Volume 29 Number 4
Improving ward-based neurogenic bladder and bowel care after acute spinal cord injury: a narrative review
Cristina Levy, Shilpa Gaikwad, Michael Whishaw, Wendy Bower
Licensed under CC BY 4.0
Keywords acute spinal cord injury, neurogenic bladder, neurogenic bowel, early spinal cord injury continence management pathway
For referencing Levy C et al. Improving ward-based neurogenic bladder and bowel care after acute spinal cord injury: a narrative review. Australian and New Zealand Continence Journal 2023; 29(4):101-108.
DOI
10.33235/anzcj.29.4.101-108
Submitted 15 September 2023
Accepted 2 November 2023
Abstract
Objectives There is a risk that early neurogenic bladder and bowel dysfunction following acute spinal cord injury (SCI) may not be adequately managed on the acute hospital wards. The aim of this paper was to review optimal assessment and management of patients with neurogenic bladder and/or bowel dysfunction during acute care immediately following SCI. A secondary aim was to translate these findings into an accessible policy for clinical staff involved in the care of acute SCI patients.
Methods A literature review was performed to identify the protocols and research describing the management of bladder and bowel dysfunction after acute SCI. The measures extracted were principles of practice, threshold measures and evaluation tools, and timelines of best care. Key findings relating to assessment, management and identification of the benefits of adhering to a protocol of clinical practice were summarised. Fidelity with evidence identified was evaluated, and practice gaps were recognised.
Results A total of 12 papers and six guidelines for providing excellent care to patients with SCI were identified. Overall, a systematic and comprehensive assessment of bladder and bowel function and neurological impairment should be completed early following SCI. Management is best individualised to both prevent known sequelae and optimise current function and quality of life. Acute SCI bladder and bowel management pathways were developed based on evidence identified.
Conclusions The study has identified that timely assessment and management of patients with spinal cord neurogenic bladder and/or bowel dysfunction during the acute phase is pivotal to optimising continence and mitigating risk of preventable harm prior to discharge to specialised spinal rehabilitation.
Background
Acute spinal cord injury (SCI) results from insults to the spinal cord and has permanent consequences, affecting independence and every aspect of life1. Individuals may develop motor and sensory impairment and autonomic dysfunction caudal to the level of injury2. The presence and severity of SCI is determined by the American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade. In patients with an AIS grade A to D3 (which correlates with one or more impairments of light touch S4–5, pin prick S4–5, deep anal pressure S4–5 or voluntary anal contraction) elimination symptoms are common.
It should be noted that spinal shock lasts for around 3 months and is characterised by a loss of spinal reflexes and muscle tone below the level of injury4. Neurogenic bladder and/or bowel dysfunction during the period of spinal shock is not indicative of function in the chronic phase. The type of neurogenic bladder and/or bowel dysfunction is determined by the Neurological Level of Injury (NLI) and whether or not the sacral outflow is impacted.
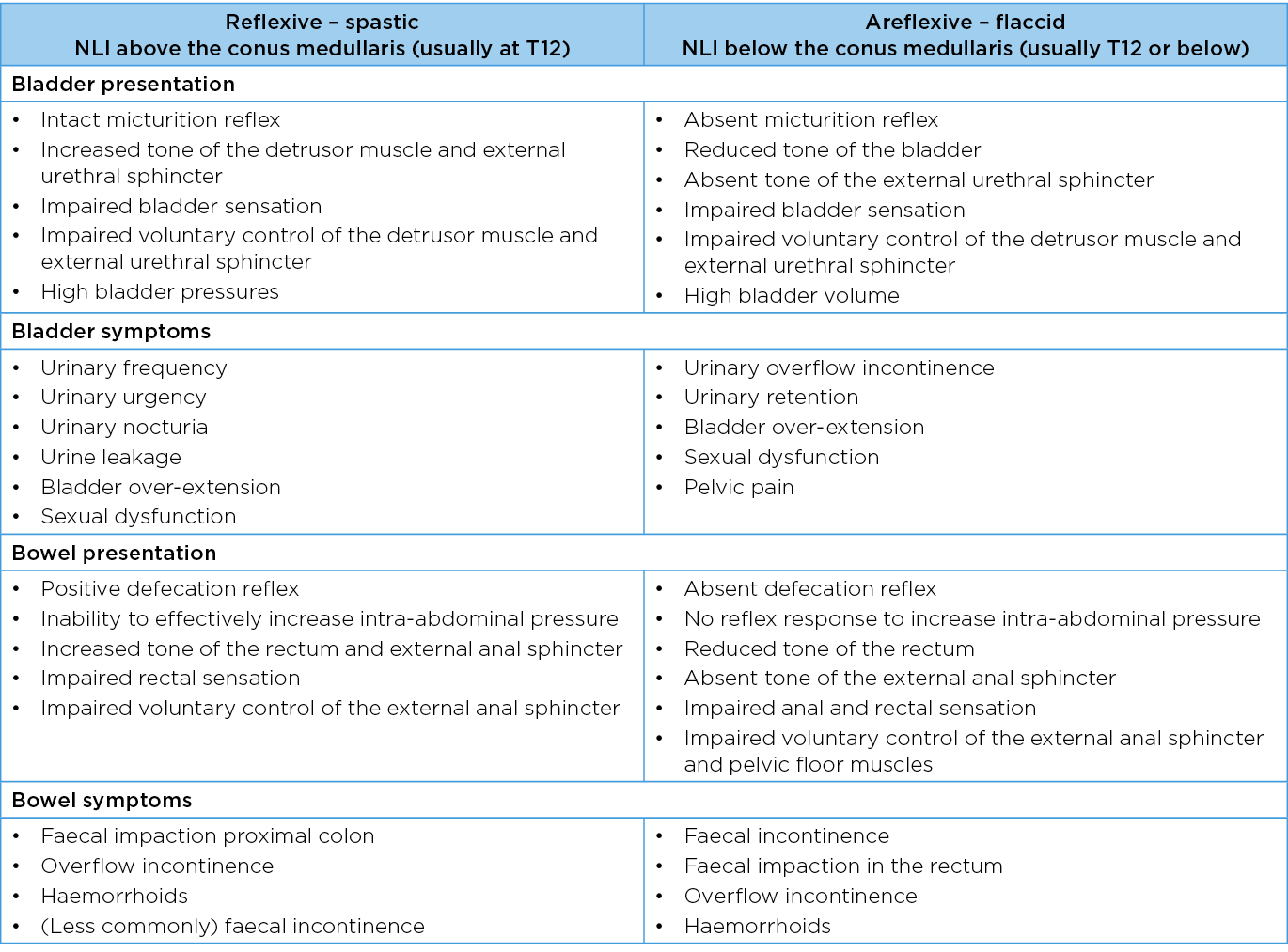
Neurogenic bladder dysfunction in patients with acute SCI is characterised by detrusor areflexia, impaired bladder sensation, non-relaxing bladder neck and proximal urethra, and possible impairment of pelvic floor muscle coordination4. Symptoms may include urinary frequency, urinary urgency, urinary incontinence4. Injuries below the conus medullaris (usually at T12 or below) usually lead to flaccid detrusor muscle and loss of innervation to the external urethral sphincter4. Filling of the bladder will occur at a low pressure; however, voiding is dysfunctional4. This results in bladder distension and high bladder volumes5. Injuries above the conus medullaris usually lead to spasticity of the muscles after resolution of spinal shock and are implicated in urinary tract infections and deterioration of the upper urinary tract5. Lesions at the level of the conus medullaris commonly cause pelvic floor dysfunction5.
Neurogenic bowel dysfunction occurs in 27–62% of patients with SCI and is characterised by dysfunction of the colon, abdominal muscles, pelvic floor muscles, rectum and external anal sphincter as well as impaired rectal and/or anal sensation6–8. Symptoms may include constipation, abdominal distention and pain, bleeding (consequence of straining) and faecal incontinence6. Injuries below the conus medullaris generally lead to flaccid pelvic floor muscles and external anal sphincter with no reflex response to increase intra-abdominal pressure. In addition, the tone of the external anal sphincter is affected7. The loss of descending inhibitory modulation significantly delays colonic transit time and may reduce compliance of the colon. Injuries above the conus medullaris lead to a reduced ability to effectively increase intra-abdominal pressure as well as an increased tone of the rectum and external anal sphincter8. Table 1 describes objective presentation and symptoms seen in the lower urinary tract and bowel after SCI.
Table 1. Important variables for bladder and bowel function in patients with SCI
The care for patients with SCI who have an acute neurogenic disruption to bladder and bowel function is complex and requires specialist knowledge and skills. Sequelae in both systems must be anticipated, recognised and managed appropriately in the acute phase. Prioritising this before patients arrive in post-acute rehabilitation minimises negative patterns and reduces the risk of complications developing4,8.
The aim of this project was twofold. Firstly, to outline optimal assessment and management of patients with bladder and/or bowel dysfunction immediately following acute SCI based on research and best clinical practice. Secondly, to translate findings into a policy applicable to all clinical staff involved in the care of patients with acute spinal cord neurogenic bladder and/or bowel dysfunction.
Methods
A literature review was performed for papers from the last decade that described management of bladder and bowel dysfunction after acute SCI. The databases of CINAHL Plus, Embase and MEDLINE were searched. Searches were restricted to English language only. A search strategy was developed that focussed on the following keywords as search terms: ‘acute spinal cord injury’, ‘bladder or urinary bladder’, ‘bowel’, ‘incontinence or urinary incontinence or bowel incontinence’, ‘complications’, ‘assessment’ and ‘management’. Search terms were established by scoping using free text terms and exploding Medical Subject Headings (MeSH).
Studies in English and from the last decade that describe bladder and or bowel care early after any type of SCI were eligible for inclusion. No limits were placed on study types. Studies describing children or adolescents, neurological conditions aside from SCI or samples of SCI patients more than 3 months post-injury were excluded from the search. Information extracted were principles of practice, threshold measures and evaluation tools and timelines of best care.
All studies identified through the search results as describing assessment and treatment of bladder and bowel dysfunction after acute SCI were reviewed. Duplicate references were identified and removed. The titles and abstracts of the search results were screened, and full texts were retrieved for the relevant articles. Two reviewers completed this process independently. Any disagreements were resolved by consensus.
Articles were scrutinised for key findings related to systems, assessment, management and benefits of adhering to a protocol. Two authors independently identified and extracted data. No risk of bias assessment was performed as all study types were included. Current ward practice at our institution was benchmarked against variables identified. Findings were presented to the multidisciplinary team caring for patients with SCI and the evidence–practice gap between published regimes and routine assessment and management was discussed.
An optimal clinical pathway informed by study findings was formulated to align with the ward culture of our institution. This was then devolved into a policy that could be integrated into the local standards of care. The new policy was reviewed by the following key institutional stakeholders: director of neurosurgery, head of neurology, senior neurosciences pharmacist, urology clinical nurse consultants (CNCs), nursing unit managers for neurology and neurosurgery wards, policy coordinator and nurse educators. Feedback was integrated into the final documents and separate pathways for bladder and bowel care in the acute period following SCI were described.
Results
In total, 31 papers were identified from the literature search; all were screened by two people. Following this, 12 papers9–20 and six guidelines from SCI services21–26 were relevant to the study aim and were included in the narrative review. The overall key findings were that a systematic and comprehensive assessment of bladder and bowel function and neurological impairment should be completed early following SCI before developing a management plan9,10,12,18,23. Table 1 summarises the status of clinically important variables that should be evaluated in both systems to confirm the impact of SCI on bladder and bowel function.
It can be seen that the level of neurological injury dictates function and bladder and bowel symptoms. AIS grade and NLI (using the International Standards for Neurological Classification of SCI (ISNCSCI)) should be established to differentiate between three possible types of spinal cord neurogenic bowel and/or bladder dysfunction – reflexive (spastic), areflexive (flaccid) or mixed (spastic and flaccid) presentation. This should be re-assessed periodically as neurological improvement during admission may change the AIS grade. The Bulbocavernosus Reflex Test, reflecting the integrity of spinal reflex arcs at sacral levels 2–4, should be conducted serially to monitor for resolution of spinal shock. Additionally, a comprehensive post-SCI assessment should identify relevant medical details and medication use (particularly those that induce constipation), onset of autonomic dysreflexia and paralytic ileus. Evaluation will also establish fluid intake, diet and cognitive and functional capacity, for example hand skills and positioning.
Key findings related to management following acute SCI prioritised early education with patients and their family/carers to engage them in establishing an optimal management plan7. This education includes alteration in anatomy and physiology, understanding the need for ongoing assessment, management options, prognosis and rehabilitation planning14. Management is ideally individualised to both prevent known sequelae and optimise current function and quality of life.
With respect to bladder function, preservation of both the upper and lower urinary tracts are inter-related. Over-distention of the bladder, a trigger for autonomic dysreflexia, is prevented by insertion of an indwelling catheter (IDC). Transitioning from an IDC to intermittent catheterisation (IC) when the patient is medically stable is important to reduce the incidence of complications such as urinary traction infections (UTI)13,14. Long-term suprapubic catheters (SPC) may be more suitable for patients with limited hand function or restricted carer support. Adherence to a daily routine minimises the risk of incontinence and UTIs. Multidisciplinary consultation will precede trial of void (TOV), ideally following the steps summarised in Figure 117. Identification of detrusor pressures during bladder filling and smooth and musculoskeletal activity during filling and any emptying are clarified by urodynamic studies performed at least 6 weeks post-SCI or after resolution of spinal shock19.

Figure 1. Trial of void process for bladder management
Bowel management should similarly identify precursors of preventable changes (for example onset of paralytic ileus) and individualise intervention to avoid incontinence, optimise stool consistency and toileting at a suitable time and place, prevent complications, and enhance quality of life10,12. This will include monitoring for signs of loose or hard stool, faecal impaction, faecal overflow incontinence, faecal incontinence and hyperkalaemia/hypernatremia. Digital rectal examination (DRE) should be performed to identify rectal fullness prior to inserting rectal stimulants or enemas and to remove rectally impacted faeces (DRF)11. Follow-up DREs may be indicated at times but could potentially be replaced by small volume rectal washouts.
Six papers9,10,13–15,17 described the benefits of adhering to a protocol of clinical practice with respect to bladder and bowel function after acute SCI. An evidenced framework will clarify status, identify complications, prevent adverse changes (for example bladder over-distension), standardise transition of care components (e.g., from IDC to IC / the TOV process) and determine the need for specialist referrals for spinal rehabilitation. From a consumer perspective, educational content to support understanding of prognosis and informed decision-making based on the individual needs were highlighted.
Acute SCI bladder and bowel management pathways were developed to provide clear guidance to assist clinicians with timely and appropriate assessment, management and treatment of patients with spinal cord neurogenic bladder and/or bowel dysfunction. The protocol was intended to be implemented within 24 hours of acute SCI and had utility for use by all clinicians involved in the care of patients with a SCI, eg, nurses, allied health clinicians, neurosurgical, neurology and urology medical clinicians and pharmacists. Separate pathways developed for bladder and bowel management are presented in Figures 2 and 3.
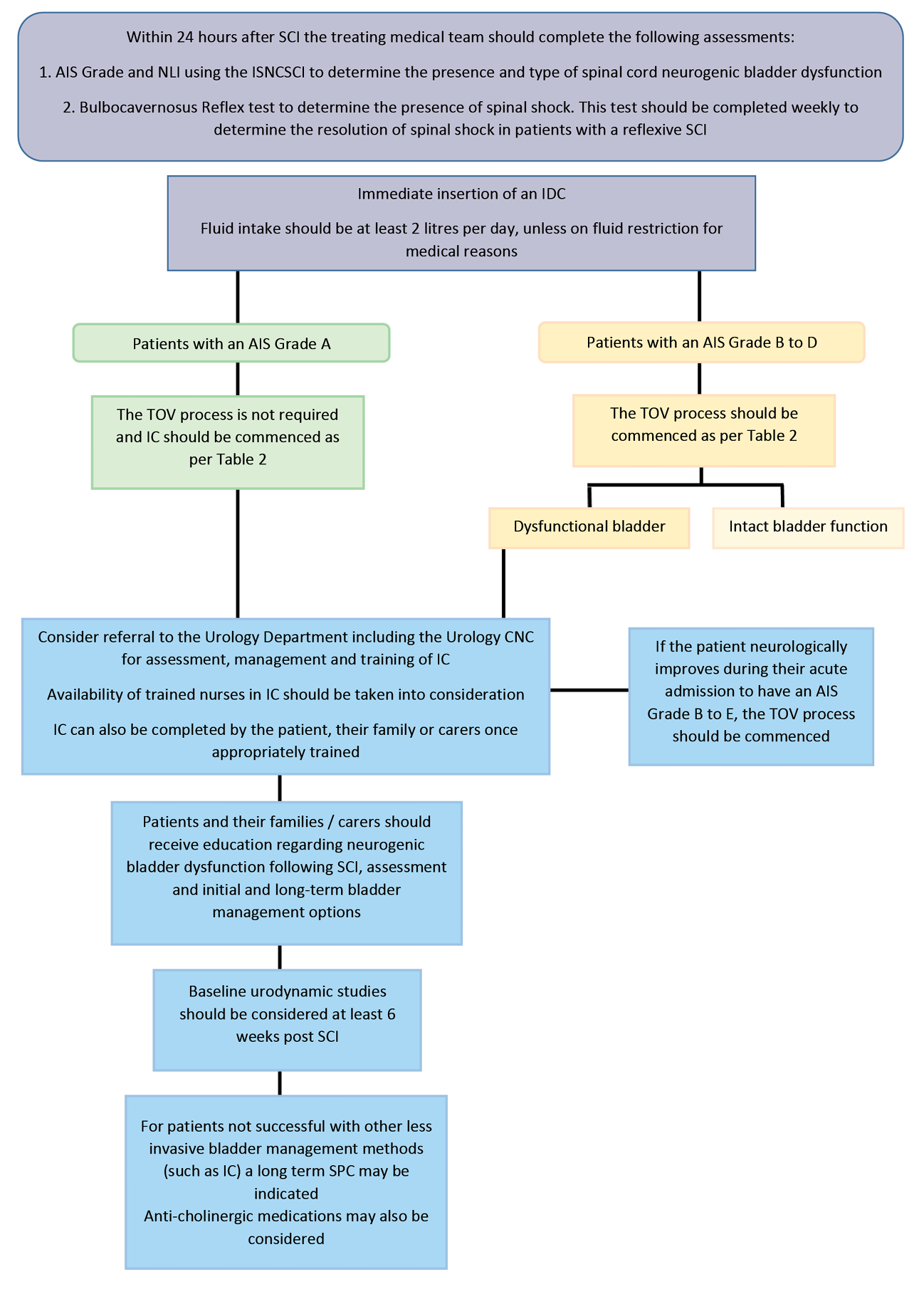
Figure 2. Bladder management in acute SCI pathway
AIS: ASIA (American Spinal Injury Association) Impairment Scale; NLI: Neurological Level of Injury; ISNCSCI: International Standards for Neurological Classification of SCI; IDC: indwelling catheter; TOV: trial of void; CNC: clinical nurse consultant; IC: intermittent catheterisation; SPC: suprapubic catheter
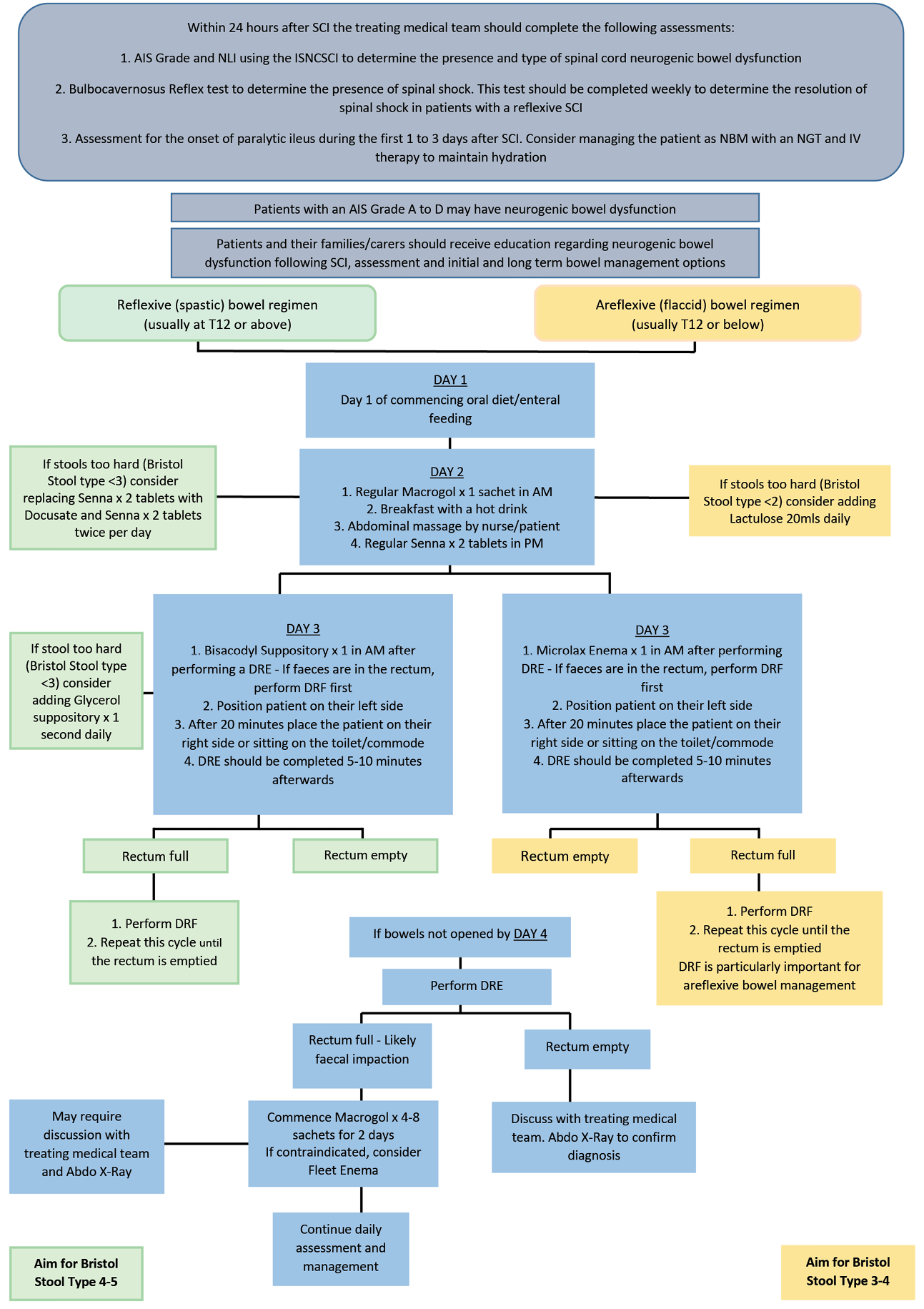
Figure 3. Bowel management in acute SCI pathway
AIS: ASIA (American Spinal Injury Association) Impairment Scale; NLI: Neurological Level of Injury; ISNCSCI: International Standards for Neurological Classification of SCI; IDC: indwelling catheter; DRE: digital rectal examination; DRF: digital removal of faeces
Discussion
Functional limitations related to neurogenic bladder and bowel symptoms are profound, being ranked higher than loss of walking17. Not only can appropriate surveillance of bladder and bowel symptoms optimise continence, it can also identify adverse changes and prevent longer term complications. As described by the recently published Australian and New Zealand clinical practice guidelines for the physiotherapy management of people with spinal cord injury27, the care of patients with SCI is complex, and bladder and bowel function is an important part of early rehabilitation. Bladder and bowel care in the presence of neurogenic disorders requires specialist knowledge and multidisciplinary skills. In the acute hospital setting, once SCI is diagnosed or suspected, neurogenic bladder and bowel dysfunction is anticipated and appropriate management commenced.
Our institution is not a recognised specialist service for people with SCI; however, spinal operations are frequently performed, generating a cohort of patients with some form of SCI. Non-specialist nurses, medical and allied health clinicians assess and plan treatment for SCI patients. As wait time for transfer to specialist spinal rehabilitation services is lengthy, patients with SCI often remain on the acute ward for an extended period. The risk of bladder or bowel complications developing during the patient’s acute admission should be appreciated. Benchmarking against evidence synthesised from the current literature review indicated that present practice on the acute wards in our non-specialised service does not align with best evidence. To address current gaps in practice recommendations, evidence gathered was tabulated as separate bladder and bowel management pathways.
Barriers and facilitators to the translation of these pathways into clinical processes of care within our institution’s unique culture and environment can be anticipated. Guihan15 reported the majority of barriers centred around current hospital workflow, policies, resources, education and training. Specifically, it is anticipated junior medical staff will require upskilling in performing ISNCSCI and Bulbocavernosus Reflex Tests and that nursing staff need more education on how to initiate and perform IC, particularly for males. This was identified by Goodes13 who noted that despite evidence supporting early implementation of IC, there were substantial delays in transitioning from IDC to IC due to staff training. Additionally, it is anticipated ward staff may be reluctant to complete invasive interventions such as DRE and DRF. The development of educational in-services, training, online learning resources and targeted competencies will be implemented to address these barriers. The upskilling of clinicians is consistent with previous research demonstrating that in-service training for staff working in the acute SCI setting resulted in improved clinician knowledge, enhanced patient knowledge and job satisfaction10.
The introduction of the newly developed clinical pathways after acute SCI will fail unless the roll out follows implementation science principles. Prior to a change in practice, clinical staff will require access to education about the underlying rationale for modification and the opportunity to be part of an iterative process that optimises acceptability9,14. The fit of the pathway process to the organisational culture should be negotiated, particularly whether additional tasks can be completed with current resources and technology. The documentation of the impact on patient status, for example the prevalence of complications after implementation, will ideally be embedded in electronic medical recording to facilitate audit and evaluation. In the interim, clinicians can endeavour to include more optimal continence strategies into their usual care of SCI patients. Future work will include the evaluation of educational components and co-design of optimal resources.
The strengths of this study include the benchmarking of current care against the latest evidence and clinical guidelines to provide a focus for change. Best practice management can potentially be individualised to target specific underlying dysfunction in each patient with acute SCI. The limitations of this work relate to our target as a single centre of care with unique culture and attributes and a low volume of acute SCI patients. The pathways require clinical champions to optimise implementation and a community of practice that prioritises bladder and bowel care in the acute phase of SCI.
In summary, this work has proactively addressed the issue of preventable complications in bladder and bowel function after acute SCI in a non-specialised hospital environment. Strategies that can be applied in the clinical context during patient assessment and management have been identified and integrated into system-specific multidisciplinary care pathways. Formal implementation to enhance continence care within our institution after SCI has been initiated, beginning with stakeholder consultation.
Author(s)
Cristina Levy*
Department of Physiotherapy, The Royal Melbourne Hospital, VIC, Australia
Email cristina.levy@mh.org.au
Shilpa Gaikwad
Department of Neurosurgery, The Royal Melbourne Hospital, VIC, Australia
Michael Whishaw
Department of Aged Care and Sub-Acute Continence Service, The Royal Melbourne Hospital, VIC, Australia
Wendy Bower
Department of Physiotherapy, The Royal Melbourne Hospital, VIC, Australia
Department of Aged Care and Sub-Acute Continence Service, The Royal Melbourne Hospital, VIC, Australia
Faculty of Medicine, Dentistry and Health Science, University of Melbourne, VIC, Australia
*Corresponding author
References
- Lohne V, Severinsson E. Hope during the first months after acute spinal cord injury. J Adv Nurs 2004;47(3):279–286.
- Furlan JC, Fehlings MG. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg Focus 2008;25(5):E13.
- Alexander M, Carr C, Alexander J, Chen Y, McLain A. Assessing the ability of the Sacral Autonomic Standards to document bladder and bowel function based upon the Asia Impairment Scale. Spinal Cord Ser Cases 2019;5:85.
- Cardozo L, Rovner E, Wagg A, Wein A, Abrams P, editors. Incontinence, 7th edition. ICI-ICS International Continence Society; 2023. Chapter 4, A. Initial assessment of urinary incontinence in adult male and female patients B. Patient-Reported Outcome Assessment, pp.308–9.
- Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol 2015 Jun 10;7:85–99.
- Beuret-Blanquart F, Weber J, Gouverneur JP, Demangeon S, Denis P. Large bowel transit time and anorectal manometric abnormalities in 19 patients with complete transection of the spinal cord. J Auton Nerv Syst 1990;25:109–112.
- Krogh K, Mosdal C, Gregersen H, Laurberg S. Rectal wall properties in patients with acute and chronic spinal cord lesions. Dis Colon Rectum 2002;45(5):641–649.
- Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord 2001;39(4):193–203.
- Bragge P, Guy S, Boulet M, Ghafoori E, Goodwin D, Wright B. A systematic review of the content and quality of clinical practice guidelines for management of the neurogenic bladder following spinal cord injury. Spinal Cord 2019;57(7):540–549.
- Borsh S, Sikka S, Callender L, Bennett M, Reynolds M, Driver S. Implementation of a neurogenic bowel program for spinal cord injury in the acute care setting: perceptions of patients and staff. Occ Ther Health Care 2019;33(3):306–322.
- Coggrave MJ, Norton C. The need for manual evacuation and oral laxatives in the management of neurogenic bowel dysfunction after spinal cord injury: a randomized controlled trial of a stepwise protocol. Spinal Cord 2010;48(6):504–510.
- Cotterill N, Madersbacher H, Wyndaele JJ, Apostolidis A, Drake MJ, Gajewski J, Heesakkers J, Panicker J, Radziszewski P, Sakakibara R, Sievert KD, Hamid R, Kessler TM, Emmanuel A. Neurogenic bowel dysfunction: clinical management recommendations of the Neurologic Incontinence Committee of the Fifth International Consultation on Incontinence. Neurourol Urodyn 2018;37(1):46–53.
- Goodes LM, King GK, Goodwin DM, Watts A, Bardley J, Middleton J, Bragge P, Dunlop SA. Barriers and facilitators to optimising inpatient bladder management after spinal cord injury. Spinal Cord 2020;58:1291–1300.
- Goodwin D, Brock J, Dunlop S, Goodes L, Middleton J, Nunn A, Wright B, Bragge, P. Optimal bladder management following spinal cord injury: evidence, practice and a cooperative approach driving future directions in Australia. Arch Phys Med Rehab 2018;99(10):2118–2121.
- Guihan M, Bosshart HT, Nelson A. Lessons learned in implementing SCI clinical practice guidelines. SCI Nurs 2004;21(3):136–142.
- Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord 2010;48(10):718–733.
- Lim V, Mac-Thiong JM, Dionne A, Begin J, Richard-Denis A. Clinical protocol for identifying and managing bladder dysfunction during acute care after traumatic spinal cord injury. J Neurotrauma 2021;38(6):718–724.
- Sekido N, Igawa Y, Kakizaki H, Kitta T, Sengoku A, Takahashi S, et al. Clinical guidelines for the diagnosis and treatment of lower urinary tract dysfunction in patients with spinal cord injury. Int J Urol 2020;27(4):276–288.
- Welk B, Schneider MP, Thavaseelan J, Traini LR, Curt A, Kessler TM. Early urological care of patients with spinal cord injury. World J Urol 2018;36(10):1537–1544.
- Yeung HY, Iyer P, Pryor J, Nicholson M. Dietary management of neurogenic bowel in adults with spinal cord injury: an integrative review of literature. Disabil Rehab 2021;43(9):1208–1219.
- Birrer K, Hobbs B, Giancarelli A, Ashworth S, Clancy R. Acute spinal cord injury management. Orlando Regional Medical Center; 2018 [cited 2022 Nov 4]. Available from: http://www.surgicalcriticalcare.net/Guidelines/Acute%20Spinal%20Cord%20Injury%202018.pdf
- Byrne C, Clements R, Mahony A. Victorian Spinal Cord Service – neurogenic bladder management. Austin Health; 2018.
- Coggrave MJ. Multidisciplinary Association of Spinal Cord Injured Professionals. Guidelines for management of neurogenic bowel dysfunction in individuals with central neurological conditions. MASCIP Middlesex; 2012 [cited 2022 Nov 4]. Available from: https://www.mascip.co.uk/wp-content/uploads/2015/02/CV653N-Neurogenic-Guidelines-Sept-2012.pdf
- Hogan M, Mullane J. Management of spinal neurogenic bladder & bowel. University College London Hospitals NHS Foundation Trust; 2019.
- Pryor J, Fisher M, Middleton J. Management of the neurogenic bowel for adults with spinal cord injuries. NSW Agency for Clinical Innovation; 2014 [cited 2022 Nov 4]. Available from: https://aci.health.nsw.gov.au/__data/assets/pdf_file/0019/155215/Management-Neurogenic-Bowel.pdf.
- Spiteri R, Brown A. Victorian Spinal Cord Service – neurogenic bowel management. Austin Health; 2009.
- Glinsky JV, Harvey LA, and the Australian and New Zealand Physiotherapy Clinical Practice Guidelines consortium. Australian and New Zealand Clinical Practice Guideline for the physiotherapy management of people with spinal cord injury. 2022 [cited 2023 May 7]. Available from: https://sciptguide.com/wp-content/uploads/2023/07/Australian-and-NZ-SCI-Physiotherapy-Clinical-Practice-Guidelines-1.1-1.pdf


