Volume 31 Number 4
Investigating cognition in people with diabetes-related foot ulcers: a study protocol
Nimantha Karunathilaka, Christina Parker, Peter A Lazzarini, Margaret MacAndrew, Kathleen Finlayson
Keywords diabetes-related foot ulcer, cognition, diabetes-related lower extremity complications, type 2 diabetes
For referencing Karunathilaka N et al. Investigating cognition in people with diabetes-related foot ulcers: a study protocol. Wound Practice and Research 2023; 31(4):197-203.
DOI
10.33235/wpr.31.4.197-203
Submitted 2 May 2023
Accepted 14 September 2023
Abstract
Aim Diabetes is associated with cognitive changes; however, it is unclear whether cognitive changes differ between those with diabetes-related foot ulcers (DFUs), or those with only diabetes-related lower extremity complications (DRLECs) that are risk factors for DFUs. Therefore, it is hypothesised that cognitive changes in people with diabetes are further influenced by the presence of DFU or DRLECs. Hence, this study aims to investigate cognition in people with a DFU compared to those with DRLECs. Secondary aims include investigating cognition over time in people with DFUs, and in those with DFUs who do and don’t heal.
Methods A case control study nested in a longitudinal study will recruit 136 participants – 68 with type 2 diabetes with DFUs (cases) and 68 with DRLECs (controls). Global cognition will be measured using the Montreal Cognitive Assessment test. The 68 cases will be followed up for 12 weeks to investigate cognition outcomes as well as to determine DFU healing.
Results The findings of this study will provide new evidence on whether cognition is further influenced by the presence of a DFU or by other DRLECs.
Conclusion These findings may be important to early detect cognitive changes in people with type 2 diabetes with DFUs or DRLECs.
Introduction
Diabetes is considered one of the most significant health challenges of the 21st century1. Diabetes-related foot ulcers (DFUs) have been identified as a top-10 leading cause of the global disability burden2,3. DFUs are defined as “foot ulcers in people with diagnosed diabetes mellitus and are usually accompanied by neuropathy (PN) and/or peripheral artery disease (PAD) in the lower extremity”4. Globally, around 20 million people have a DFU at any one time2 and will have poorer quality of life and increased risks of hospitalisation, amputation and mortality compared to those without DFUs2,5,6. Moreover, recent evidence also suggests that diabetes and DFUs may be associated with detrimental cognitive changes7–9.
Cognition is defined as the “brain’s ability to acquire, process, store, and retrieve information”10. For people with diabetes with diabetes-related complications, the cognitive domains reported to be affected include executive function, psychomotor speed, memory, attention, concentration, verbal fluency and reaction time9,11. These cognitive changes are believed to be caused by multiple factors, including defects in insulin signalling, autonomic function, neuroinflammatory pathways, mitochondrial metabolism, increased inflammatory and oxidative stress pathways, and vascular deficits7,12–14. In turn, these cognitive changes can detrimentally influence self-care management in people with diabetes15,16.
However, studies to date in those with DFU report conflicting findings related to cognition, likely due to the different study designs, populations, outcomes and follow-up periods used17,18. Therefore, it is still unclear whether cognitive changes in people with diabetes are worsened by the presence of DFU or by other diabetes-related complications that are risk factors for DFU such as PN or PAD.
The primary aim of this study is to investigate cognition in people with type 2 diabetes with a DFU (cases), compared to those who do not have a foot ulcer but do have diagnosed type 2 diabetes and are accompanied by other diabetes-related lower extremity complications (DRLECs) such as PN and/or PAD (controls). Secondary aims include investigating changes in cognition over time (12 weeks) in people with diabetes with a DFU and in subgroups of people with a DFU who heal compared to those who do not heal. Therefore, it is hypothesised that cognitive changes in people with diabetes are further influenced by the presence of DFU or DRLECs. Therefore, cases are defined as people who have a foot ulcer with diagnosed type 2 diabetes (DFU) and are accompanied by PN and/or PAD in the lower extremity4, while controls are defined as people who do not have a foot ulcer but do have diagnosed type 2 diabetes and are accompanied by PN and/or PAD in the lower extremity (DRLECs). The findings of this study will provide new evidence on whether cognition is further influenced in people with diabetes by a DFU or by other diabetes-related complications that are risk factors for a DFU.
Methods
Study design
The study design for the project combines a case control study nested in a 12-week prospective longitudinal study. The case control study will investigate cognition in people with type 2 diabetes with DFUs (cases), compared to those with DRLECs (controls). A prospective longitudinal study will then further investigate changes in cognition over 12 weeks of follow-up for those people with type 2 diabetes with a DFU (cases).
Settings
The study setting will be outpatient diabetic foot services (eight facilities), including hospitals and community health services in Australia.
Participants
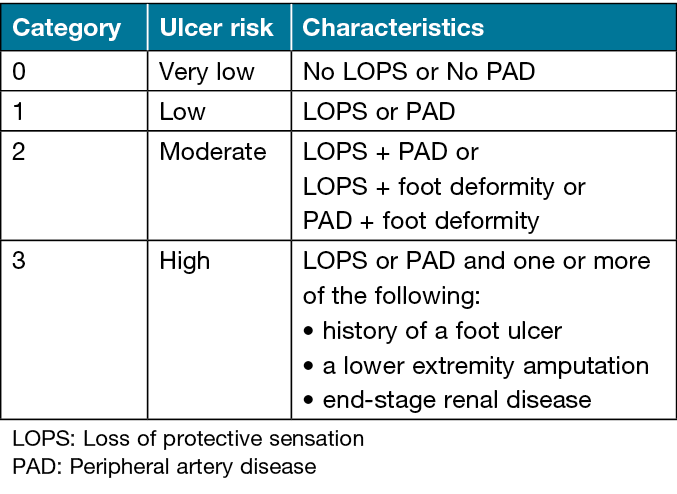
Eligible participants will be those aged 18 years and over who are diagnosed with type 2 diabetes with DFUs (cases), and people with type 2 diabetes with DRLECs (controls). Figure 1 displays the definitions of the terms for participants’ selection4,19. Exclusion criteria will be those previously diagnosed with cognitive impairment (mild, moderate or severe), dementia, cerebrovascular accident, neurodegenerative diseases or those who are pregnant. Participants will be allocated to one of two groups: those with DFUs (cases); and those with DRLECs (controls). The International Working Group of the Diabetic Foot (IWGDF) risk classification system20 (Table 1) will be used to assign controls to the categories of moderate (category 2) or high (category 3) ulcer risk. The control group will be matched in age and sex with the case group during recruitment.
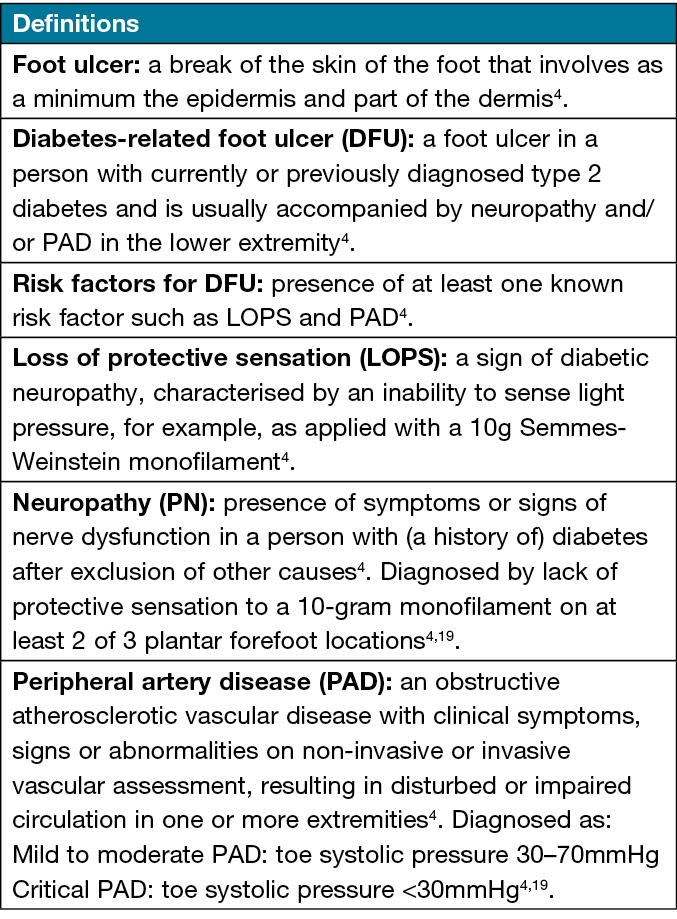
Figure 1. Definitions of the terms for participants’ selection
Table 1. IWGDF 2019 risk classification20
Sample size calculation
The primary hypothesis is that there is a significant difference in cognition in people with type 2 diabetes with DFUs compared to people with type 2 diabetes with DRLECs. This primary hypothesis was used to calculate the sample size for this study. A search of the literature was unable to locate any similar previous case controlled studies with similar comparison groups to estimate the sample size by using exposed and unexposed percentages, odds ratio, risk/prevalence ratio or risk/ prevalence differences. Therefore, a medium level of effect size was assumed to estimate the sample size (d=0.5). The cases to control allocation ratio were taken as 1:1. G*Power (ver. 3.1.9.4) was used to calculate the sample size21 and the calculated sample size for a one-tail test is 57 for each group by using the independent sample t-test with 80% power and an overall significance of 0.05. As there is a number of hypotheses, including a prospective longitudinal follow-up, we inflated the sample size by 20% to account for the likely attrition rate during the 12-week follow-up. Hence, the sample size recruited for each group will be 68 participants.
Variables of interest
Baseline variables of demographic information (age, gender, ethnicity, marital status and education level), weight and height, and data related to diabetes and DFUs (comorbidities and foot-related conditions) will be obtained (see Figure 2 for variable definitions). Clinical examination records will be utilised to collect medical history related to comorbidities and foot-related conditions. The foot-related conditions include the presence/absence of a previous foot ulcer, previous amputation, PN, PAD, acute Charcot foot, depth of ulcer, infection and ulcer size. All participants will be weighed using an electronic portable scale while height will be measured using a stadiometer, ensuring that participants are barefoot with the heels, hips and shoulders touching the vertical scale bar, the chin straight and the inion touching the back of the vertical scale. The horizontal sliding measure will be lowered to the highest point of the head to lightly touch the top of the head. Weight and height will be used to calculate the body mass index (BMI) by dividing the body mass (kg) by the square of the height (m2) of each participant.
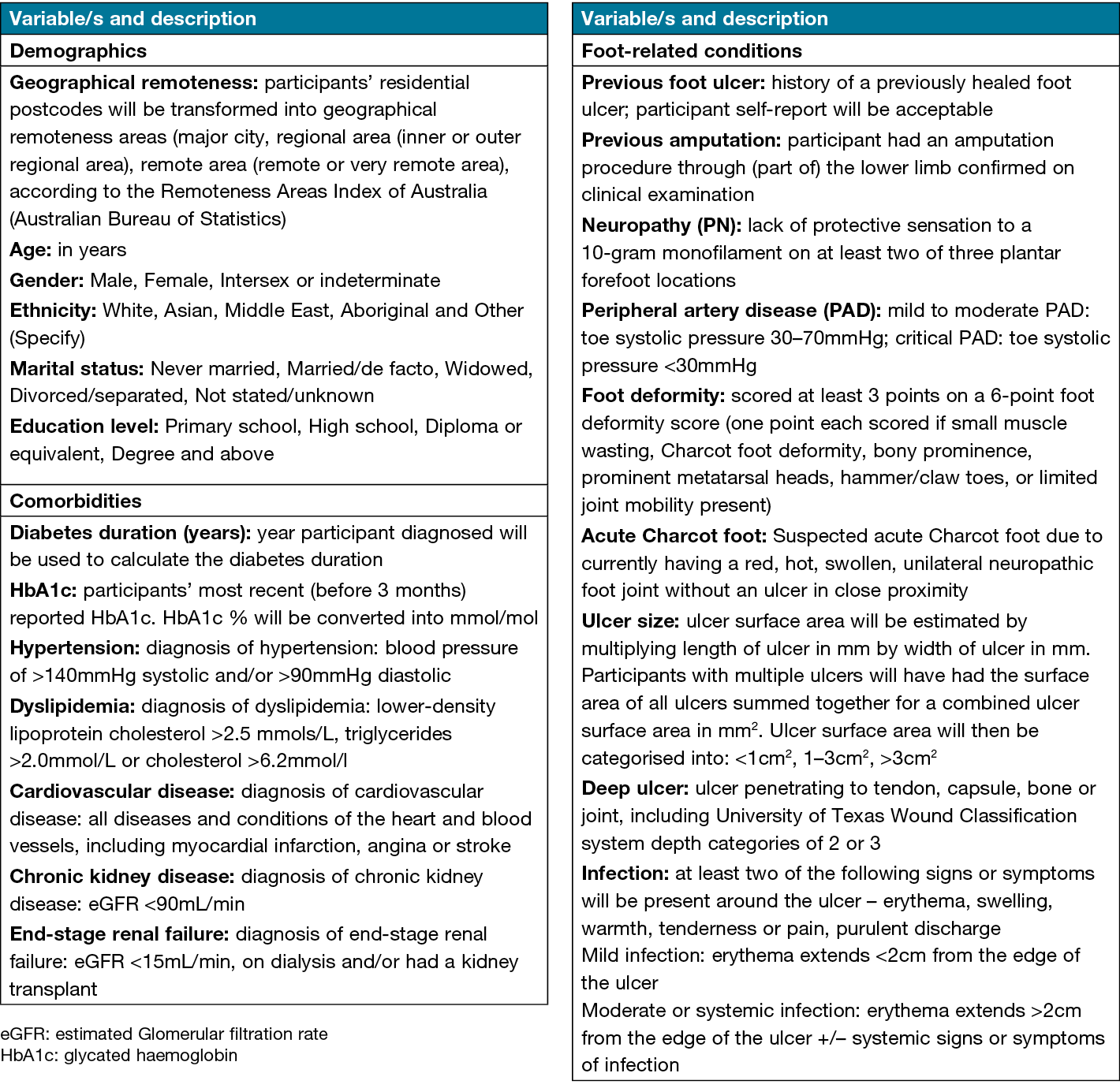
Figure 2. Descriptions of demographic data
Covariates
Cognition is influenced by several confounders such as demographics (gender, age and education level22,23), cardiovascular factors (blood pressure, cholesterol level, presence of carotid plaque)24–26, depression27 and physical activity and sedentary lifestyle28. Therefore, data will be collected on these items. The level of depression and physical activity will be assessed through the Patient Health Questionnaire-Depression (PHQ-9)29,30 and the Yale Physical Activity Survey (YPAS)31 respectively in both baseline and follow-up data collection periods. The effect of demographic and cardiovascular factors on cognition will be controlled as covariates during the analysis.
The self-administered PHQ-9 survey is a validated nine-item depression survey widely used for assisting primary care clinicians in diagnosing depression and monitoring treatment29–30. It is widely used among healthcare professionals caring for people with diabetes for screening for depression32,33. The survey is scored from 0 to 27, with a higher score indicating a higher probability of depression29. Furthermore, based on the raw PHQ-9 score, the level of depression is categorised into mild depression, moderate depression, moderately severe depression, and severe depression, by ranging PHQ-9 scores from 5 to 9, 10 to 14, 15 to 19, and 20–27 respectively29.
The self-administered YPAS was developed to determine the type, amount and patterning of physical activity/exercise which may influence cognition in older adults31. The tool is composed of two sections – “the amount of physical activity/exercise performed during a typical week in the past month” and “activities performed in the past month” – to estimate weekly energy expenditure31. Furthermore, the total time spent on those activities in a week is converted to weekly energy expenditure (kcal·wk-1) and total time index per week (h·wk-1) for measuring the level of physical activity34. The YPAS has shown acceptable validity35 and reliability36,37. Furthermore, the YPAS has also been previously used and found reliable in chronic wound research in Australian settings38.
Outcomes of interest
The primary outcome (global cognition) will be measured using the Montreal Cognitive Assessment tool (MoCA)39. The MoCA is a widely used validated screening test for assessing global cognition that is composed of 30 questions (score range 0–30)39,40. The MoCA has several categories based on the level of cognition; 26–30 is considered normal cognition, 18–25 mild cognitive impairment (MCI), 10–17 moderate cognitive impairment and 0–10 severe cognitive impairment39,40. The MoCA is recommended for use to assess cognitive changes in clinical settings41,42. Furthermore, the internal consistency of the MoCA is good, with a Cronbach’s alpha of 0.8339. Moreover, sensitivity and specificity to identifying MCI of MoCA among people with type 2 diabetes have been noted to be 67% and 93%43.
Study procedures
Participants who fulfil the inclusion criteria will be recruited from the participating diabetic foot services as a convenience sample. Figure 3 displays a summary of the study procedures.
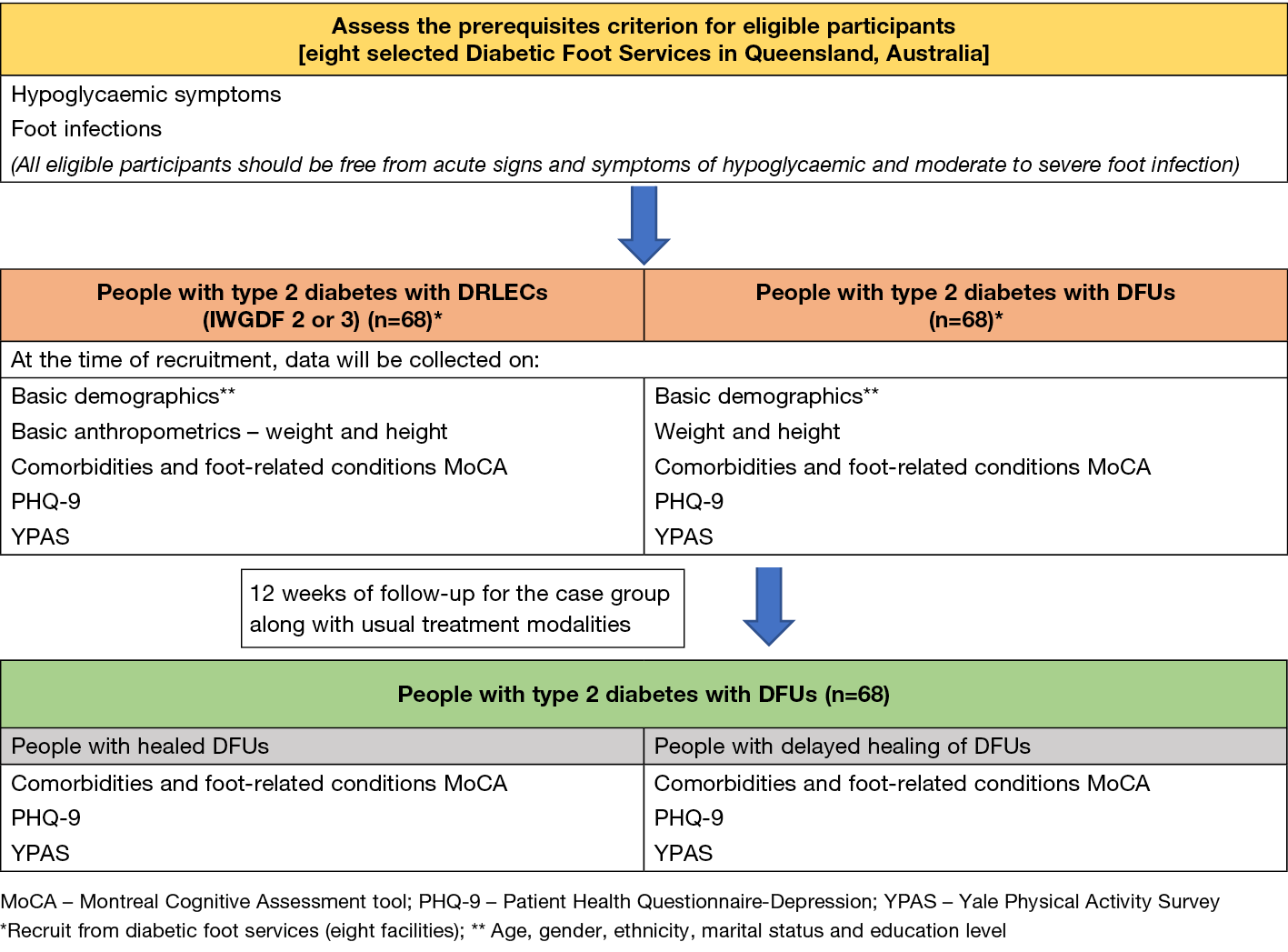
Figure 3. Summary of the study processes
Prerequisite eligibility criteria
All eligible consenting participants will be initially screened to ensure they are free from acute signs and symptoms of hypoglycaemia (clinical signs and symptoms) and moderate to severe foot infection (from medical records and clinical signs and symptoms) at their baseline study visit as these conditions are known to confound cognitive changes44,45. If a participant has any signs or symptoms of these conditions, they will not have baseline measurements performed and instead be invited to return for a future baseline visit.
Baseline measurements
Baseline measurements will be gathered from both cases and controls that include demographic information, comorbidities, foot-related conditions, BMI, MoCA, PHQ-9 and YPAS.
Follow-up measurements
The case group will be followed up 12 weeks after baseline data has been collected. At week 12, comorbidities, foot-related conditions, MoCA, PHQ-9 and YPAS will be collected from cases. The study process is depicted in Figure 3.
Statistical analyses
The data will be analysed using the Statistical Package for Social Sciences (SPSS) (version 29). The descriptive categorical data will be presented as counts and frequencies while descriptive continuous data will be presented as mean (SD) or median (IQR). All primary and secondary outcome variables will first be assessed graphically using scatter and boxplots and mean/median analyses to look at the between-group differences in data. Explanatory continuous variables will be compared between case and control groups using independent t-tests (parametric test) or Mann-Whitney U tests (non-parametric test) based on the test results of Shapiro-Wilk (normality test). Furthermore, a regression analysis will be performed to investigate the outcome of cognition among cases and controls, adjusting for the covariates (e.g., duration of diabetes, education, depression, physical activity, obesity and cardiovascular factors [presence/absence of hypertension, dyslipidaemia, cardiovascular diseases]).
During follow-up, the difference in cognition changes over time for cases will be analysed using generalised linear mixed models, utilising time as the primary independent variable, and controlling for covariates (e.g., duration of diabetes, education, depression, physical activity, obesity and cardiovascular factors) to assess changes in cognition. Furthermore, logistic regression, adjusted for duration of diabetes, education, depression, physical activity, obesity and cardiovascular factors, will be performed to assess any difference in cognition among cases who have healed compared to those not healed during the follow-up period of 12 weeks.
Ethical considerations
This protocol has been approved by two human research ethics committees – participating hospitals and health services (Hospital HREC/89344) and university ethics committees (University HREC Administration approval – 6859). Furthermore, governance approval has been received from each of the diabetes foot services for granting permission for data collection.
Discussion
The relationships between cognition and people with type 2 diabetes and DFUs are unclear due to the few relevant empirical studies reporting inconsistent findings. Therefore, it is still unclear if DFUs influence cognition among people with diabetes and how ulcer healing may influence cognition over time. Therefore, this case control study nested in a prospective longitudinal study is planned to address this existing evidence gap.
Implication for practice
This study will provide novel evidence on how cognitive changes may differ between those with DFUs compared to those with only DRLECs. Results should indicate which groups may, or may not, benefit from regular assessment of cognition to help clinicians in detecting early cognitive changes among people with diabetes with DFUs/DRLECs. Cognitive changes may affect self-care behaviour, including physical activity, healthy diet plans, self-monitoring of glucose levels, and adherence to treatment and medication46. For those at increased risk of cognitive impairment with DRLECs/DFUs, interventions to provide additional support to both the person with DRLECs/DFUs and their carer to manage their chronic condition could be implemented as part of primary prevention to mitigate the impact on self-care behaviour and adherence to treatment processes among people with type 2 diabetes.
Strengths
A case control study nested in a prospective longitudinal study is designed to assess cognitive changes between those with DFUs and those with only DRLECs. The robust methodology will be used to overcome limitations of the previous studies7–10 in areas of participant selection, data collection and controlling potential confounders as covariates during the analysis.
Limitations
The proposed 12-week follow-up time is based on the literature which suggests that around 50% of DFUs will be completely epithelialised within this time47,48 and complete epithelialisation without any drainage of a previous foot ulcer site is defined as a healed foot ulcer4.
Furthermore, it is expected that the number of participants recruited in each follow-up subgroup (i.e., for each group n=20–30) should provide statistically significant differences49. However, a limitation is that there is inadequate time to follow up with all patients until healing. Furthermore, there is no reliable evidence of a timeframe to repeat the MoCA assessment with meaningful cognitive changes. The proposed study has limited resources to look at differences between people with type 2 diabetes with and without DFU but does not consider other diabetes-related complications individually (i.e., PN, PAD). Foot-related conditions are assessed from medical records by following the clear guidance of the Queensland High Risk-Foot Form (QHRFF) which has been shown to have appropriate reliability and validity. QHRFF has also been recognised as a standardised instrument for collecting foot-related conditions data50 and is used in other studies for research purposes19,51. Additionally, the PN and PAD data from the QHRFF is captured by clinicians who have been trained to do these assessments at research standards (i.e., PN – 10-gram monofilament test and PAD – toe systolic pressure).
However, clinical data such as PN, PAD, ulcer characteristics and medical co-morbidities are not specifically collected for the purpose of this study which may affect the reliability of findings. Furthermore, the impact of certain medications (except hypoglycaemic drugs) on cognition is also not considered in this study. Nevertheless, as there is a lack of any evidence in this research field, the findings of this study will provide important evidence to inform larger studies investigating how cognition influences different diabetes-related complications that are risk factors for DFU.
Acknowledgments
The first author acknowledges the support of the Queensland University of Technology (QUT) as this study has been undertaken in partial fulfilment of a Doctor of Philosophy.
Conflict of interest
No conflicts of interest to report.
Ethics statement
This protocol has been approved by two human research ethics committees: participating hospital and health services (Hospital HREC/89344) and university ethics committees (University HREC Administration approval – 6859).
Funding
This publication was supported by QUT Postgraduate Research Award and QUT Higher Degree Research tuition fee scholarships.
Authors’ contributions
All authors conceived and designed the study. NK wrote the first draft of the manuscript while KF, PAL, CP and MM critically reviewed the manuscript.
Author(s)
Nimantha Karunathilaka*1–3, Christina Parker1,3, Peter A Lazzarini1,4,5, Margaret MacAndrew1,3, Kathleen Finlayson1,3
1Centre for Healthcare Transformation, Queensland University of Technology, Brisbane, QLD, Australia
2Department of Nursing & Midwifery, Faculty of Allied Health Sciences, General Sir John Kotelawala Defence University, Ratmalana, Sri Lanka
3School of Nursing, Queensland University of Technology, Brisbane, QLD, Australia
4School of Public Health and Social Work, Queensland University of Technology, Brisbane, QLD, Australia
5Allied Health Research Collaborative, The Prince Charles Hospital, Brisbane, QLD, Australia
*Corresponding author email nimantha.durage@hdr.qut.edu.au
References
- Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 2016;12:616–22.
- Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care 2020;43:964–74.
- Riandini T, Pang D, Toh MPHS, Tan CS, Liu DYK, Choong AMTL, et al. Diabetes-related lower extremity complications in a multi-ethnic Asian population: a 10 year observational study in Singapore. Diabetologia 2021;64:1538–49.
- Netten V, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev 2020;36 (Suppl 1):e3268.
- Lazzarini PA, Pacella RE, Armstrong DG, van Netten JJ. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability Diabet Med 2018.
- Lazzarini PA, Cramb SM, Golledge J, Morton JI, Magliano DJ, Van Netten JJ. Global trends in the incidence of hospital admissions for diabetes-related foot disease and amputations: a review of national rates in the 21st century. Diabetologia 2023;66:267–87.
- Kim HG. Cognitive dysfunctions in individuals with diabetes mellitus. Yeungnam Univ J Med 2019;36:183–91.
- Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GEHM, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol 2015;14:329–40.
- Natovich R, Kushnir T, Harman-Boehm I, Margalit D, Siev-Ner I, Tsalichin D, et al. Cognitive dysfunction: part and parcel of the diabetic foot. diabetes care 2016;39:1202–7.
- Khera T, Rangasamy V. Cognition and pain: a review. Front Psychol 2021;12.
- Bouché C, Zucchello A, Troude P, Sarron T, Dumurgier J, Gautier JF. Patients with diabetes and foot ulcer present cognitive dysfunction and express fewer needs in terms of educational support. Diabetes Metab 2019;45:491–3.
- Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW. Diabetes and cognitive impairment. Curr Diab Rep 2016;16:87.
- van Bussel FCG, Backes WH, Hofman PAM, van Oostenbrugge RJ, van Boxtel MPJ, Verhey FRJ, et al. Cerebral pathology and cognition in diabetes: the merits of multiparametric neuroimaging. Front Neurosci 2017;11:1–10.
- Ma L, Wang J, Li Y. Insulin resistance and cognitive dysfunction. Clin Chim Acta 2015;444:18–23.
- Brognara L, Volta I, Cassano VM, Navarro-Flores E, Cauli O. The association between cognitive impairment and diabetic foot care: role of neuropathy and glycated hemoglobin. Pathophysiol 2020;27(1):14–27.
- Doucet J, Le Floch JP, Bauduceau B, Verny C, SFD/SFGG Intergroup. GERODIAB: glycaemic control and 5-year morbidity/mortality of type 2 diabetic patients aged 70 years and older: 1. Description of the population at inclusion. Diabetes Metab 2012;38:523–30.
- Kloos., Hagen F, Lindloh C, Braun A, Leppert K, Müller N, et al. Cognitive function is not associated with recurrent foot ulcers in patients with diabetes and neuropathy. Diabetes Care 2009;32:894–6.
- Willrich A, Pinzur M, McNeil M, Juknelis M, Lavery L. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation: a preliminary study. Foot Ankle Int 2015;26(2):128–34.
- Zhang Y, Cramb S, McPhail SM, Pacella R, van Netten JJ, Cheng Q, et al. Multiple factors predict longer and shorter time-to-ulcer-free in people with diabetes-related foot ulcers: survival analyses of a large prospective cohort followed-up for 24-months. Diabetes Res Clin Pract 2022:109239.
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA, et al. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 (Suppl) 1:e3266.
- Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:171–91.
- Au B, Dale-McGrath S, Tierney MC. Sex differences in the prevalence and incidence of mild cognitive impairment: a meta-analysis. Ageing Res Rev 2017;35:176–99.
- Lipnicki DM, Makkar SR, Crawford JD, Thalamuthu A, Kochan NA, Lima-Costa MF, et al. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: a COSMIC collaboration cohort study. PLoS Med 2019;16(7):e1002853.
- Liu Y, Zhong X, Shen J, Jiao L, Tong J, Zhao W, et al. Elevated serum TC and LDL-C levels in Alzheimer’s disease and mild cognitive impairment: a meta-analysis study. Brain Res J 2020;1727:146554.
- Álvarez-Bueno C, Cavero-Redondo I, Bruno RM, Saz-Lara A, Sequí-Dominguez I, Notario-Pacheco B, et al. Intima media thickness and cognitive function among adults: meta-analysis of observational and longitudinal studies. J Am Heart Assoc 2022;11:e021760.
- Anbar R, Sultan SR, Al Saikhan L, Alkharaiji M, Chaturvedi N, Hardy R, et al. Is carotid artery atherosclerosis associated with poor cognitive function assessed using the Mini-Mental State Examination? A systematic review and meta-analysis. BMJ Open 2022;12:e055131.
- Hudon C, Escudier F, De Roy J, Croteau J, Cross N, Dang-Vu TT, et al. Behavioral and psychological symptoms that predict cognitive decline or impairment in cognitively normal middle-aged or older adults: a meta-analysis. Neuropsychol Rev 2020;30:558–79.
- Rojer AGM, Ramsey KA, Amaral Gomes ES, D’Andrea L, Chen C, Szoeke C, et al. Objectively assessed physical activity and sedentary behavior and global cognitive function in older adults: a systematic review. Mech Ageing Dev 2021;198:111524.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med 2001;16:606–13.
- Costantini L, Pasquarella C, Odone A, Colucci ME, Costanza A, Serafini G, et al. Screening for depression in primary care with Patient Health Questionnaire-9 (PHQ-9): a systematic review. J Affect Disord 2021;279:473–83.
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. N. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 1993;25:628–42.
- Cichon E, Kiejna A, Kokoszka A, Gondek TM, Radzio R, Jastrzebski A, et al. People with diabetes need a lower cut-off than others for depression screening with PHQ-9. PLoS One 2020;15:e0240209.
- Blanquisco L, Abejero JE, Buno Ii B, Trajano-Acampado L, Cenina A, Santiago D. Factors associated with mild cognitive impairment among elderly Filipinos with type 2 diabetes mellitus. J ASEAN Fed Endocr Soc 2017;32:145–50.
- Young DR, Jee SH, Appel LJ. A comparison of the Yale Physical Activity Survey with other physical activity measures. Med Sci Sports Exerc 2001;33(6):955–61.
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc 2001;33:962–70.
- Kolbe-Alexander TL, Lambert EV, Harkins JB, Ekelund U. Comparison of two methods of measuring physical activity in South African older adults. J Aging Phys Act 2006;14:98–114.
- Washburn RA. Assessment of physical activity in older adults. Res Q Exerc Sport 2000;71(sup2):79–87.
- Finlayson KJ, Parker CN, Miller C, Edwards HE, Campbell J. Decreased mobility, lack of social support, haemosiderosis and use of antidepressant medications may predict recurrent venous leg ulcers within 12 months of healing: a prospective longitudinal study. Phlebol 2021;37:206–15.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
- Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med 2015;175:1450–8.
- Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimer’s Dis Other Dement 2018;33:500–7.
- Bentvelzen A, Aerts L, Seeher K, Wesson J, Brodaty H. A comprehensive review of the quality and feasibility of dementia assessment measures: the dementia outcomes measurement suite. J Am Med Dir Assoc 2017;18:826–37.
- Alagiakrishnan K, Zhao N, Mereu L, Senior P, Senthilselvan A. Montreal Cognitive Assessment is superior to Standardized Mini-Mental Status Exam in detecting mild cognitive impairment in the middle-aged and elderly patients with type 2 diabetes mellitus. Biomed Res Int 2013:186106.
- Nevo-Shenker M, Shalitin S. The impact of hypo- and hyperglycemia on cognition and brain development in young children with type 1 diabetes. Horm Res Paediatr 2021;94:115–23.
- Muzambi R, Bhaskaran K, Brayne C, Davidson JA, Smeeth L, Warren-Gash C. Common bacterial infections and risk of dementia or cognitive decline: a systematic review. J Alzheimers Dis 2020;76:1609–26.
- Yang J, Zhang Z, Zhang L, Su Y, Sun Y, Wang Q. Relationship between self-care behavior and cognitive function in hospitalized adult patients with type 2 diabetes: a cross-sectional study. Diabetes Metab Syndr Obes 2020;13:207–214.
- Patry J, Tourigny A, Mercier MP, Dionne CE. Outcomes and prognosis of diabetic foot ulcers treated by an interdisciplinary team in Canada. Int Wound J 2021;18:134–46.
- Jeffcoate WJ, Bus SA, Game FL, Hinchliffe RJ, Price PE, Schaper NC. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality. Lancet Diabetes Endocrinol 2016;4:781–8.
- Dalmaijer ES, Nord CL, Astle DE. Statistical power for cluster analysis. BMC Bioinformatics 2022;23:205.
- Lazzarini PA, Ng V, Kinnear EM, Kamp MC, Kuys SS, Hurst C, et al. The Queensland high risk foot form (QHRFF) – is it a reliable and valid clinical research tool for foot disease? J Foot Ankle Res 2014;7:7
- Lazzarini PA, Hurn SE, Kuys SS, Kamp MC, Ng V, Thomas C, et al. Foot complications in a representative Australian inpatient population. J Diabetes Res 2017;2017:4138095.



