Volume 32 Number 1
Skin integrity, antimicrobial stewardship and infection control: a critical review of current best practice
Joanna Blackburn, Zlatko Kopecki, Karen J Ousey
Keywords skin integrity, antimicrobial stewardship, infection control
For referencing Blackburn J, Kopecki Z, Ousey KJ. Skin integrity, antimicrobial stewardship and infection control: a critical review of current best practice. Wound Practice and Research. 2024;32(1):34-43.
DOI
10.33235/wpr.32.1.34-43
Submitted 14 February 2024
Accepted 1 March 2024
Abstract
‘Skin integrity’ refers to intact, unbroken, and healthy skin. Disruption of skin integrity can be caused by intrinsic and extrinsic factors including altered nutritional status, vascular disease, diabetes, and tissue injury, and this is often associated with development of localised clinical infection. Skin health and hygiene is important for preventing wounds and development of localised clinical infection or sepsis. Clinical wound infection is an increasing problem in healthcare, with the potential for increasing the burden of antimicrobial resistance (AMR), if antimicrobials are overused to treat wound infection.
In this review we discuss skin integrity and wound infection prevention and outline the guiding principles of antimicrobial resistance and antimicrobial stewardship for infection control. Additionally, we provide a critical review of current best practice, highlighting the pathway to guide management of patients at risk of infection development, and discuss the latest research progress on antimicrobial resistance and skin integrity.
Introduction
Optimal skin health is crucial for protecting the body against disease and infection, as well as serving to regulate body temperature and preventing damage to the internal organs. Skin integrity has been defined by the Department of Health, Australia,1 as the skin being “whole, intact and undamaged”, maintenance of skin integrity can be disrupted through processes such as natural aging2 or from extrinsic factors including external damage, trauma, or infection.3,4 Impaired skin integrity occurs when the body fails to protect itself. Defined by the North American Nursing Diagnosis Association5 as an “altered epidermis and/or dermis, destruction of skin layers (dermis) and disruption of skin surface (epidermis),” impaired skin integrity can occur in response to, or be caused by, infection and inflammation, which can lead to complications such as disease, skin tears and pressure damage.2,3 Clinical wound infection is an increasing problem in healthcare, with the potential for increasing the burden of antimicrobial resistance (AMR) if antimicrobials are overused to treat wound infection. Appropriate treatment and management of wound infection and ensuring the appropriate prescribing of antibiotics is crucial to limiting the spread of AMR. Antimicrobial Stewardship (AMS) is a coordinated approach to ensuring the appropriate use of antimicrobials (including antibiotics) to improve patient outcomes, and includes strategies such as raising awareness of AMR and appropriate antimicrobial prescribing. AMS is one way of reducing the problem of AMR in healthcare.
Skin integrity and wound infection prevention
The Wounds UK Best Practice Statement on Maintaining Skin Integrity6 highlights who is most at risk of complications from skin damage. The list includes older people and patients suffering from long-term conditions.6 Several inflammatory, autoimmune and genetic skin conditions, such as eczema, psoriasis and dermatitis, which affect the skin’s ability to act as a barrier against infection also predispose people to compromised skin integrity.6
Although some patients maybe be predisposed to developing complications due to impaired skin integrity, it is the responsibility of health care professionals to ensure all patients have an adequate holistic skin assessment to help prevent breakdowns in skin function.7 Skin assessments can help to identify potential risks to the patient and prevent skin damage occurring or escalating, as well as providing an opportunity to implement appropriate treatment strategies, if necessary. A holistic approach to help prevent breakdowns in skin function should include gathering details of the patients’ medical history, including overall health, mobility, and nutrition.8 Appropriate training and education on the risk factors of impaired skin integrity and undertaking skin assessments are vital to the prevention of wound development.9 The Wounds UK Best Practice Statement on Maintaining Skin Integrity6 presents the following elements that should be included in a comprehensive skin assessment:
- Patient medical history,
- Assessment of skin condition, texture and temperature,
- Assessment of intrinsic or wound related factors,
- Assessment of patient’s knowledge about their skin condition, and 4) history of the skin condition.6
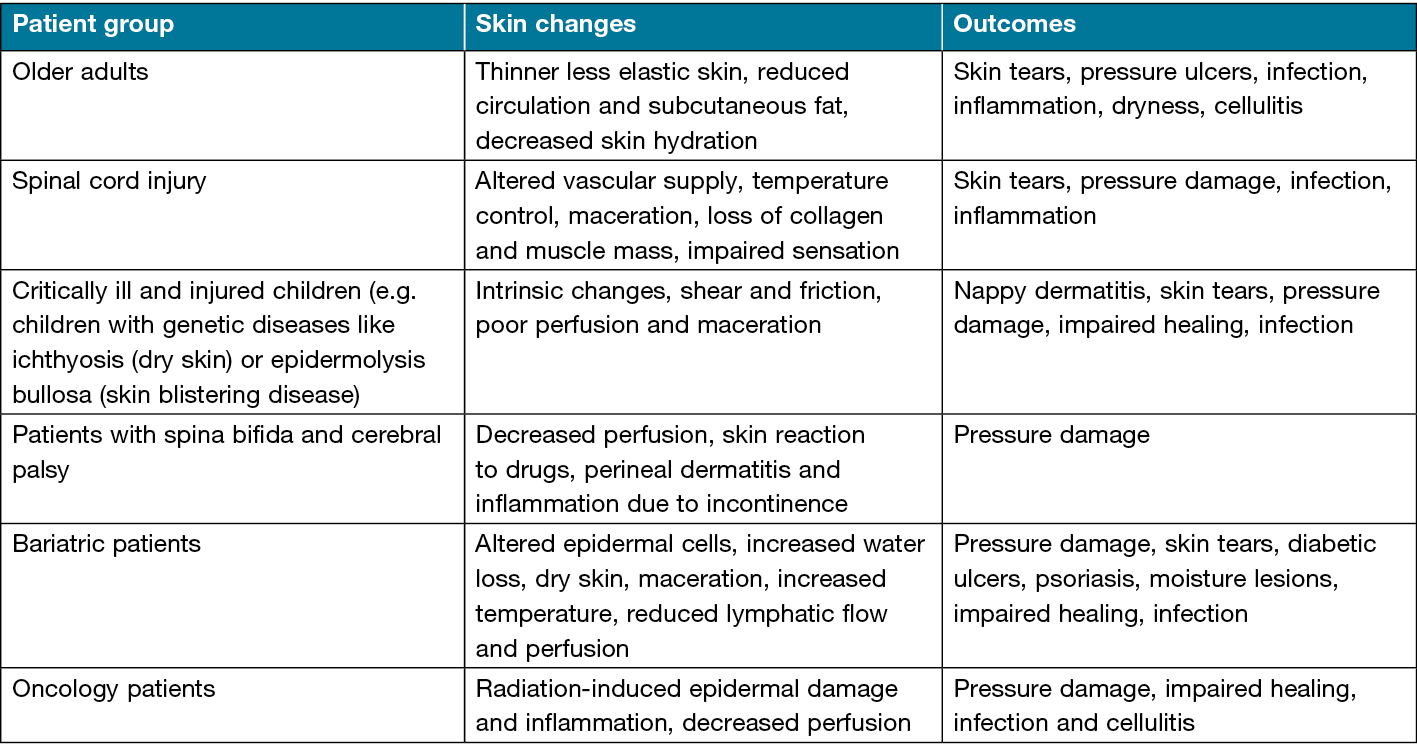
Most common skin conditions that affect the skin integrity may include following: rash (irritation or medically induced), inflammatory skin conditions (such as eczema, psoriasis), genetic conditions (such as ichthyosis, epidermolysis bullosa), pruritus, cellulitis, skin cancer (such as melanoma, squamous cell carcinoma), lipodermatosclerosis.6 Patients at high risk of disrupted skin integrity and associated skin changes are outlined in Table 1.
Table 1. Patient groups at high risk of disrupted skin integrity, associated skin changes and potential outcomes. Reproduced with permission from The Wounds UK Best Practice Statement on Maintaining Skin Integrity.6
Skin health and good skin hygiene are important for preventing wounds and development of localised clinical infection or sepsis. Clinical wound infection has been defined by the Wound Infection Institute10 as “the invasion of a wound by proliferating microorganisms to a level that invokes a local and/or systemic response in the host”. Clinical wound infection has been highlighted as an increasingly emerging medical problem with profound impacts on the healthcare system, with the potential for severe and enduring complications for patients and associated financial burdens, if early identification and appropriate treatment interventions are not implemented promptly. The IWII Wound Infection Continuum (WIC)10 demonstrates the phases of wound infection through five stages from contamination to systemic infection and describes the symptoms associated with each phase (Figure 1). Factors it claims are associated with increased risk of infection include:
- Host risk factors, such as chronic diseases like diabetes, peripheral neuropathy, radiation or chemotherapy, immune system propblems and connective tissue disorders, malnutrition, obesity, and alcohol and drug abuse;
- Wound risk factors (chronicity, duration, type of injury, anatomical location, foreign body presence or necrotic tissue, increased oedema, impaired perfusion, deep tissue involvement); and
- Environmental factors, such as an unhygienic environment, hospitalisation, inadequate moisture management, and interface pressure.
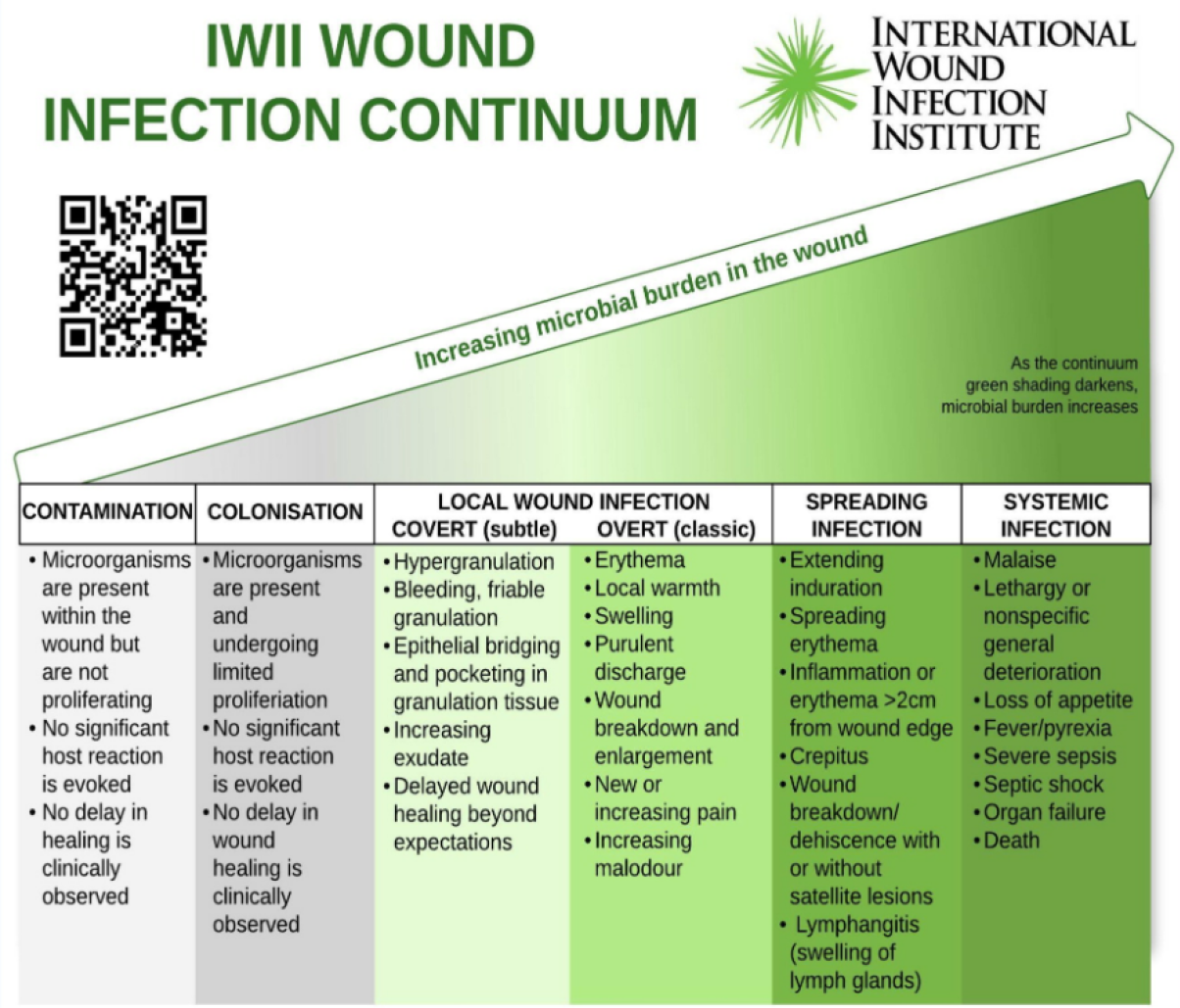
Figure 1. International Wound Infection Institute (IWII) Wound infection Continuum (IWII-WIC). Reproduced with permission from IWII10
While many sample tools have been developed to assess the risk of infection many are validated against certain wound types, dependent on numerous risk variables, and have low to moderate predictive power. Hence, the expertise of the healthcare professional is relied upon to diagnose critical colonisation early, before localised clinical infection develops.
Antimicrobials is a general term for a set of treatments designed to reduce infection. It can include antibiotics, antiseptics, antivirals, antifungals and anti-parasitic.11 Antimicrobial Resistance (AMR) occurs when microorganisms, through repeated exposure to medicines, develop a resistance and no longer respond to antimicrobial treatments, even at high concentrations.11 Infections that develop resistance to commonly available antimicrobials become harder to treat and increase the risk and spread localised wound infection to sepsis. AMR has a significant impact on the public, patients, and healthcare system.11 Antimicrobial resistant bacteria increase the risk of disease and mortality and increase healthcare costs, due to longer hospital stays and treatment. Furthermore, it is estimated that over 5 million deaths worldwide each year are due to AMR11 and this figure is expected to increase over time.
Antimicrobial resistance (AMR) and antimicrobial stewardship (AMS)
Studies to date have shown that AMR can be accelerated by: the overuse and misuse of antibiotics in humans and animals; health care transmission; environmental contamination; and suboptimal vaccination. A lack of newly available antibiotics to treat infections also significantly increased the risk of serious illness and death in the community.12 To address the problem of AMR, the World Health Organization (WHO)13 developed the Global Action Plan (GAP) which outlines the global priorities for tackling AMR and focuses on five main strategic objectives:
- Improving AMR awareness and understanding
- Strengthening knowledge through AMR surveillance and research
- Reducing the incidence of clinical infection
- Optimising the use of antimicrobial medicines
- Ensuring sustainable investment in tackling AMR
Central to the GAP is AMS; a coordinated approach to ensuring the appropriate use of antimicrobials (including antibiotics) to improve patient outcomes, reduce AMR, and decrease the spread of infections caused by multidrug-resistant organisms. AMS and infection control are critical elements of healthcare practices aimed at promoting the effective use of antimicrobial agents to treat infections. AMS has been defined by several healthcare organisations focused on education about the treatment of infectious diseases and infection prevention to limit the burden and spread of AMR. The National Institute for Care and Health Excellence (NICE) defines AMS as “an organisational or healthcare-system-wide approach to promoting and monitoring judicious use of antimicrobials to preserve their future effectiveness”.14 While WHO defines AMS as “a coherent set of integrated actions, which promote the response and appropriate use of antimicrobials to help improve patient outcomes across the continuum of care”.15 WHO also has a distinct definition of an AMS program which is “an organisational or system-wide health-care strategy to promote appropriate use of antimicrobials through the implementation of evidence-based interventions”.15 The GAP is also supported by the WHO Global Framework for the Development and Stewardship to Combat AMR,16 which aims to provide a set of evidence-based recommendations to drive integrated AMS activities within organisations, to preserve antimicrobials. Central to this is the integration of infection control measures, such as appropriate hand hygiene measures and access to clean water (particularly in low to middle income countries) which can minimise the emergence and spread of AMR.16
The guiding principles of the World Health Organization (WHO) policy15 for integrated AMS activities are:
- Give due consideration to national and local context and the structure of the health system in carrying out AMS activities.
- Focus on prioritising implementation of activities that are likely to provide the greatest benefits based on national and facility needs assessment.
- Strengthen and use existing national and subnational platforms and coordinating mechanisms and resources to implement integrated AMS activities.
- Ensure strong and effective linkages and synergies between relevant areas and disciplines related to AMR, including national infectious diseases and infection prevention programmes.
AMS and wound care
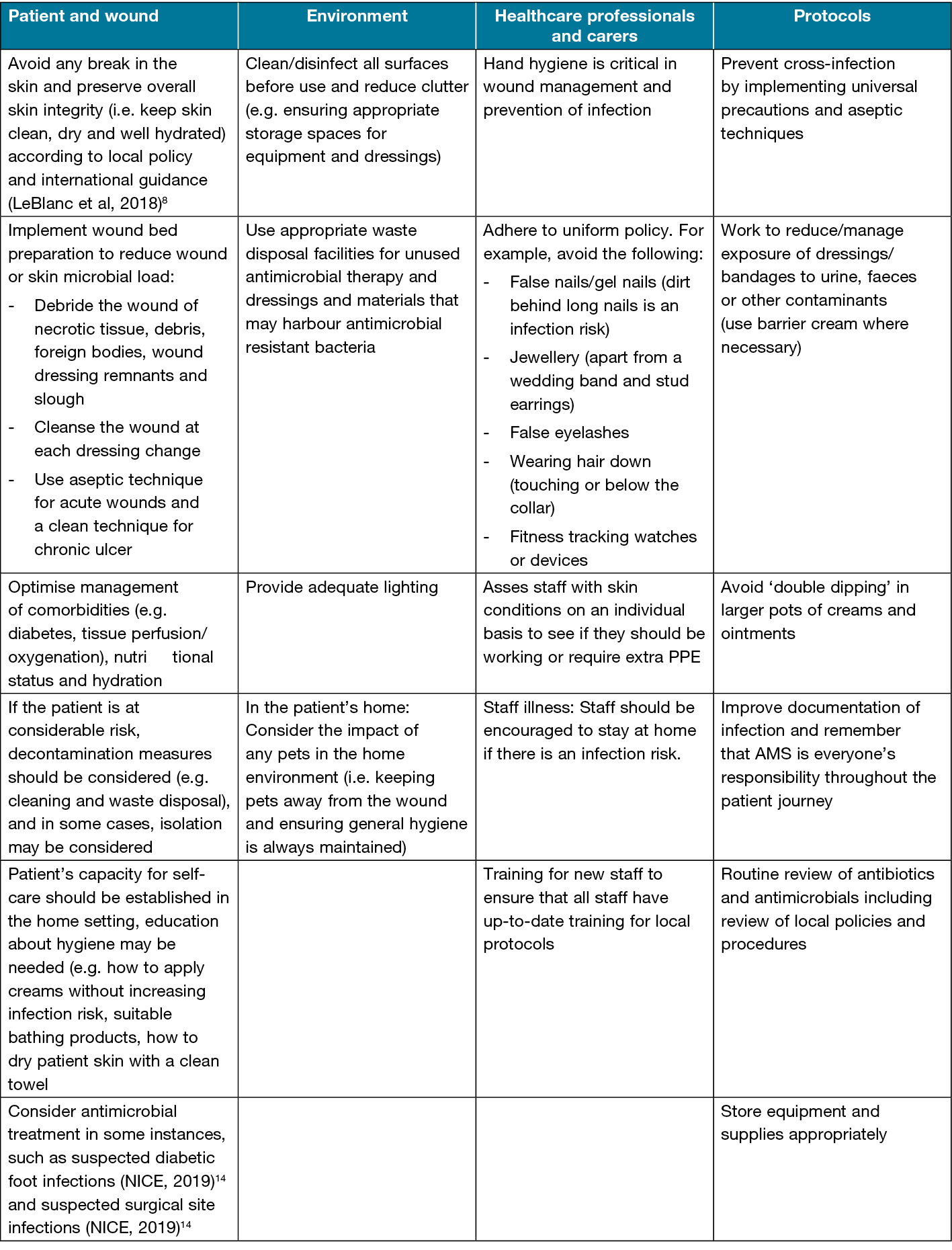
The Wounds UK Best Practice Statement on AMS for wound management17 describes the important role AMS can play in maintaining good skin integrity and provides useful advice on infection prevention practices. The multimodal approach17 to antimicrobial stewardship (AMS) practice is underpinned by good education including:
Increased public awareness, implementing changes to local and processes and systems;
- Having good knowledge of multidisciplinary teams;
- Accurate assessment of clinical signs and symptoms of infection;
- Minimising the use of unnecessary broad spectrum antibiotics;
- Use of dressings with a physical mode of action, which act to bind bacteria and fungi, in conjunction with antiseptics and topical agents for wound care; and
- Understanding the responsibilities and expectations of patient self-care.
The foundation of infection prevention in wound care should focus on a back-to-basics approach of good hand hygiene, use of personal protective equipment, good waste management, comprehensive documentation, and management of the patient’s environment.
Table 2 provides a detailed summary describing the infection protection and AMS practice in wound care as outlined below:
Several barriers to implementing effective antimicrobial stewardship (AMS) strategies have been identified in the literature. For example, Limato et al,18 found that healthcare professionals (including microbiologists, pharmacists, physicians, surgeons, and hospital managers) described how ineffective resources, competing priorities and insufficient medical facilities prevented AMS strategies from being effective. Other research highlighted that although awareness of AMS in wound care has been found to be high, the measurement of the impact of AMS programs or strategies is low,19 therefore increasing education around how AMS activities can be measured is an important aspect of reducing the impact of AMR in healthcare.
The International Wound Infection Institute (IWII) Wound Infection in Clinical Practice consensus document10 states that AMS activities should center around education on appropriate prescribing and monitoring the use of antimicrobial usage. At an individual level, this could involve better education of patients and caregivers surrounding signs of infection, the importance of adherence to treatments and medications, when to seek medical attention, and on suitable alternatives to the inappropriate use of antimicrobials. At a system level, this could involve ongoing healthcare professional education and regular auditing of antimicrobial prescribing.10 The IWII also recommends establishing an AMS advisory group to guide and monitor antimicrobial use, including antibiotic practices within organisations to drive change and improvements in current practice and limit the use of unnecessary prescribing. This is important, since evidence from the UK demonstrates a significant amount of antibiotic prescribing occurs in primary care with approximately 20% of antibiotics being prescribed inappropriately.20 Alarmingly, that figure was even higher in Australia where 33−73% of prescriptions in primary care were not appropriately prescribed, compared to 23% in hospitals,21 with level of experience, use of AMR guidelines and clinical setting being the biggest driving factors in antibiotic prescribing.22 The integration and monitoring of AMS strategies at all organisational levels is necessary for AMS activities to drive change in antibiotic prescribing in wound care.23 Health care professionals are expertly placed to advance change in their own practices and provide education to patients on AMR, as insufficient knowledge has been found to drive inappropriate prescribing worldwide.24 A multidisciplinary collaborative approach with experts in wound care, including tissue viability nurses, wound nurse practitioners, podiatrists and community nurses having input into AMS strategies is highly recommended.25 Edward-Jones26 described the importance of AMS education for those working in wound care and advocated for exploration of other methods of wound care rather than simply prescribing antibiotics, with topical antiseptics being one proven method of reducing the impact of AMR.26
Table 2. Summary of infection prevention and AMS practice considerations. Reproduced with permission.17
AMS and antiseptics
The WHO Access, Watch, Reserve (AWaRe)11 antibiotic guidance document defines antibiotics as “antimicrobial products used to slow or stop the growth of microorganisms”.In the vast majority of cases, antibiotics are not necessary, despite a significant proportion of patients who present in primary care with an infection still being prescribed them.27 The international consensus document on the use of wound antiseptics in clinical practice28 provides an overview on the potential benefits of using antiseptics to prevent and treat wound infection, alongside practical guidance on how to use them safely and effectively in a clinical practice. This document states that antiseptics are grossly underutilised as a method of infection management29 and that they are an effective alternative to antimicrobials.30,31 Topical antiseptics commonly used in wound management can include wound dressings, lotions, and cleansers.28 Choosing the right antiseptic for the patient is crucial to avoid causing unnecessary problems, including additional skin irritation.32
Consistently, Blackburn et al34 explored the effects of using topical antimicrobials on AMR and found that there was very limited evidence to prove efficacy, with most clinical evidence focusing on exploring the effectiveness of topical antimicrobials on infection and subsequent wound healing. The authors suggested that understanding the contribution of topical antimicrobials in AMR remains an important issue that is yet to be fully investigated.
Antimicrobial wound treatments
Antimicrobial wound treatment should be guided by a holistic wound assessment and identification of the infection causing micro-organisms to ensure appropriate treatment is prescribed; that it is specific to the wound infection; and that it is only prescribed for a limited period of time.25,33
The expertise of the treating clinician is critical to recognise whether a clinical assessment reveals that a wound is clinically infected, and that topical antimicrobial agents or wound dressings should not be used as a form of treatment.28
Wound infection diagnosis should be based on a clinical diagnosis supported by microbiological findings.35 The Wounds UK Best Practice Statement on AMS strategies for wound management refers to the ‘five rights’ of drug administration which have been modified for appropriate prescribing of antibiotics in wound care.17 The five rights emphasise the importance of appropriate identification and treatment strategies to ensure the most appropriate antimicrobial is administered promptly, at the right dose and for the optimal length of time to treat the infection. The five rights are the:
- Right diagnosis and care plan
- Right antimicrobial and the right delivery system
- Right time to initiate antimicrobial treatment
- Right antimicrobial dose
- Right duration of antimicrobial treatment
AMS and sepsis
WHO12 defines sepsis as a life-threatening condition due to the body’s response to infection. Sepsis is unfortunately a frequent consequence of many infectious diseases (including wound infections) and can result in organ failure and death.12 It is particularly common in older adults and in those with immunosuppressive disorders. Sepsis is common in the aging population, and it disproportionately affects patients with cancer and underlying immunosuppression. Septic shock occurs when this response results in impaired blood supply to organs requiring specific treatments to maintain adequate perfusion.36,37 There are approximately 48.9 million cases and 11 million sepsis-related deaths worldwide, accounting for an estimated 20% of all global deaths.38 An estimated 918,000 people in the UK have sepsis each year, with around 48,000 deaths.39 AMR poses a significant challenge to the treatment of sepsis; the UK Sepsis Trust states that sepsis claims more lives than some of the most severe types of cancers40 and that an estimated 5% of emergency admissions are due to sepsis. Approximately 70% of cases of sepsis occur in primary care.41 Additionally, despite the importance of early medical attention and excessive costs of sepsis to the Australian healthcare system (direct costs of $700 million, and indirect costs of $4 billion) the community awareness of sepsis is extremely low.42 As antibiotics form the initial treatment strategy for sepsis, ensuring wounds are properly managed is key to managing infection and limiting wound deterioration. The decision to prescribe antibiotics for sepsis management is often a clinical decision and while AMS and sepsis management coincide, following local sepsis guidelines can help minimise the impact of AMR, while maximising wound healing and patient outcomes. This further highlights the importance of an appropriate AMS strategy in relation to management of skin and wound infections. Figure 2 outlines the pathway to guide management of patients with wounds, with or without infection risk, considering the principles of antimicrobial stewardship.17
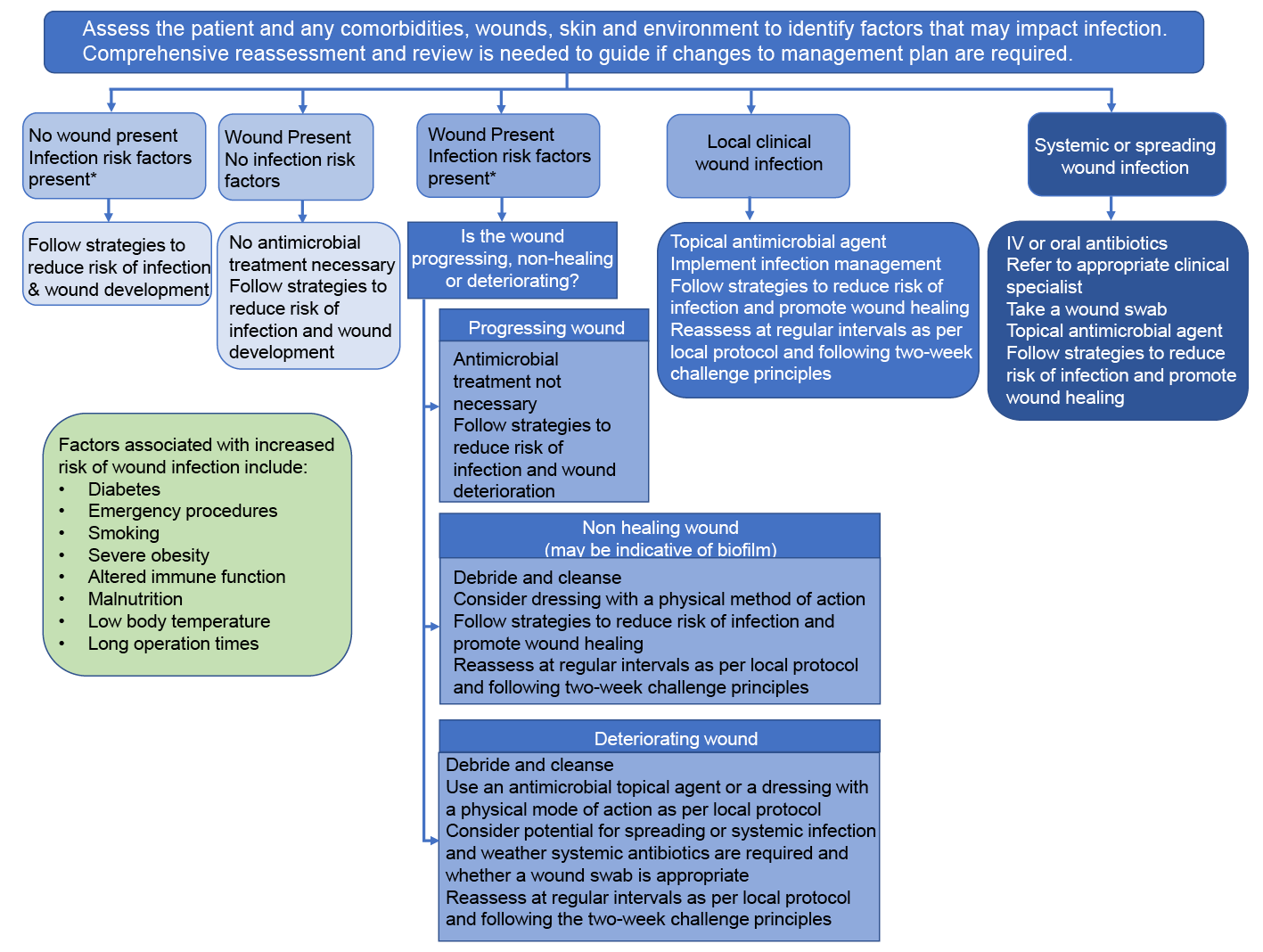
Figure 2. Pathway to guide the management of patients with wounds with or without infection considering the principles of antimicrobial stewardship. IV = intravenous. Figure reproduced with permission from Wounds UK 17 Best Practice Statement: Antimicrobial stewardship strategies for wound management. Wounds UK, London.
Current research on AMR and skin integrity
Research is essential for guiding practice to address AMR and can help further our knowledge on improving wound infection diagnosis and treatment. WHO36 published the first global research agenda for AMR in human health, highlighting the research priorities required to inform policy and practice, spanning 11 AMR areas of concern, across five themes to include prevention, diagnosis, treatment and care, cross-cutting and drug-resistant TB. This was in part due to the fact that limited progress has been made on raising awareness of AMR and AMS since the introduction of the GAP.43 Furthermore, in a recent systematic review exploring the interventions used to implement antimicrobial stewardship practices among hospitalised patients in least-developed countries, Mzumara et al44 concluded that measuring a range of outcomes including prescribing, patient, and microbiological outcomes, are all crucial to adequately evaluate the effectiveness of AMS interventions in wound care.44 The current literature suggests varying levels of inappropriate prescribing of antibiotics in both inpatient and outpatient settings with some studies reporting figures as high as 50%45, 46 and 20% in UK primary care,47 double the levels reported for parts of Scandinavia and the Netherlands.48 This problem is further reinforced by the British Society for Antimicrobial Chemotherapy (BSAC) and European Wound Management Association (EWMA) position paper25 stating that AMS is fundamental to promoting the appropriate use of antimicrobials, including antibiotics, and in reducing AMR.25,33 Given that wound management accounts for 16.4% of all systemic antibiotic prescriptions18 varying levels of clinical knowledge have been shown to further contribute to the inconsistent use of antimicrobials.48 Improving professional education, particularly around furthering clinician understanding of the need for more sustainable use of antibiotics is, therefore, a core focus of the UK Department of Health’s 2019-2024 five-year antimicrobial stewardship strategy,49 which suggested that such education should be supported by current policies and local guidelines25 and by the development of local standardised measurements to document patient wound care status and care planning. Likewise, the Australian Government released a 20-year vision and strategy document called Australia’s National Antimicrobial Resistance Strategy 2020 and Beyond.49 It supported seven key objectives aimed at tackling AMR:
- Clear governance for AMR Initiatives;
- Prevention and control of infection and the spread of resistance;
- Greater engagement in the combat against resistance;
- Appropriate usage and stewardship practices;
- Integrated surveillance and response to resistance and usage;
- A strong collaborative research agenda across all sectors; and
- Strengthening global collaboration and partnerships.
Prioritising action in developing a collaborative research agenda will facilitate a flexible AMR research agenda that aims for innovation, coordination, shared research and development activities, as well as dedicated funding for a national research agenda and support for translation of research findings into new approaches, application and policies that combat AMR.49
From a prescribing perspective, over-cautious decision making can contribute to the problem of AMR, something which was particularly evident during the COVID-19 pandemic in the UK, which resulted in an increase of antibiotic prescribing (up 6.71%) despite a reduction in face-to-face GP appointments (reduction of 51.5%) and an increase in telephone appointments (increase of 270.45%).50
Although AMS is an important strategy for managing the problem of AMR, evidence suggests that many AMS interventions do not accurately measure or assess their impact; for example, in a pilot survey of nurses attending a webinar on AMS, Ousey et al35 explored the effectiveness and impact of AMS programmes. A total of 987 nurses completed the survey (including advanced nurse practitioners; wound care specialists; podiatrists; tissue viability specialists; and wound, ostomy and continence nurses). The results showed that although many participants were completely, or partially aware of AMS (35.1% and 57.9% respectively), with most having an AMS strategy within their practice, almost 65% of participants (64.3%) stated that they did not measure the impact their AMS strategy. This means it is difficult for them to accurately determine the effectiveness of their AMS programme in managing AMR outcomes.35 In 2023 The Australian Commission on Safety and Quality in Health Care released a report called Antimicrobial Use and Resistance in Australia Surveillance System (AURA). It looked at antimicrobial resistance in human health and noted that the most common indications for antimicrobial prescriptions in aged care include non-surgical wound infections. This was the fourth most common indication for number of prescriptions issued.51 Alarmingly, the report found that 31.3% of hospital prescriptions for non-surgical wounds and 22.5% for wound infection were not compliant with guidelines for antimicrobial use in Australian hospitals.51
In the context of wound management, there remains a clear lack of knowledge surrounding the role of biofilms in non-healing wounds with a tendency to adopt in-vitro based models for how bacteria grow in non-healing wounds. Current research is, therefore, focused on gaining a better understanding to bridge the preclinical findings into clinical applications. Additionally, there is a continued evaluation and research focus on timely diagnosis of wound infection using technologies such as infrared and digital imaging. Preliminary research surrounding Dynamic Infrared Thermography (DIRT) had demonstrated that perforator mapping using DIRT could be a potentially valuable tool for stratification of high-risk patients in evidence-based antibiotic prophylaxis52 while fluorescent imaging tools have been shown to be useful in early detection of bacterial colonisation of wounds and dermal templates.53 Additionally, a number of studies have focused on developing wound dressings using smart biomaterials that can deliver antimicrobials in response to changes in wound pH and temperature in a stimuli-responsive manner to both reduce associated toxicity to mammalian cells and decrease AMR development.54,55 Research has also focused on practical assessment of antimicrobial dressings evaluating their efficacy during storage and after opening to better understand their effects on AMR development and cost-effectiveness, while preserving clinical efficacy and safety.56 In the context of skin integrity, researchers are continuously generating and implementing evidence-based wound care tools, educational resources, including best practice statements, and skin integrity prevention models to facilitate clinical translation and uptake of evidence-based practice.57,58,59, 60 Lastly, researchers and clinicians are also exploring standardisation of methods to calculate effectiveness of antimicrobial dressings against both planktonic and biofilm bacteria, and microbial communities associated with wounds.61 Probst et al33 highlighted that the primary endpoint should be defined either as prevention of clinical infection or clinical resolution of infection when choosing a topical antimicrobial treatment for wound care and that researchers should adhere to standard research guidelines to support improved uniformity and comparability of clinical studies.
Conclusions
Strategies to tackle the global crisis of AMR include implementation of AMS strategies across healthcare settings, including in clinical wound management, with a focus on maintaining skin integrity. Educational strategies play a critical part in raising awareness of AMR in wound management to support wound care practitioners and facilitate better understanding and implementation of AMS programs in clinical practice. This is critical to the maintenance of skin integrity and tackling the global challenges of wound infection.
Acknowledgements
Z.K. is supported by the Channel 7 Children’s Research Foundation Mid-Career Fellowship for Childhood Wound Infections.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author contribution
All authors contributed equally to writing and editing of the manuscript, approve the final submission and share responsibility for integrity of the work.
Author(s)
Joanna Blackburn*1, Zlatko Kopecki2, Karen J Ousey3*
1Institute for Skin Integrity and Infection Prevention, Department of Nursing and Midwifery, University of Huddersfield, UK.
2Future Industries Institute, University of South Australia, Australia.
3Institute for Skin Integrity and Infection Prevention, Department of Nursing and Midwifery, University of Huddersfield, UK.
*Corresponding author email J.Blackburn3@hud.ac.uk
References
- Department of Health Australia. Best care for older people everywhere: The toolkit. 2015 [cited 2024 Feb 4]. Available from: www.health.vic.gov.au/older/toolkit/index.htm
- Bonifant H, Holloway S. A review of the effects of ageing on skin integrity and wound healing. Br J Community Nurs. 2019; 24(3):S28–S32.
- Bianchi J, Cameron J. Assessment of skin integrity in the elderly 1. Br J Community Nurs. 2008;13(3):S26–S32.
- Wounds International. International review: Pressure ulcer prevention: pressure, shear, friction and microclimate in context. 2010 [cited 2024 Feb 4]. Available from: https://tinyurl.com/y8m65fapj
- North American Nursing Diagnosis Association. Nursing Diagnoses, Definitions and Classification. 2018. Available from: www.nanda.org/nanda-i-publications/
- Wounds UK. Best Practice Statement Maintaining skin integrity. 2018. London: Wounds UK. Available from: www.wounds-uk.com.
- The All Wales Guidance for the Prevention and Management of Skin Tears. All Wales Tissue Viability Nurse Forum. Wounds UK. Available at: https://wounds-uk.com/wp-content/uploads/sites/2/2023/02/8c3fb4c6d12806f7724aeaba1a410a90.pdf. [accessed 14.03.24].
- LeBlanc K, Campbell K, Beeckman D. ISTAP Best practice recommendations for the prevention and management of skin tears in aged skin. Wounds International. 2018 [cited 2024 Feb 4]. Available from: https://woundsinternational.com/best-practice-statements/istap-best-practice-recommendations-prevention-and-management-skin-tears-aged-skin/
- Idensohn P, Beeckman D, Campbell KE, Gloeckner M, LeBlanc K, Langemo D, Holloway, S. Skin tears: A case-based and practical overview of prevention, assessment and management. J Community Nursing, 2019;33(2).
- International Wound Infection Institute (IWII) Wound Infection in Clinical Practice. Wounds International. 2022.
- World Health Organization. The WHO AWaRe (Access, Watch, Reserve) antibiotic book. 2022 [cited 2024 Feb 4]. Available from: https://www.who.int/publications/i/ item/9789240062382 [accessed 31.01.2024].
- WHO factsheet Sepsis. WHO. 2023 [cited 2024 Jan 11]. Available from: https://www.who.int/news-room/fact-sheets/detail/sepsis
- Global action plan on antimicrobial resistance. Geneva: WHO. 2015 [cited 2024 Feb 4]. Available from: https://apps.who.int/iris/handle/10665/193736
- The National Institute for Care and Health Excellence NICE (2015). Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. NICE guideline [NG15]. 2015. [cited 2024 Feb 4]. Available from: www.nice.org.uk/guidance/ng15
- WHO. Policy guidance on integrated antimicrobial stewardship activities. Geneva: WHO. 2021 [cited 2024 Jan 31]. Available from: https://infectionlearninghub.co.uk/wp-content/uploads/2021/06/WHO-policy-guidance-on-integrated-antimicrobial-stewardship-activities.pdf
- WHO. Global framework for development and stewardship to combat antimicrobial resistance. 2018. Available from: https://www.who.int/groups/framework-development-stewardship-AMR
- Wounds UK. Best Practice Statement: Antimicrobial stewardship strategies for wound management. 2020. Wounds UK, London.
- Limato R, Broom A, Nelwan EJ, Hamers RL. A qualitative study of barriers to antimicrobial stewardship in Indonesian hospitals: governance, competing interests, cost, and structural vulnerability. Antimicrob Resist Infect Control. 2022;11(1):85.
- Ousey K, Rippon M, Rogers A, Stephenson J. (2022). Antimicrobial stewardship in wound care implementation and measuring outcomes: Results of an e-survey. J Wound Care, 2022;31(1), 32−39. https://doi.org/10.12968/jowc.2022.31.1.32
- Christiaan F, Dolk K et al. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother, 2018;73(S2)ii2–ii10.
- Australian Commission on Safety and Quality in Health Care. AURA 2017: Second Australian report on antimicrobial use and resistance in human health. 2017 [cited 2024 Feb 1]. Available from: https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-2017
- Laka M, Milazzo A, Merlin T. (2021). Inappropriate antibiotic prescribing: understanding clinicians. Australian Health Review. 2021;46. DOI:10.1071/AH21197.
- Ousey K, and Blackburn J. Understanding antimicrobial resistance and antimicrobial stewardship in wound management. Wounds UK. 2020;16(2)36−39.
- Md Rezal RS, Hassali MA, Alrasheedy AA, Saleem F, Md Yusof FA, Godman, B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: A systematic review of the literature, Expert Review of Anti-infective Therapy, 2015;13(5)665−680. DOI: 10.1586/14787210.2015.1025057
- Lipsky BA, Dryden M, Gottrup F, et al. Antimicrobial stewardship in wound care: A position paper from the British Society for Antimicrobial Chemotherapy and European Wound Wanagement Association. J Antimicrob Chemother. 2016;71: 3026e35
- Edwards-Jones V. Antimicrobial resistance: Challenges for the 21st century. Wounds UK; 2018;14:46−51.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ. 2010;340:c2096. https://doi.org/10.1136/bmj.c2096
- Nair HKR et al (2023) International Consensus Document: Use of wound antiseptics in practice. Wounds International. Available from: www.woundsinternational.com
- Roberts CD, Leaper DJ, Assadian O. The role of topical antiseptic agents within antimicrobial stewardship strategies for prevention and treatment of surgical site and chronic open wound infection. Adv Wound Care (New Rochelle). 2017;6(2): 63−71.
- World Union of Wound Healing Societies (WUWHS). Consensus Document: Wound exudate — effective assessment and management. Wounds International. 2019.
- Kramer A, Dissemond J, Kim S, et al. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol Physiol. 2018;31(1): 28−58.
- Matiasek J, et al. An intra-individual surgical wound comparison shows that octenidine-based hydrogel wound dressing ameliorates scar appearance following abdominoplasty. Int Wound J. 2018;15:914−920.
- Probst S, Apelqvist J, Bjarnsholt T, Lipsky BA, Ousey K, Peters EJG. Antimicrobials and non-healing wounds: An Update. J Wound Management, 2022;23:S1-S33. DOI:10.35279/jowm2022.23.03.sup01
- Blackburn J. Ousey K, Patton D, Moore Z, Avsar P. What is the evidence that there is antimicrobial resistance associated with the use of topical antimicrobial preparations. Wound Practice and Research. 2023;31(1):40−48. https://doi.org/10.33235/wpr.31.1.40-48
- Ward D, Holloway S. Validity and reliability of semi-quantitative wound swabs. Br J Community Nurs, 2019;24(Sup12): S6−S11.
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016 May 23;353:i1585. doi: 10.1136/bmj.i1585
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315(8):801−810.
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet (London, England). 2020;395(10219):200−211.
- Academy of Medical Royal Colleges. Working party position statement on the initial antimicrobial treatment of sepsis.Academy of Medical Royal Colleges. 2022.
- The UK Sepsis Trust. About Sepsis. Available from: About — The UK Sepsis Trust.
- Markwart R, Saito H, Harder T, Tomczyk S, Cassini A, Fleischmann-Struzek C, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systematic review and meta-analysis. Intensive Care Med. 2020 Aug;46(8):1536−1551. doi: 10.1007/s00134-020-06106-2
- The George Institute for Global Health. Cost of sepsis in Australia. 2021 [cited 2024 Feb 1]. Available from: https://www.georgeinstitute.org/sites/default/files/cost-of-sepsis-in-australian-report.pdf
- WHO. Global Database for Tracking Antimicrobial Resistance (AMR) country self-assessment survey (TrACSS) [website]. Geneva: WHO. 2023 [cited 2024 Feb 4]. Available from: https:// amrcountryprogress.org/
- Mzumara, GW, Mambiya M, Iroh Tam, PY. Protocols, policies and practices for antimicrobial stewardship in hospitalized patients in least-developed and low-income countries: a systematic review. Antimicrob Resist Infect Control. 2023;12:131. https://doi.org/10.1186/s13756-023-01335-8
- Wise R, Hart T, Cars O. Antimicrobial resistance Is a major threat to public health. BMJ 1998;317(7159):609–610.
- Nicolle LE. Antimicrobial stewardship in long term care facilities: what is effective? Antimicrob Resist Infect Control. 2014;3(1):6
- Smieszek T, Pouwels KB, Dolk FCK, Smith DRM, Hopkins S, Sharland M, Hay AD, Moore MV, Robotham JV. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018;73(s2):ii36-ii43. doi: 10.1093/jac/dkx500
- Australian Government. Australia’s National Antimicrobial Resistance Strategy — 2020 and Beyond. 2020 [cited 2024 Feb 1]. Available from: https://www.amr.gov.au/sites/default/files/2022-11/australia-s-national-antimicrobial-resistance-strategy-2020-and-beyond_0.pdf
- UK Department of Health. Five year antimicrobial resistance strategy 2019-2024. 2019 [cited 2024 Feb 1]. Available from: https://www.gov.uk/government/publications/uk-5-year-action-plan-for-antimicrobial-resistance-2019-to-2024
- Armitage R, Nellums LB. The COVID-19 response must be disability inclusive. The Lancet Public Health, 2020;5(5):e257. https://doi.org/10.1016/S2468-2667(20)30076-1
- Australian Commission on Safety and Quality in Health Care. Fifth Australian report on antimicrobial use and resistance in human health. 2023 [cited 2024 Feb 1]. Available from: https://www.safetyandquality.gov.au/sites/default/files/2023-11/aura_2023_fifth_australian_report_on_antimicrobial_use_and_resistance_in_human_health.pdf
- Childs C, Nwaizu H. Bullivant E. Willmott J. Davies M. Ousey K. Soltani H. Jacques R. Cutaneous perfusion dynamics of the lower abdomen in healthy normal weight, overweight and obese women: methods development using infrared thermography with applications for future wound management after caesarean section. Int J Environ Res Public Health. 2023; 20:5100. https:// doi.org/10.3390/ijerph20065100
- Redmond S, Lewis C. Rowe J, Raby E, Rea S. The use of MolecuLight™ for early detection of colonisation in dermal templates. Burns. 2019;45(8):1940−1942. https://doi.org/10.1016/j.burns.2019.10.011.
- Browning-Monroe MB, Fikhman DA. Mini-review: Antimicrobial smart materials: the future’s defense against wound infections. Front Biomater Sci. (Sec. Bio-interactions and Bio-compatibility). 2023;2. https://doi.org/10.3389/fbiom.2023.1285386
- Haidari H, Vasilev K, Cowin AJ, Kopecki Z. Bacteria-activated dual pH- and temperature-responsive hydrogel for targeted elimination of infection and improved wound healing. ACS Appl Mater Interfaces. 2022 Nov 23;14(46):51744−51762. doi: 10.1021/acsami.2c15659
- May A, Kopecki Z, Carney B, Cowin, A. Practical extended use of antimicrobial silver (PExUS). ANZ Journal of Surgery. 2022;92: 1199−1205. https://doi.org/10.1111/ans.17598
- Raepsaet C, Alves P, Cullen B, Gefen A, Lázaro-Martínez JL, Lev-Tov H, Najafi B, Santamaria N, Sharpe A, Swanson,T, Woo K, Beeckman D. The development of a core outcome set for clinical effectiveness studies of bordered foam dressings in the treatment of complex wounds, J Tissue Viability. 2023;32(3):430−436. https://doi.org/10.1016/j.jtv.2023.04.008.
- Gardiner L, Lampshire S, Biggins A, McMurray A, Noake N, van Zyl M, Vickery J, Woodage T, Lodge J, Edgar M. Evidence-based best practice in maintaining skin integrity. Wound Practice and Research. 2008;16(2):5-15.
- Edwards, H, Finlayson, K, Parker, C, Jensen, B, Finlayson, K. Improving wound management for residents in residential aged care facilities: National dissemination and implementation of the evidence based Champions for Skin Integrity Program - Final Report. Report to the Australian Government Department of Social Services. Queensland University of Technology, Australia. 2015 [cited 2024 Feb 1]. Available from: https://eprints.qut.edu.au/215401/
- Kilroy-Findley A, Ousey, K. Outcomes for non-healing wounds. Wounds UK. 2023;19(3), 22−28. Available from: https://wounds-uk.com/journal-articles/implementing-an-evidence-based-pathway-to-improve-outcomes-for-non-healing-wounds/
- Bowler P, Westgate S. An insight into in vitro microbiological testing of wound dressings. Wounds UK, 2022;18(1):75−80.



