Volume 32 Number 1
Electrical stimulation therapy for wound-related pain: a WHAM evidence summary
Emily Haesler
For referencing Haesler E for Wound Healing and Management Collaborative. Electrical stimulation therapy for wound-related pain: a WHAM evidence summary. Wound Practice and Research 2024;32(1):44-48.
DOI 10.33235/wpr.32.1.44-48
Clinical question
What is the best available evidence for electrical stimulation therapy (EST) for reducing wound-related pain?
Summary
Electrical stimulation therapy is a biophysical modality through which an electromagnetic current is delivered to the wound with the intention of promoting wound healing. The electrical current is thought to influence healing by increasing blood flow to the wound bed. Level 1 evidence1-4 indicated mixed results for EST applied via microcurrent amplitudes using various electroceutical devices in reducing wound-related pain. In some studies, EST was associated with statistically significant reduction in wound-related pain.1, 4 In other studies, there was no significant difference in impact on pain of EST versus placebo or standard care.2, 3 The evidence was generally at moderate-to-high risk of bias, pain scores were often not high at baseline,1 and the level of pain reduction achieved was of questionable clinical significance in some studies.1,4 Only one study3 used a multidimensional pain assessment tool and none of the studies included pain assessment techniques that might identify nociceptive pain. Level 3 and 4 evidence from observational studies reported benefits of EST for wound-related pain.5-7 Using EST as an adjunct to best practice wound treatment is associated with improved wound healing outcomes,8 and this might contribute to reduction in wound-related pain for some people.
Clinical practice reccommendations
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
| There is insufficient evidence to recommend electrical stimulation therapy primarily to manage wound-related pain. Pain management might be experienced when using electrical stimulation therapy to promote wound healing in hard-to-heal wounds that have not responded to best practice wound treatment. |
Sources of evidence
This summary was conducted using methods published by the Joanna Briggs Institute.9-12 The summary is based on a systematic literature search combining search terms related to wounds and EST. Searches were conducted in Embase, Medline, Cochrane Library and Google Scholar with inclusion limited to evidence published from January 2013 to December 2023 in English. Levels of evidence for intervention studies are reported in the table below.
Background
Electrical stimulation therapy involves applying an electrical current to the wound. The current is generally generated by a battery-type device at various electrical frequencies, amplitudes, polarities, and either in a direct, alternating or pulsed (monophasic or biphasic) current. The electrical stimulation is applied by placing at least two electrodes on the skin (with at least one applied to either the wound bed or the peri-wound skin) to conduct the electrical current through the wound tissue.
Electrical stimulation therapy can be broadly categorised based on the response the amplitude elicits in the individual. Higher amplitudes (300-400 milliamps [mA]; e.g. electrical muscle stimulation [EMS]) generate a motor response (e.g., muscle contraction); however, this level of stimulation is generally not required in wound care.18 Electrical stimulation therapy at an amplitude of 150-250 mA (e.g., trans-epidermal nerve stimulation [TENS]) leads to a sensory response (e.g., tingling or prickling) and at less than 100 mA the stimulation is sub-sensory (i.e., the recipient does not sense the stimulation).18 Sub-sensory electrical stimulation at the lowest of amplitude (e.g., below 60 mA) is referred to as microcurrent stimulation.18 Most EST is delivered in sessional treatments and using a range of regimens (regularity, duration, etc.).
Electrical stimulation therapy is used to promote wound healing and to reduce wound pain. The mechanism through which electrical current might promote wound healing is suggested to be promoting increased blood flow and reducing tissue oedema, which positively influences tissue oxygenation and cell proliferation.13, 19, 20 Any reduction in wound pain that might be achieved is likely to be associated with the stimulation of cell activity and reduction in tissue oedema that leads to wound healing.18,19 Because higher amplitude EST elicits motor response and sensations that are often described as tingling or uncomfortable, EST at lower amplitudes (i.e., TENS or microcurrent stimulation) is more often associated with pain relieving outcomes.18
Clinical evidence
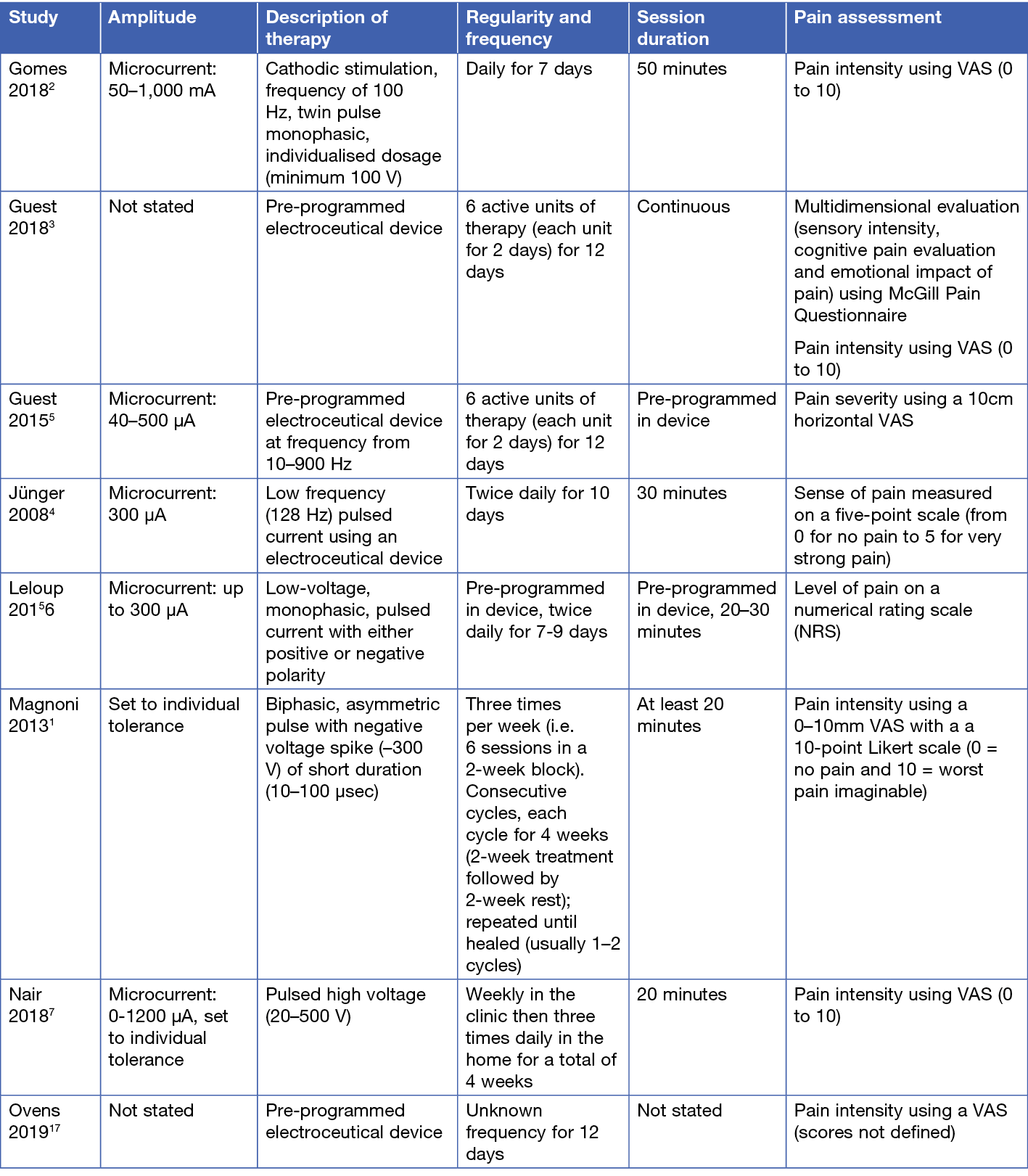
The best clinical evidence on EST used to address wound-related pain comes from small trials that investigate electroceutical devices delivering microcurrent EST directly to the wound bed. The studies assessed pain using a unidimensional pain assessment tool to evaluate pain intensity or severity. Only one study3 also included a multidimensional pain assessment tool, and none of the studies included an evaluation of pain quality that might identify characteristics of nociceptive, neuropathic and or mixed pain experience. The different regimens are summarised in Table 1. Level 1 evidence comes from four RCTs1-4 with mixed findings on the impact of EST on pain:
- An RCT3 at low risk of bias compared EST to placebo therapy for VLUs, with pain as one of the patient-reported outcome measures. The EST was applied with an electroceutical device (Accel-Heal) for 12 days, with outcomes measured at various intervals up to 24 weeks for the 90 participants. There were no statistically significant differences in visual analogue scale (VAS) scores or McGill Pain Questionnaire scores at any time point between people who received the EST device and those receiving a placebo device. Mean pain scores decreased over time in both groups3 (Level 1).
- An RCT1 at moderate risk of bias found a significant effect for Frequency, Rhythmic Electrical Modulation System (FREMS) in reducing pain associated with chronic leg ulcers (n = 60, different aetiologies). The FREMS EST was applied via electrodes at five different locations on the lower limb, including the peri-wound skin. Pain intensity was measured at 2-week intervals using a 0–10 mm VAS. Compared with no treatment, the FREMS regimen was associated with a significantly greater reduction in VAS scores, with statistical significance noted for all time intervals after the first treatment cycle1 (Level 1). However, the mean pain intensity score was 3 mm at study commencement, suggesting pain was not a major concern for the participants.
- Another small RCT2 at moderate risk of bias explored EST for managing pain at skin graft donor sites. The EST was applied using the Neurodyn High Volt (IBRAMED) device. Participants (n = 30) received either EST or sham therapy. Pain was measured using a VAS (score 0–10) before and after treatment, each day for 7 days. The same pain experience pattern was observed in both groups, with pain intensity at its highest immediately following donor site harvest and decreasing over time, to a negligible pain level at day 3 in the EST group and by day 7 in the sham therapy group2 (Level 1).
- An RCT4 at high risk of bias explored pulsed EST using an electroceutical device (Dermapulse®). Participants with VLUs (n = 39) of at least 3 months’ duration received either the EST or placebo therapy. Pain was scored monthly using a 5-point scale. At month 4 there was a statistically significant (p = 0.049) difference in pain reduction between the groups, favouring the EST4 (Level 1). However, the average reduction in pain was approximately 1-point on the scale, which might not have been clinically significant.
Four observational studies5-7, 17 provided additional evidence that EST can reduce wound pain, but this evidence is at moderate-to-high risk of bias, the studies are small and positive results are sometimes negligible:
- A cost effectiveness study5 at high risk of bias compared clinical and cost outcomes for people with chronic venous leg ulcers (VLUs, n = 30) over the 12 months prior to EST and 12 months with EST added to the standard regimen. The EST was applied with an electroceutical device (Accel-Heal). Pain scores on a 0–10 VAS after 12 months of EST significantly decreased (mean pain score before therapy 3.6, 95% confidence interval [CI] 2.4 to 4.8 versus mean pain score after therapy 0.63, 95% CI 0 to 1.3, p < 0.001)5 (Level 3). However, there was no control for change over time that might be expected over 12 months of treatment.
- In an observational study6 at high risk of bias, people (n = 73) with hard-to-heal wounds (median duration 12 months, different aetiologies) were treated with electrical stimulation therapy delivered with an electroceutical device (WoundEL®). Pain was measured using a numeric rating scale (NRS) at commencement of EST, at day 3 and at conclusion of treatment (day 7 or day 9). Median NRS score decreased significantly over time from day 0 (median 6, SD 3.3), to day 3 (median 3, SD 2.8; p < 0.001) and to day 7 or 9 (median 2, SD 2.2; p < 0.001). The decrease in pain was also associated with a decrease in analgesic treatment (Level 3). However, there was no comparison group in this study6.
- A case series7 at moderate risk of bias reported the use of pulsed EST as an adjunct to standard care for 100 chronic wounds, primarily DFUs of more than 12 months’ duration. The EST in this study was a pulsed microcurrent delivered using a system (BEST, Biofeedback Electro-Stimulation Technology) that allowed application of the electrical stimulation wirelessly without touching the skin (i.e., therapy was delivered through clothes and bandages). The EST was delivered weekly in the clinic at the time of wound dressing changes, and three times daily at home. Of those reporting wound pain at baseline (n = 89), there was a statistically significant reduction in the mean VAS score from a pre-treatment mean score of 6.0 ± 1.75 to post-treatment score of 2.2 ± 1.47 (p < 0.001). Over the course of the 4-week study, 59% of these participants experienced at least a 50% reduction in pain score7 (Level 4). This is likely to have been clinically significant.
- In a small observational study17 at high risk of bias, EST was applied with an electroceutical device (Accel-Heal) for 12 days to VLUs (n = 8). Progress was followed for up to 20 weeks. Of the three VLUs reported as painful at the commencement of the study, all three reported rapid reduction in pain, with a mean reduction of 84% observed in the first two weeks and pain resolution within eight weeks for all VLUs17 (Level 4).
Table 1. Electrical stimulation therapy regimens
Considerations for use
- Electrical stimulation therapy should not replace best standard of wound care.21
- Reduction in wound pain is associated with other positive clinical outcomes, including reduction in use of analgesia and improved sleep quality.7
- Evaluate the capacity of the individual to adhere to treatment when selecting adjunct therapies, therapy device and the treatment regimen.18
- Standards of wound practice22 and evidence-based clinical guidelines23, 24 outline that health professionals should collaborate with an interdisciplinary team when selecting adjuvant therapies, and have appropriate education and training before selecting or delivering EST, or teaching individuals to self-administer.
Adverse effects and complications
Some complications/adverse events are associated with treating wounds with EST. A small number of people treated with electrical stimulation therapy reported dizziness and delusions, but these were not attributed to the EST intervention.13,14 Skin redness, irritation, slight discomfort, tingling or burning sensations have also been reported13,14,16, but the certainty that these events were associated with EST is low.13 A minor burn has also been reported in one person.14
Use caution when applying high voltage monophasic pulsed current (HVPMC) to wounds in people with Raynaud’s syndrome.15 Increased wound pain has been reported but more research is required on this potential adverse event.15
Funding
The development of WHAM evidence summaries is supported by a grant from The Western Australian Nurses Memorial Charitable Trust.
Conflicts of interest
The author declares no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
About WHAM evidence summaries
WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information.
WHAM evidence summaries are developed using methodology consistent with that published by Joanna Briggs Institute9-12. Evidence underpinning a WHAM recommendation is identified via a PICO search strategy, assigned a level of evidence and evaluated for risk of bias. All WHAM evidence summaries are peer-reviewed by an international Expert Reference Group. For more information on the methods and the WHAM Expert Reference Group, visit the website: www.WHAMwounds.com.
Copyright © Wound Healing and Management Collaborative, Curtin University, and the authors.
Author(s)
Emily Haesler, PhD, P Grad Dip Adv Nurs (Gerontics), BN, Fellow Wounds Australia
Adjunct Professor, Curtin University, Curtin Health Innovation Research Institute, Wound Healing and Management (WHAM) Collaborative
Email emily.haesler@curtin.edu.au
References
- Magnoni C, Rossi E, Fiorentini C, Baggio A, Ferrari B, Alberto G. Electrical stimulation as adjuvant treatment for chronic leg ulcers of different aetiology: an RCT. J Wound Care, 2013; 22(10): 525-33.
- Gomes RC, Guirro ECO, Gonçalves AC, Farina Junior JA, Murta Junior LO, Guirro RRJ. High-voltage electric stimulation of the donor site of skin grafts accelerates the healing process. A randomized blinded clinical trial. Burns, 2018; 44(3): 636-45.
- Guest J, Singh H, Rana K, Vowden P. Cost-effectiveness of an electroceutical device in treating non-healing venous leg ulcers: results of an RCT. J Wound Care, 2018; 27(4): 230-43.
- Jünger M, Arnold A, Zuder D, Stahl H-W, Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse®): A prospective, placebo controlled, double blind trial. Wound Repair Regen, 2008; 16(4): 480-7.
- Guest JF, Ayoub N, Greaves T. Clinical outcomes and cost-effectiveness of an externally applied electroceutical device in managing venous leg ulcers in clinical practice in the UK. J Wound Care, 2015. Dec;24(12):572, 4-80.
- Leloup P, Toussaint P, Lembelembe J-P, Célérier P, Maillard H. The analgesic effect of electrostimulation (WoundEL®) in the treatment of leg ulcers. Int Wound J, 2015; 12(6): 706-9.
- Nair H. Microcurrent as an adjunct therapy to accelerate chronic wound healing and reduce patient pain. J Wound Care, 2018; 27(5): 296-306.
- Haesler E. WHAM evidence summary: Electrical stimulation therapy for wound healing Wound Practice and Research, 2024.
- Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. https://synthesismanual.jbi.global: Joanna Briggs Institute; 2020.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. New JBI Grades of Recommendation. Adelaide, Australia: Joanna Briggs Institute, 2013.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. Adelaide, Australia: Joanna Briggs Institute, 2014.
- Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach. Worldviews Evid Based Nurs, 2015; 12(3): 131-8.
- Arora M, Harvey LA, Glinsky JV, Nier L, Lavrencic L, Kifley A, Cameron ID. Electrical stimulation for treating pressure ulcers. Cochr Database of Sys Rev, 2020 (1).
- Khouri C, Kotzki S, Roustit M, Blaise S, Gueyffier F, Cracowski J-L. Hierarchical evaluation of electrical stimulation protocols for chronic wound healing: An effect size meta-analysis. Wound Rep Regen, 2017; 25(5): 883-91.
- Girgis B, Carvalho D, Duarte J. The effect of high-voltage monophasic pulsed current on diabetic ulcers and their potential pathophysiologic factors: A systematic review and meta-analysis. Wound Repair Regen, 2023; 31(2): 171-86.
- Lala D, Spaulding SJ, Burke SM, Houghton PE. Electrical stimulation therapy for the treatment of pressure ulcers in individuals with spinal cord injury: A systematic review and meta-analysis. Int Wound J, 2016; 13(6): 1214-26.
- Ovens L. Application of Accel-Heal for patients with chronic venous leg ulcers: an evaluation in a community UK NHS trust. Wounds UK, 2019; 15: 78-84.
- Milne J, Swift A, Smith J, Martin R. Electrical stimulation for pain reduction in hard-to-heal wound healing. J Wound Care, 2021; 30(7): 568-80.
- Forrester I. Electrotherapy and wound healing. Wounds Middle East, 2018; 5(2): 18-25.
- Barnes R, Shahin Y, Gohil R, Chetter I. Electrical stimulation vs. standard care for chronic ulcer healing: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Invest, 2014; 44(4): 429-40.
- Rayman G, Vas P, Dhatariya K, Driver V, Hartemann A, Londahl M, Piaggesi A, Apelqvist J, Attinger C, Game F. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev, 2020;36 (S1): (e3283).
- Haesler E, Carville K. Australian Standards for Wound Prevention and Management: Australian Health Research Alliance, Wounds Australia and WA Health Translation Network, 2023.
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan-Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Haesler E (editor): EPUAP/NPIAP/PPPIA, 2019.
- Rivolo M, Dionisi S, Olivari D, Ciprandi G, Crucianelli S, Marcadelli S, Zortea RR, Bellini F, Martinato M, Gabrielli A, Pomponio G. Heel pressure injuries: Consensus-based recommendations for assessment and management. Adv Wound Care, 2020; 9(6): 332-47.



