Volume 28 Number 4
Ageing and the bladder mucosa: A scoping review of recent animal model studies
Charlotte Phelps and Christian Moro
Keywords overactive bladder, lamina propria, underactive bladder, urogenital diseases, urothelium
For referencing Phelps C & Moro C. Ageing and the bladder mucosa: A scoping review of recent animal model studies. Australian and New Zealand Continence Journal 2022; 28(4):77-83.
DOI
https://doi.org/10.33235/anzcj.28.4.77-83
Submitted 11 May 2022
Accepted 4 October 2022
Abstract
The incidence of lower urinary tract symptoms increases with age. People with bladder disorders who are older than 60 years are more likely to comply and persist with treatment regimens, such as antimuscarinic medications for overactive bladder. Suggestions for these differences include lifestyle factors, greater compliance with medication prescription, or pharmacological and physiological changes to the receptors within the bladder tissue itself. This scoping review focused on the latter and sought to identify if there is recent evidence to support a prominent role for the internal lining of the urinary bladder, the bladder mucosa (urothelium with lamina propria), in age-related alterations. Although there continues to be substantial urological research in humans, animal models remain highly important for the assessment of physiological and pharmacological changes in the bladder. Recent work in this field brings insights into the overall understanding of bladder function and dysfunction. The PUBMED database was searched for studies published between 2018 and 2022. From the 25 articles identified, 10 were eligible for inclusion in the evaluation, and a risk of bias assessment was performed. Studies reported a variety of age-related alterations in the bladder mucosa. The most pronounced changes appear to be an inhibition of the mucosal barrier function and signalling pathways with ageing. Ageing also appeared to inhibit receptor systems associated with contraction inhibition, potentially leading to enhanced contractions. The overall evidence suggests that the increased prevalence of bladder contractile disorders seen in ageing may be due, in some part, to physiological alterations occurring within the bladder tissue itself.
Introduction
During ageing, the bladder undergoes various anatomical and physiological changes that may alter its ability to maintain normal function1. Marked changes in the urinary bladder include impaired contractility, sensitivity and storage2,3. As a result, there is an increase in the prevalence of lower urinary tract symptoms (LUTS), including urinary incontinence, overactive bladder (OAB), nocturia, urgency and underactive bladder (UAB) 2,3. The risk of bladder dysfunction tends to increase linearly with age, and becomes particularly problematic in frail elderly patients4, with an estimated 70% of the elderly population presenting with at least one LUTS5. The pathophysiology of age-related LUTS includes comorbid medical illnesses6, lifestyle factors7 and medicine use8. With the ageing population in Australia, the prevalence of LUTS as it affects the elderly is expected to place a significant burden on health services and the economy9. Interestingly, 48% of Australians consider incontinence to be an inevitable consequence of ageing10.
Current front-line pharmaceutical treatment options for bladder contractile disorders target the muscarinic receptors in the urinary bladder, for example antimuscarinics such as fesoterodine, oxybutynin, propiverine, solifenacin, tolterodine and trospium for the treatment of OAB11. Despite this, a lack of persistence with antimuscarinics has been identified as a major problem when treating OAB, with between 65–86% of patients discontinuing therapy within 1 year, depending on the antimuscarinic prescribed12. This has been attributed to the occurrence of adverse side effects and lower than expected treatment outcomes13, which are also found with the use of parasympathomimetics (muscarinic agonists) in the treatment of UAB14. A determinant in persistence and adherence to antimuscarinics is age, where older people are more likely to persist with antimuscarinic treatments for longer13,15. This may be due to pharmacological changes to the receptors or systems within the bladder tissue that respond to agonists and antagonists, or even due to alterations of cells within the tissue itself; however, this remains largely unclear.
The lack of compliance with treatment regimens due to the low effectiveness of current pharmaceutical options and the occurrence of adverse side effects highlights that a better understanding of the mechanisms underlying bladder contractions is warranted. Furthermore, the demonstrated successes for combination therapies, such as the use of an antimuscarinic with an α-adrenoceptor antagonist16 or an antimuscarinic with a β3-adrenoceptor agonist17 highlights the importance of continuing research in this field.
Animal models, in particular from pig and rodent tissues, have offered insights into potential future medications, the receptors to target, and other directions for clinical application. The research question underpinning this study was: is there a clinical application from the knowledge of OAB and UAB gained from animal model studies published within the last 5 years? The objective of this review was to investigate the impacts of ageing on the physiology of the urinary bladder mucosa, using information obtained from animal investigations to draw conclusions.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Systematic Reviews (PRISMA-ScR) was followed for the review’s outline18. Prior to commencement, an a priori protocol was prepared but not published. A comprehensive review of peer-reviewed English language full text articles published in the last 5 years (January 2018 to September 2022) was searched. Studies were identified through the available literature via PubMed (MEDLINE database) to identify original research papers on age-related physiological changes to the urinary bladder in animal models. All studies assessing any measurement of mucosal function with ageing were included. Both in vitro and in vivo studies were included, and all studies on bladder mucosa were included, with no requirement to have a formal control animal for assessments.
Search strategy
The following search strategy (formatted for the PUBMED database) was employed: (ageing[tiab] OR aging[tiab] OR “aging”[mesh] OR “age-related”[tiab]) AND (bladder[tiab] OR “urinary bladder”[tiab] OR “urinary bladder”[mesh]) AND (urothelium[tiab] OR mucosa[tiab] OR “lamina propria”[tiab]) AND (physiolog*[tiab] OR receptor*[tiab] OR function*[tiab] OR “muscle contraction”[tiab] OR “muscle contraction”[mesh] OR “overactive bladder”[tiab] OR “underactive bladder”[tiab]). A forward–backwards search of reference lists of retrieved articles was also undertaken (CP) to identify additional studies not captured from the database search.
Study selection
Screening of the literature was conducted by two authors independently (CP and CM), first by title/abstract, and subsequently in full text. Discrepancies were to be referred to an additional academic if required, although this was not necessary for this study.
Risk of bias
SYRCLE’s risk of bias tool for animal studies19, an adapted version of the Cochrane Risk of Bias tool, was used for the critical appraisal of the sources of evidence. Each study was assessed to each criterion in the risk of bias tool by the first author (CP) with reference to the second author (CM) to collaborate on grading any ambiguities. No contact was required with any included publication authors to obtain more data or information regarding the presented results. Each potential source of bias from the 10-item SYRCLE assessment was graded as low risk, high risk or unclear risk.
Results
Search results
The electronic search identified 22 articles, and the forward–backwards citation searches identified an additional three articles. After a title and abstract search, the full texts for 13 articles were obtained and analysed. From this collection, two were excluded due to wrong animal species (both on humans), and one was excluded due to wrong tissue (intact with detrusor rather than isolated mucosa). The results from the remaining 10 articles informed the recommendations in this review20–29 (Figure 1, Table 1).
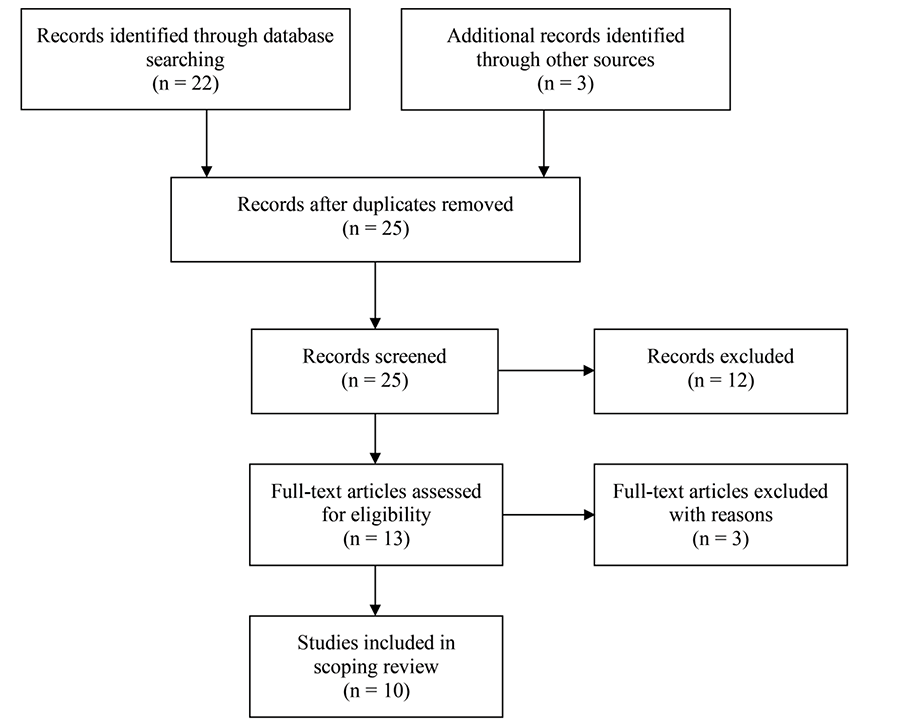
Figure 1.PRISMA flow chart
Table 1. General characteristics of included studies
Risk of bias
Eight of the 10 studies clearly reported that the baseline characteristics of animals were balanced, including animal model and gender. All studies had a formal control group, where ‘young’ was compared with ‘old’. Due to the nature of the studies, and limited information provided, only one study27 reported random housing of animals during the experiment, one study27 reported random selection of animals for outcome assessment, and one study29 reported blinding of investigators. All studies were free of other problems that could result in high risk of bias (Figure 2).
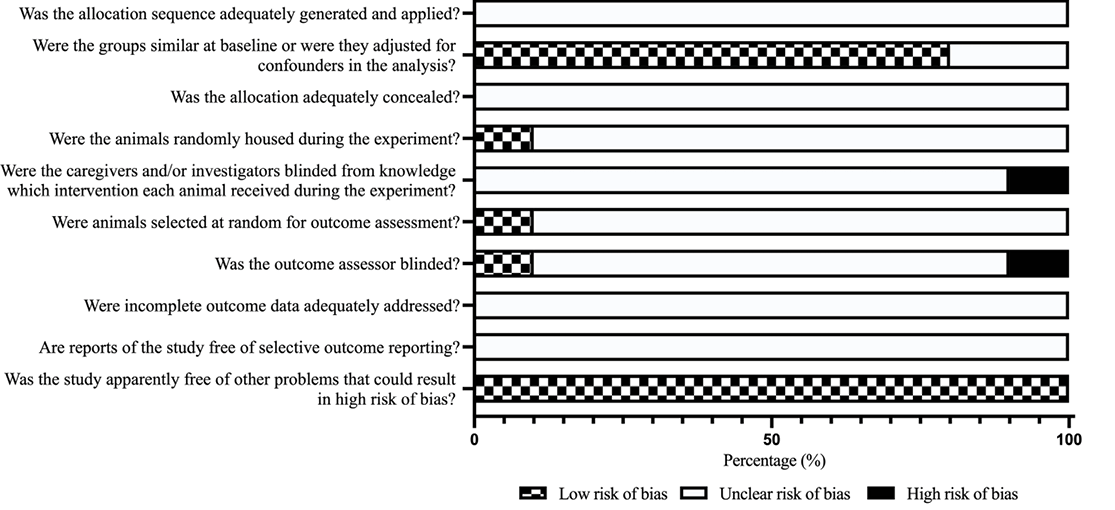
Figure 2. Risk of bias assessment results
Evaluation of the literature
The developing body of research into the role of the mucosa in overall bladder contractile activity highlights the potential role of this layer as a target for the development of novel therapeutics. Previously characterised for its protective functions, the bladder mucosa is now understood to play a key role in regulating, mediating and modulating overall bladder function through its signalling properties. Isolated urothelium with lamina propria tissue is capable of developing spontaneous phasic activity30, suggesting mechanisms where it might underlie bladder contractile disorders. Although past research on the mucosa has focussed on traditional mediators of contraction, such as acetylcholine31, noradrenaline32 and nitric oxide33, recent research has considered histamine34, prostaglandins35–37, neurokinin-A38 and angiotensin II39 as key mediators of contraction in the mucosa. However, the impact of ageing on these receptor systems is not often included in research outcomes.
Insights from animal studies have identified that the activity of contractile mediators of the urinary bladder may be altered throughout the lifespan. Stromberga and colleagues27 investigated the effects of age on the function of the histaminergic receptor system in porcine mucosa. The findings identified the histamine receptor system as having the potential to induce diseased states of the bladder due to its alterations by ageing. In particular, although the H1 receptor was responsible for contraction34, results suggest that the H2 receptor may have an inhibitory role. However, this was only in juvenile bladders, and aged bladders did not exhibit any response from H2 receptor activation. This could mean that the H2 receptor subtype plays an active part in maintaining bladder contractile stability in the young, and reduced activity with ageing could stimulate enhanced contractions, which would be particularly noticeable during the filling phase34. This potentially age-dependent alteration to the histamine receptor system highlights the potential for further research into this pathway as a possible future therapeutic target.
Other studies show the release of stretch-induced and stimulation-induced adenosine triphosphate (ATP) in mucosa-intact bladder wall decreases with ageing, which could be a contributing factor to symptoms of urgency and OAB in the elderly25. The ageing process can deteriorate the barrier and signalling properties of the bladder mucosa, as shown by significant increases in cellular senescence and oxidate stress22. Age-related bladder dysfunction could also be caused by a decrease in the cell signalling processes caveolin 1 and 2, which was found by an immunohistochemical study in the urothelium of aged rat bladders24. The study also reported cystometric results, showing that aged bladders exhibited a decreased voiding interval and pressure24, symptoms commensurate with OAB which is highly prevalent in the ageing population. In addition, Truschel and colleagues28 found an age-related decrease in lysosomal function in the urothelium of rats, which may have significant effects on bladder function. The authors reported this could alter the homeostatic chemical balance of the urothelium and cellular communication with underlying layers, manifesting various clinical conditions observed in the elderly28. The regenerative capability of the urothelium in aged mice bladders is slightly delayed compared to younger models, but there is no difference in the restoration of urothelial function23. However, some regenerative functions appear to deplete during ageing. For example, reduced recruitment of collagen fibres across the lamina propria occurs with age, impacting the overall elasticity of the bladder wall20. This could also have a role in diminishing sensitivity in the mucosa and its ability to release mediators when required.
Underactive bladder is an increasingly prevalent problem in the elderly population. Age-related alterations to bladder function leading to the development of an underactive bladder include sensory impairment, decreased voiding efficiency, and impaired bladder contractility40. The developing interest in the sensory role of the mucosa has led to the recognition of sensory molecules in these tissue layers. One is the transient receptor potential vanilloid-4 (TRPV4) channel, a key sensory protein in the bladder urothelium involved in stretch-induced ATP release from the urothelium, which modulates afferent nerve activity in response to bladder filling41. A recent study found that TRPV4 expression was reduced in the bladder urothelium of older female mice compared to young mice, which may play a role in the pathophysiology of UAB21. Similarly, Wen and colleagues29 reported a down-regulation of urothelial TRPV4 expression in older female rats accompanied by diminished sensory functioning of the bladder. However, in aged male guinea pig bladders, the expression of TRPV4 was shown to be upregulated in the bladder mucosa, with an increase in the quantity of urothelial ATP release, which could potentially become a contributing factor in OAB26. These insights highlight that TRPV4 signalling in the bladder mucosa may be altered with age; however, there may be gender and species differences.
Limitations of the review
Overall, the findings of the 10 studies reviewed related to alterations in the bladder mucosa with age highlight this as an area of developing interest. Although limited by the short 5-year timeframe, and inclusion of only English language articles, this scoping review has identified a variety of age-related alterations in the bladder mucosa which could contribute to bladder dysfunction.
Conclusion
Understanding the physiological mechanisms that are impaired in the urinary bladder mucosa with age can provide insights into novel therapeutic targets to manage bladder contractile disorders. Marked changes in receptor systems, barrier properties, and signalling pathways are shown in animal studies to play a significant role in age-related bladder dysfunction. These findings are of importance as there is an increasing interest in finding potential targets to use for combination therapies in the treatment of contractile disorders. Further research into using animal models to investigate additional impacts of ageing on bladder dysfunction is needed as we continue to build an understanding of the variations across bladder mechanisms which may present insights into age-specific treatment options for older people presenting with lower urinary tract dysfunctions.
Conflict of interest
The authors declare no conflicts of interest.
Funding
Christian Moro acknowledges research support from the Australian Bladder Foundation Grant managed by the Continence Foundation of Australia (2016– 2017). Charlotte Phelps is a recipient of an Australian Government Research Training Program Scholarship (2021 to present).
Author(s)
Charlotte Phelps*
Faculty of Health Sciences and Medicine, Bond University, Gold Coast, QLD 4229, Australia
Email cphelps@bond.edu.au
Christian Moro
Faculty of Health Sciences and Medicine, Bond University, QLD 4229, Australia
Member, Australia and New Zealand Continence Journal Editorial Committee
Author ORCID ID
Charlotte Phelps: 0000-0002-4217-0214
Christian Moro: 0000-0003-2190-8301
*Corresponding author
References
- Ellsworth P, Marschall-Kehrel D, King S, et al. Bladder health across the life course. Int J Clin Pract 2013;67(5):397–406.
- Wehrberger C, Madersbacher S, Jungwirth S, et al. Lower urinary tract symptoms and urinary incontinence in a geriatric cohort – a population-based analysis. BJU Int 2012;110(10):1516–21.
- Suskind AM. Frailty and lower urinary tract symptoms. Curr Urol Rep 2017;18(9):67.
- Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol 2020;38(8):1895–904.
- Przydacz M, Golabek T, Dudek P, et al. Prevalence and bother of lower urinary tract symptoms and overactive bladder in Poland, an Eastern European Study. Sci Rep 2020;10(1):19819.
- Coyne KS, Wein A, Nicholson S, et al. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract 2013;67(10):1015–33.
- Smith DP, Weber MF, Soga K, et al. Relationship between lifestyle and health factors and severe lower urinary tract symptoms (LUTS) in 106,435 middle-aged and older Australian men: population-based study. PLoS One 2014;9(10):e109278.
- Hashimoto M, Hashimoto K, Ando F, et al. Prescription rate of medications potentially contributing to lower urinary tract symptoms and detection of adverse reactions by prescription sequence symmetry analysis. J Pharm Health Care Sci 2015;1:7.
- Powell LC, Szabo SM, Walker D, et al. The economic burden of overactive bladder in the United States: a systematic literature review. Neurourol Urodyn 2018;37(4):1241–9.
- Continence Foundation of Australia. 2020 Annual Report. Victoria, Australia; 2020. Available from: https://www.continence.org.au/sites/default/files/2020-10/CFA008-Annual-Report-Final-2020.pdf.
- Robinson D, Cardozo L. Managing overactive bladder. Climacteric 2019;22(3):250–6.
- Wagg A, Compion G, Fahey A, et al. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012;110(11):1767–74.
- Yeowell G, Smith P, Nazir J, et al. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): a systematic literature review. BMJ Open 2018;8(11):e021889.
- Moro C, Phelps C, Veer V, et al. The effectiveness of parasympathomimetics for treating underactive bladder: a systematic review and meta-analysis. Neurourol Urodyn 2021;41(1):127–39.
- Carlson KV, Rovner ES, Nair KV, et al. Persistence with mirabegron or antimuscarinic treatment for overactive bladder syndrome: findings from the PERSPECTIVE registry study. Low Urin Tract Symptom 2021;13(4):425–34.
- Yamanishi T, Yasuda K, Kamai T, et al. Combination of a cholinergic drug and an α-blocker is more effective than monotherapy for the treatment of voiding difficulty in patients with underactive detrusor. Int J Urol 2004;11(2):88–96
- Lightner DJ, Gomelsky A, Souter L, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline amendment 2019. J Urol 2019;202(3):558–63.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–73.
- Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43.
- Cheng F, Birder LA, Kullmann FA, et al. Layer-dependent role of collagen recruitment during loading of the rat bladder wall. Biomech Model Mechanobiol 2018;17(2):403–17.
- de Oliveira MG, Alexandre EC, Bonilla-Becerra SM, et al. Autonomic dysregulation at multiple sites is implicated in age-associated underactive bladder in female mice. Neurourol Urodyn 2019;38(5):1212–21.
- de Rijk MM, Wolf-Johnston A, Kullmann AF, et al. Aging-associated changes in oxidative stress negatively impacts the urinary bladder urothelium. Int Neurourol J 2022;26(2):111–8.
- Erzar E, Kerec Kos M, Lakota K, et al. Does the urothelium of old mice regenerate after chitosan injury as quickly as the urothelium of young mice? Int J Mol Sci 2020;21(10).
- Kim JH, Yu SH, Kim SO. Decreased urothelial expression of caveolin 1 and 2 in aging rats showing detrusor overactivity: potential association with aging bladder. Investig Clin Urol 2021;62(6):690–6.
- Nishikawa N, Chakrabarty B, Kitney D, et al. Stretch- and carbachol-induced ATP release from bladder wall preparations of young and aged mice. Neurourol Urodyn 2020;39(6):1644–52.
- Roberts MWG, Sui G, Wu R, et al. TRPV4 receptor as a functional sensory molecule in bladder urothelium: Stretch-independent, tissue-specific actions and pathological implications. FASEB J 2020;34(1):263–86.
- Stromberga Z, Chess-Williams R, Moro C. Alterations in histamine responses between juvenile and adult urinary bladder urothelium, lamina propria and detrusor tissues. Sci Rep 2020;10(1):1–9.
- Truschel ST, Clayton DR, Beckel JM, et al. Age-related endolysosome dysfunction in the rat urothelium. PLoS One 2018;13(6):e0198817.
- Wen J, Zu S, Chen Z, et al. Reduced bladder responses to capsaicin and GSK-1016790A in retired-breeder female rats with diminished volume sensitivity. Am J Physiol Renal Physiol 2018;315(5):F1217-f27.
- Moro C, Phelps C. Urothelium removal does not impact mucosal activity in response to muscarinic or adrenergic receptor stimulation. Tissue Barrier 2022:2099214.
- Moro C, Uchiyama J, Chess-Williams R. Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology 2011;78(6):e9–15.
- Moro C, Chess-Williams R. Non-adrenergic, non-cholinergic, non-purinergic contractions of the urothelium/lamina propria of the pig bladder. Auton Autacoid Pharmacol 2012;32(3):53–9.
- Moro C, Leeds C, Chess-Williams R. Contractile activity of the bladder urothelium/lamina propria and its regulation by nitric oxide. Eur J Pharmacol 2012;674(2–3):445–9.
- Stromberga Z, Chess-Williams R, Moro C. Histamine modulation of urinary bladder urothelium, lamina propria and detrusor contractile activity via H1 and H2 receptors. Sci Rep 2019;9(1):3899.
- Stromberga Z, Chess-Williams R, Moro C. The five primary prostaglandins stimulate contractions and phasic activity of the urinary bladder urothelium, lamina propria and detrusor. BMC Urol 2020;20(1):48
- Stromberga Z, Chess-Williams R, Moro C. Prostaglandin E2 and F2alpha modulate urinary bladder urothelium, lamina propria and detrusor contractility via the FP receptor. Front Physiol 2020;11(705):1–11.
- Stromberga Z, Moro C. Which of the primary prostaglandin receptors might play a role in lower urinary tract dysfunction? A narrative review. ANZCJ 2020;26(3):58–60.
- Grundy L, Chess-Williams R, Brierley SM, et al. NKA enhances bladder-afferent mechanosensitivity via urothelial and detrusor activation. Am J Physiol Renal Physiol 2018;315(4):F1174-f85.
- Phelps C, Chess-Williams R, Moro C. The dependence of urinary bladder responses on extracellular calcium varies between muscarinic, histamine, 5-HT (serotonin), neurokinin, prostaglandin, and angiotensin receptor activation. Front Physiol 2022;13.
- Santos-Pereira M, Charrua A. Understanding underactive bladder: a review of the contemporary literature. Porto Biomed J 2020;5(4):e070.
- Wu Y, Qi J, Wu C, et al. Emerging roles of the TRPV4 channel in bladder physiology and dysfunction. J Physiol 2021;599(1):39–47.