variables had a minor influence – McKlelvey and Zavoina’s R2=0.43 (all variables model) versus 0.41 (significant model) – therefore only significant variables remain in the final model (Table 3).
Table 3. Odds ratios for independent predictors of a high impact of VLU* when measured with the Wound-QoL tool
Clients were asked to rate from 1 for ‘no impact’ to 5 for ‘large impact’ on their QoL compared to QoL before they had their VLU. On average, clients reported a score of 3.2, with a large proportion (31%) reporting a ‘large impact’ (Figure 4).
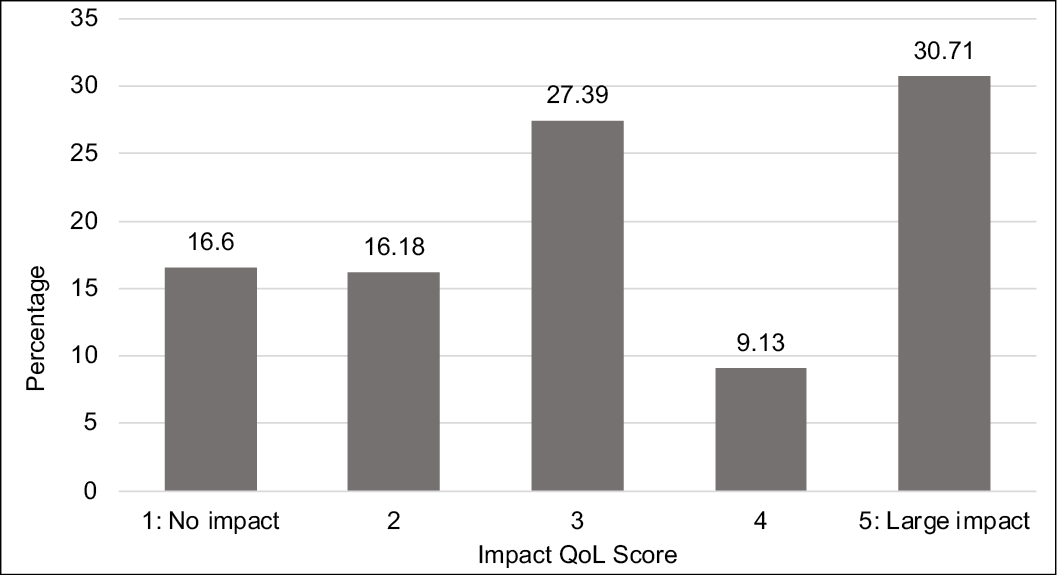
Figure 4. Perception of QoL compared to before VLU
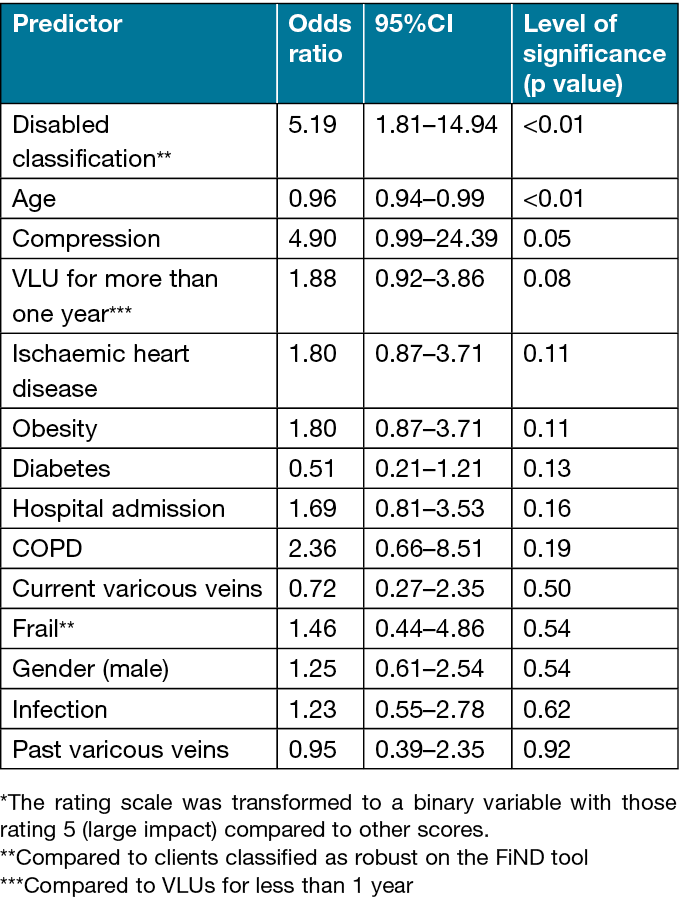
Multivariable logistic regression showed that the odds of a perceived large impact of the VLU on patients’ QoL compared to prior to their VLU increased by: being classified as disabled compared to being classified as robust; or having compression therapy compared to no compression, whilst a 1-year increase in age decreased the odds of a perceived large impact on QoL (Table 4). Removal of non-significant variables had an influence – McKlelvey and Zavoina’s R2=0.35 (all variables model) versus 0.28 (significant model) – where the variance accounted for by the model was reduced by 20% with the removal of the non-significant variables; therefore, they remain in the final model.
Table 4. Odds ratios for independent predictors of clients’ perception of a large impact on QoL as compared to QoL prior to having a VLU*
Discussion
This study describes valuable information about a community cohort of clients with current VLUs. The point of care digital survey data collection methodology achieved a high response rate of 96% of eligible clients, hence the sample is likely to be representative of clients being treated in the community for VLUs more broadly.
Our study participants reported a mean global Wound-QoL score of 15 (SD=12.9), with the psyche domain having the largest mean score of 6.5 (SD=5.2). The global score is comparable to a homecare VLU population in the Netherlands who reported a mean global Wound-QoL of 15 (SD=10.4), n=2026. Importantly 31% of our participants reported a large impact on their QoL compared to their QoL prior to the VLU. Similar QoL impacts were found in a study that compared the general population norms for the SF-36 in New Zealand to individuals with VLUs27. Clients reported their VLU reduced QoL associated with physical functioning, bodily pain, general health, vitality, social functioning and mental health27. The New Zealand study found that a younger age group (<65 years) were impacted more than older individuals27. In our study, age was found to be an independent predictor of improved QoL, with Wound-QoL scores decreasing as clients aged.
Disabled or frail clients reported a greater impact of the VLU on QoL than did robust clients. More than half (54.5%) of the participants in the study were classified as disabled, with disability defined by difficulties in walking and climbing stairs23. Despite this, only a small proportion rated the impact of their VLU on moving (8.2%) or climbing stairs (13.5%) as problematic. However, disability was an independent predictor of QoL after adjusting for multiple confounders (AOR=13.8, 95%CI=2.89–65.86).
Over two-thirds of participants were classified as obese (48.9%) or overweight (19%) according to BMI. Although the proportion of overweight and obese individuals in this population was like the general WA population (67%)28 and Australia wide (67%)29, our VLU cohort had a significantly larger proportion of morbidly obese individuals compared to the general Australian population (25.3% versus 11.7% respectively)28,29. It is possible that obese participants associated their reduced mobility with their high BMI rather than the presence of an VLU. Previous research examining the relationship between QoL and obesity indicates that obese individuals have a significantly worse QoL than non-obese individuals and obesity is strongly related to decreased mobility30. Our study also identified that obesity and classification of disability and frailty were independent predictors of reduced QoL. Additionally, overweight, obesity and decreased mobility are reported risk factors for the development of VLUs31–35 but, conversely, a VLU can impair mobility with subsequent increase in weight or obesity36. Furthermore, impaired mobility has also been demonstrated to impair healing of VLUs37–40, largely due to inefficient calf muscle pump function and valvular incompetence34,40. Therefore, QoL deficits for individuals with a VLU could be exaggerated when immobility, obesity and delayed healing are presenting clinical factors.
While a large proportion (37%) of individuals with VLUs reported that frustration over the time that it was taking for the VLU to heal impacted on their QoL, time to heal was not an independent predictor of high impact on QoL. VLUs can take weeks to years to heal41. While the VLUs of participants in this study were not yet healed, over half had the VLU for 6 months or more and over 15% for 2 years or more. Healing in chronic wounds can be inhibited by a variety of factors, not least of them comorbidities that affect vascular perfusion or immunity42 such as ischaemic heart disease and diabetes which were relatively prevalent in our study participants despite not being identified as independent predictors of reduced QoL.
A total of 20% of the participants reported a history of a DVT. The sequelae of post-thrombotic syndrome and CVI is associated with initial development and recurrence of a VLU32,33,45,46. Furthermore, Utne and colleagues45 found QoL in DVT/post-thrombotic clients was significantly impacted in the long-term. Such impairment in QoL may be related to painful and unsightly varicose veins due to valvular incompetence and venous hypertension associated with CVI. While not found to be independent predictors of QoL, 32% of our study participants stated they had varicose veins at the time of the survey and 45% reported history of varicose veins, amongst which 50% stated they had reoccurred after treatment. VLUs, varicose veins and concomitant lower leg oedema can be unsightly and impair cosmesis and QoL.
It is well recognised that compression therapy in the form of bandages, stockings or wraps is a gold standard management of VLUs and prevention of recurrence44,46,47. In this study, 91% of participants were wearing compression therapy and, when compared to clients without compression therapy, it was a borderline (p=0.05) independent predictor (AOR=4.9, 95%CI=0.99–24.39) of having a high impact on QoL compared to prior to having the VLU, albeit with a wide confidence interval. Compression therapy is well recognised as having an impact on an individual’s ability to perform activities of daily living6. In particular, compression bandages may inhibit the wearing of an individual’s preferred clothing and footwear9,11,44,45 and their ability to shower or bathe independently51,52. In our study, 37% of participants identified that the VLU impacted their ability to shower, and 36% identified an impact on wearing preferred clothes and footwear. Differences in the type of compression bandaging (e.g., wraps versus bandages or stocking) was not explored further but may be a factor in the impact of compression therapy on QoL. Additionally, further research is required to investigate the small proportion (9%) of participants who were not on compression therapy at the time of the survey in terms of how long they had a VLU, recurrence, and reasons for not using compression therapy.
The literature identifies wound malodour36,51–53, excessive exudate44,52, pain10,53,54 and cost of treatment55–57 to be significant factors with subsequent impacts on individuals’ QoL. However, amongst our study participants, few reported malodour (2%), exudate (7%) and cost (7%) concerns. Cost may be less of a consideration in our cohort than in other studies as the organisation provides evidence-based wound care products and wound management services free of charge to clients as a component of care provision. Access to advanced wound management products may have also had an impact in controlling pain as only 10% of participants reported that pain impaired their QoL compared to 64% of clients attending a leg ulcer clinic in Sweden or the United Kingdom in a study by Hofman et al58. Pain is recognised to inhibit physical mobility59 and decreased mobility is a reported risk factor for the development or impaired healing of VLUs, which can lead to a vicious cycle for clients32,33.
Participants reported greater impacts of their VLU in the psyche and everyday life domains of the Wound-QoL15 than the physical. A total of 20% of participants reported that their VLU impacted on their life by limiting their recreational activity. While ‘recreational’ is not further described, it is assumed that recreational activities would include some form of social and/or physical activity. Other research has reported that the negative impacts of the VLU on social activities and other physical activities is a result of increased pain60, fear of further injuring the wound61, and restrictions due to compression therapy61. In addition, impact on recreational activities may have been impacted further by participants’ mobility issues, with more than half of the participants having markers of disability and a significant proportion presenting with obesity.
Limitations
Information about the participants’ VLU background and clinical history were not obtained from clinical notes but through a survey administered by RNs to clients. This was aimed at encouraging the participant to think about their VLU and the impact it had on their current QoL. However, a limitation is the clients’ recall and potential inaccuracies. Additionally, whilst the survey had a high response rate, the participants are from a single state service and there should therefore be some caution with extrapolation of results.
Conclusions
The results of this study clearly show that VLUs impact on clients’ QoL. Being frail, disabled and obese were factors that were found to be independent predictors of increased impact of VLUs on the QoL in this cohort. Additionally, the major impacts on the QoL for these clients identified by the survey were the frustration over the time taken for the VLU to heal and the difficulties the VLU caused in wearing desired clothing and footwear or being able to shower or bathe. These aspects are often not addressed by nurses in care plans which tend to focus on the management of the wound. QoL should therefore be considered in client care planning for optimal outcomes and targeted for active intervention as much as practicable.
Acknowledgements
The authors would like to acknowledge the time spent by our clients with VLU who completed the survey and the RNs who collected the data.
Conflict of interest
The authors declare no conflicts of interest
Ethics statement
Ethical approval was provided by the Silverchain Human Research Ethics Committee (ref App-138).
Funding
The authors received no funding for this study.
Author contribution
The authors confirm equal joint responsibility for the following – study conception and design, analysis and interpretation of the results, and draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Author(s)
Joanna Smith1*, Keryln Carville1,2, Cate Maguire1, Karen Smith1,3, Toby Richards4
1Silver Chain Group Limited (Silverchain), Perth, WA, Australia
2Curtin University School of Nursing, Perth, WA, Australia; Curtin Health Innovation Research Institute (CHIRI), Perth, WA, Australia
3School of Population Health Curtin., Perth, WA, Australia; School of Medicine Monash University, Melbourne, Vic, Australia;
School of Nursing QUT, Brisbane, QLD, Australia
4Curtin University School of Medicine, Perth, WA, Australia
*Corresponding author email joanna.smith@silverchain.org.au
References
- Carville K, Alan J, Smith J. Best practice, best products, best outcomes in community wound care: three descriptive cohorts. Wound Pract Res 2022;30(4):196–206. doi:10.33235/wpr.30.4.196–206.
- The Australian Wound Management Association Inc., The New Zealand Wound Care Society Inc. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers; 2011. Available from: https://www.nzwcs.org.nz/images/luag/2011_awma_vlug.pdf
- Parker CN, Finlayson KJ, Shuter P, et al. Risk factors for delayed healing in venous leg ulcers: a review of the literature. Int J Clin Pract 2015;69:967–977. doi:10.1111/ijcp.12635.
- Vowden KR, Vowden P. Preventing venous ulcer recurrence: a review. Int Wound J 2006;3:11–21. doi:10.1111/j.1742-4801.2006.00180.x.
- Nelzen O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphol 2008;15:143–150.
- Boxall SL, Carville K, Leslie GD, et al. Controlling compression bandaging pressure in leg ulcer research trials: a summary of the literature. Phlebology 2019;34:501–514. doi:10.1177/0268355519825590.
- Bainbridge P. Why don’t patients adhere to compression therapy? Br J Comm Nurs 2013;18:S35-S40. doi:10.12968/bjcn.2013.18.Sup12.S35.
- Brown A. Achieving concordance with compression therapy. Nurs Resident Care 2011;13:537–540. doi:10.12968/nrec.2011.13.11.537.
- Dereure O, Vin F, Lazareth I, et al. Compression and peri-ulcer skin in outpatients’ venous leg ulcers: results of a French survey. J Wound Care 2005;14:265–271. doi:10.12968/jowc.2005.14.6.26787.
- Mandal A. The concept of concordance and its relation to leg ulcer management. J Wound Care 2006;15:339–341. doi:10.12968/jowc.2006.15.8.26947.
- Edwards LM. Why patients do not comply with compression bandaging. Br J Nurs 2003;12:S5-S16. doi:10.12968/bjon.2003.12.Sup2.11327.
- World Health Organization. WHOQOL: measuring quality of life; cited 2010 Jan 21. Available from: https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/
- Liu S, Team V, Qiu Y, et al. Investigating quality of life instrument measurement properties for adults with active venous leg ulcers: a systematic review. Wound Rep Regen 2022;30:468–486. doi:10.1111/wrr.13034.
- Aoun S, O’Connor M, Skett K, et al. Do models of care designed for terminally ill ‘home alone’ people improve their end-of-life experience? A patient perspective. Health Soc Care Comm 2012;20:599–606. doi:10.1111/j.1365-2524.2012.01074.x.
- Blome C, Baade K, Sebastian Debus E, et al. The ‘Wound-QoL’: a short questionnaire measuring quality of life in patients with chronic wounds based on three established disease-specific instruments. Wound Repair Regen 2014;22:504–514. doi:10.1111/wrr.12193.
- Bland JM, Dumville JC, Ashby RL, et al. Validation of the VEINES-QOL quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC Cardiovasc Disorder 2015;15:85. doi:10.1186/s12872-015-0080-7.
- Augustin M, Herberger K, Rustenbach SJ, et al. Quality of life evaluation in wounds: validation of the Freiburg Life Quality Assessment-wound module, a disease-specific instrument. Int Wound J 2010;7:493–501. doi:10.1111/j.1742-481X.2010.00732.x.
- Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–17. doi:10.1111/j.1742-481x.2004.00007.x.
- Engelhardt M, Spech E, Diener H, et al. Validation of the disease-specific quality of life Wuerzburg Wound Score in patients with chronic leg ulcer. Vasa 2014;43:372–379. doi:10.1024/0301-1526/a000378.
- Smith JJ, Guest MG, Greenhalgh RM, et al. Measuring the quality of life in patients with venous ulcers. J Vasc Surg 2000;31:642–649. 2001/02/07. doi:10.1067/mva.2000.104103.
- Silver Chain Group. ComCare wound module data 2018–2019 financial year. Silver Chain Group; 2019.
- Blome C. Wound-QoL short manual; cited 2019 Dec 20. Available from: https://www.wound-qol.com/wp-content/uploads/Wound-QoL-ShortManual-2018-05.pdf
- Cesari M, Demougeot L, Boccalon H, et al. A self-reported screening tool for detecting community-dwelling older persons with frailty syndrome in the absence of mobility disability: the FiND questionnaire. Plos One 2014;9:e101745. doi:10.1371/journal.pone.0101745.
- StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017.
- Department of Health and Aged Care. Body mass index (BMI) and waist measurement; 2021. Available from: https://www.health.gov.au/topics/overweight-and-obesity/bmi-and-waist
- Zorge NE, Scheerders ERY, Dudink K, et al. A prospective, multicentre study to assess frailty in elderly patients with leg ulcers (GERAS study). J Eur Acad Dermatol Venereol 2023;37:428–435. 20221014. doi:10.1111/jdv.18586.
- Jull A, Muchoney S, Parag V, et al. Impact of venous leg ulceration on health-related quality of life: a synthesis of data from randomized controlled trials compared to population norms. Wound Repair Regen 2018;26:206–212. doi:10.1111/wrr.12636.
- Western Australian Department of Health. WA healthy weight action plan 2019–2024. Perth: Health Networks, Western Australian Department of Health; 2019.
- Australian Government: Cancer Australia. Overweight and obesity – adults; 2020 [cited 202 Feb 5]. Available from: https://ncci.canceraustralia.gov.au/prevention/overweight-and-obesity/overweight-and-obesity-adult
- Forhan M, Gill SV. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrin Metab 2013;27:129–137. doi:10.1016/j.beem.2013.01.003.
- Robertson L, Lee AJ, Gallagher K, et al. Risk factors for chronic ulceration in patients with varicose veins: a case control study. J Vasc Surg 2009;49:1490–1498. doi:10.1016/j.jvs.2009.02.237.
- Abelyan G, Abrahamyan L, Yenokyan G. A case-control study of risk factors of chronic venous ulceration in patients with varicose veins. Phlebol 2018;33:60–67. doi:10.1177/0268355516687677.
- Vlajinac H, Marinkovic J, Maksimovic M, et al. Factors related to venous ulceration: a cross-sectional study. Angiol 2014;65:824–830. doi:10.1177/0003319713508218.
- Collins L, Samina S. Diagnosis and treatment of venous ulcer. Am Fam Phys 2010;81:989–996.
- Barber GA, Weller CD, Gibson SJ. Effects and associations of nutrition in patients with venous leg ulcers: a systematic review. J Adv Nurs 2018;74:774–787. doi:10.1111/jan.13474.
- Green J, McKinley R, Rebecca J, et al. Chronic venous leg ulcer care – are we missing a vital piece of the jigsaw? Wounds UK 2017;13.
- Chaby G, Viseux V, Ramelet AA, et al. Refractory venous leg ulcers: a study of risk factors. Dermatolog Surg 2006;32:512–519. doi:10.1111/j.1524-4725.2006.32104.x.
- Meagher H, Ryan D, Clarke-Moloney M, et al. An experimental study of prescribed walking in the management of venous leg ulcers. J Wound Care 2012;21:421–430. doi:10.12968/jowc.2012.21.9.421.
- Clarke-Moloney M, Godfrey A, O’Connor V, et al. Mobility in patients with venous leg ulceration. Eur J Vasc Endovasc Surg 2007;33:488–493. doi:10.1016/j.ejvs.2006.11.032.
- Simka M. Calf muscle pump impairment and delayed healing of venous leg ulcers: air plethysmographic findings. J Dermatol 2007;34:537–544. doi:10.1111/j.1346-8138.2007.00327.x.
- Franks PJ, Barker J, Collier M, et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care 2016;25:S1-S67. doi:10.12968/jowc.2016.25.Sup6.S1.
- Carville K. Wound care manual (7th ed). Osborne Park: Silverchain Foundation; 2023.
- Finlayson K, Wu M-L, Edwards HE. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: a longitudinal study. Int J Nurs Stud 2015;52:1042–1051. doi:10.1016/j.ijnurstu.2015.02.016.
- Gohel MS, Taylor M, Earnshaw JJ, et al. Risk factors for delayed healing and recurrence of chronic venous leg ulcers: an analysis of 1324 legs. Euro J Vasc Endovasc Surg 2005;29:74–77. doi:10.1016/j.ejvs.2004.10.002.
- Utne KK, Tavoly M, Wik HS, et al. Health-related quality of life after deep vein thrombosis. SpringerPlus 2016;5:1278. doi:10.1186/s40064-016-2949-z.
- Taylor RJ, Taylor AD, Smyth JV. Using an artificial neural network to predict healing times and risk factors for venous leg ulcers. J Wound Care 2002;11:101–105. doi:10.12968/jowc.2002.11.3.26381.
- Edwards H, Finlayson K, Courtney M, et al. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC Health Serv Res 2013;13:86. doi:10.1186/1472-6963–13-86.
- O’Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012. doi:10.1002/14651858.CD000265.pub3.
- Nelson EA, Bell-Syer SEM. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2014. doi:10.1002/14651858.CD002303.pub3.
- Ratliff C, Yates S, McNichol R, et al. Compression for primary prevention, treatment, and prevention of recurrence of venous leg ulcers. J WOCN 2016;43:347–364.
- Greaves T, Ivins N, Stephens C. A compression bandage system that helps to promote patient wellbeing. J Comm Nurs 2014;28:8–30.
- Moffatt C, Kommala D, Dourdin N, et al. Venous leg ulcers: patient concordance with compression therapy and its impact on healing and prevention of recurrence. Int Wound J 2009;6:386–393. doi:10.1111/j.1742-481X.2009.00634.x.
- Furlong W. Venous disease treatment and compliance: the nursing role. Br J Nurs 2001;10:S18-S35. doi:10.12968/bjon.2001.10.Sup2.12342.
- Heinen MM, Achterberg Tv, Reimer WSo, et al. Venous leg ulcer patients: a review of the literature on lifestyle and pain-related interventions. J Clin Nurs 2004;13:355–366. doi:10.1046/j.1365-2702.2003.00887.x.
- Annells M, O’Neill J, Flowers C. Compression bandaging for venous leg ulcers: the essentialness of a willing patient. J Clin Nurs 2008;17:350–359. doi:10.1111/j.1365-2702.2007.01996.x.
- Barnsbee L, Cheng Q, Tulleners R, et al. Measuring costs and quality of life for venous leg ulcers. Int Wound J 2019;16:112–121. doi:10.1111/iwj.13000.
- Cheng Q, Kularatna S, Lee XJ, et al. Comparison of EQ-5D-5L and SPVU-5D for measuring quality of life in patients with venous leg ulcers in an Australian setting. Qual Life Res 2019;28:1903–1911. doi:10.1007/s11136-019-02128-6.
- Hofman D, Ryan TJ, Arnold F, et al. Pain in venous leg ulcers. J Wound Care 1997;6:222–224. doi:10.12968/jowc.1997.6.5.222.
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcome 2007;5:44. doi:10.1186/1477-7525-5-44.
- Miertová M, Dlugošová K, Ovšonková A, et al. Chosen aspects of quality of life in patients with venous leg ulcers. Central Eur J Nurs Midwif 2016;7:527–533. doi:10.15452/CEJNM.2016.07.0025.
- Phillips P, Lumley E, Duncan R, et al. A systematic review of qualitative research into people’s experiences of living with venous leg ulcers. J Adv Nurs 2018;74:550–563. doi:10.1111/jan.13465.



