Volume 29 Number 3
Outcomes of bladder neck Onabotulinumtoxin A injection in the treatment of men and women with primary bladder neck obstruction
David C T Homewood, Andrew W Silagy, Christopher C K Ip, Henry H Yao, Sophie Plagakis, Vincent Tse, Lewis Chan, Johan Gani, Helen E O’Connell
Licensed under CC BY 4.0
Keywords lower urinary tract symptoms, Onabotulinumtoxin A, bladder outlet obstruction, primary bladder neck obstruction
For referencing Homewood DCT, Silagy AW, Ip CCK, et al. Outcomes of bladder neck Onabotulinumtoxin A injection in the treatment of men and women with primary bladder neck obstruction. Australian and New Zealand Continence Journal 2023; 29(3):53-58.
DOI
https://doi.org/10.33235/anzcj.29.3.53-58
Submitted 19 May 2023
Accepted 8 August 2023
Abstract
Objectives This study aims to determine the clinical outcomes of bladder neck injection of Onabotulinumtoxin A as treatment for adult men and women with primary bladder neck obstruction (PBNO).
Methods Patients at participating institutions who underwent bladder neck injection of Onabotulinumtoxin A for PBNO between May 2011 and May 2018 were included in this retrospective case series study. All patients underwent cystoscopy to exclude anatomical causes and had video urodynamics to diagnose PBNO. Clinico-pathological data were collected from medical records. The primary outcome was subjective improvement in symptoms on clinical follow-up. Proportions were compared using Fisher’s exact test.
Results This study had 13 patients with a median age of 40. There were five men and eight women. Nine patients had failed alpha-blocker therapy previously, and none had undergone prior surgery for PBNO. Of the 12 patients who attended at least one follow-up appointment, 75% reported improvement in symptoms. The response rate was significantly higher in men compared with women (100% vs 57.1%, p=0.034). There were no significant complications reported on follow-up. We have subsequently developed a novel practical management algorithm from our clinical experience.
Conclusions Bladder neck injection of Onabotulinumtoxin A for the treatment of PBNO has a good response rate with minimal risk of complications and is a reasonable alternative or precursor to bladder neck incision surgery for patients wishing to avoid complications associated with surgery. Further prospective comparative studies on its use are warranted.
Introduction
Primary bladder neck obstruction (PBNO) is a functional cause of bladder outlet obstruction (BOO), where there is inadequate bladder neck opening without an anatomical obstruction such as bladder neck contracture, stricture or sling1,2. PBNO symptomatology includes voiding symptoms, storage symptoms, recurrent urinary infections, pelvic discomfort or pain. Video urodynamic studies (VUDS) is required to distinguish PBNO from other causes of obstructive voiding such as dysfunctional voiding or Fowler’s syndrome3. Characteristic features of PBNO include high voiding pressures, normal urethral relaxation, and the absence or delay of bladder neck opening during permitted voiding despite well-sustained detrusor contraction on fluoroscopy4. In contrast, absence of proximal urethral dilatation may occur with downstream pathologies such as Fowler’s syndrome (obstructive external sphincter). The prevalence of PBNO is unknown, and its incidence has only been reported in patient series with abnormal VUDS. In men younger than 55 years with storage symptoms who undergo VUDS, 33–54% were diagnosed with PBNO5,6, whilst women with PBNO only account for 4.6–8.7% of women with abnormal VUDS7,8.
Treatment options for men and women with PBNO include watchful waiting, lifestyle modifications, pharmacotherapy, clean intermittent self-catheter-isation (CISC) and surgery. The mainstay of pharmacotherapy has been alpha-blocker therapy. However, unlike benign prostatic hyperplasia in older men, alpha-blockers in younger men with PBNO are less successful6. There are also issues regarding tolerance and compliance as only 30% of men with PBNO who responded to alpha-blocker continue treatment for more than 1 year9. In females with PBNO, success rates of alpha-blockers have been reported to be 50% at 30 days10. Surgery such as bladder neck incision (BNI) for PBNO can have sustained results, although it has to be used judiciously due to the risk of permanent complications3,10. In young men undergoing BNI, the risk of developing retrograde ejaculation is the main concern, and has been reported in at least 27% of men after BNI9. In females, a study by Zhang et al. reported 15% risk of postoperative complications following BNI, including 5% who had irreversible stress incontinence or vesicovaginal fistula11.
Onabotulinumtoxin A (BoNTA) blocks acetylcholine transmitter release from pre-synaptic nerves, and has been demonstrated to relax urethral smooth muscle and reduce bladder outflow resistance12. BoNTA injection to the bladder neck for men with PBNO has only been studied in one series of 30 patients with disease refractory to alpha-blockers13. The study used 200 units of BoNTA, and after two months the reported mean International Prostate Symptom Score (IPSS) decreased from 21.9 to 7.813. The study also reported a high patient satisfaction, with few significant side effects and 86% of patients willing to recommend the treatment to others with PBNO13. In women, there has only been a single small pilot study of seven patients treated with bladder neck BoNTA for functional BOO including PBNO, dysfunctional voiding and Fowler’s syndrome14. The study found a 62% reduction in post-void residual volumes (PVR) and reported effects that lasted an average of 16.8+ weeks14. The benefit of BoNTA is its reversibility with time, thus avoiding permanent retrograde ejaculation or incontinence.
Given the limited published literature on the use of bladder neck BoNTA injection for PBNO, especially in female patients, this study aims to determine the success rate of this operation in a case series involving both male and female patients using a dose of 100 units of BoNTA.
Methods
Patients at participating institutions who underwent bladder neck injection of BoNTA for PBNO between May 2011 and May 2018 were included in this retrospective case series study. All patients underwent cystoscopy to exclude anatomical causes of BOO and had VUDS to diagnose PBNO. Imaging modality for VUDS included both fluoroscopy (Figure 1) and ultrasound. Urethral electromyography (EMG) was performed in select patients and included in the study.
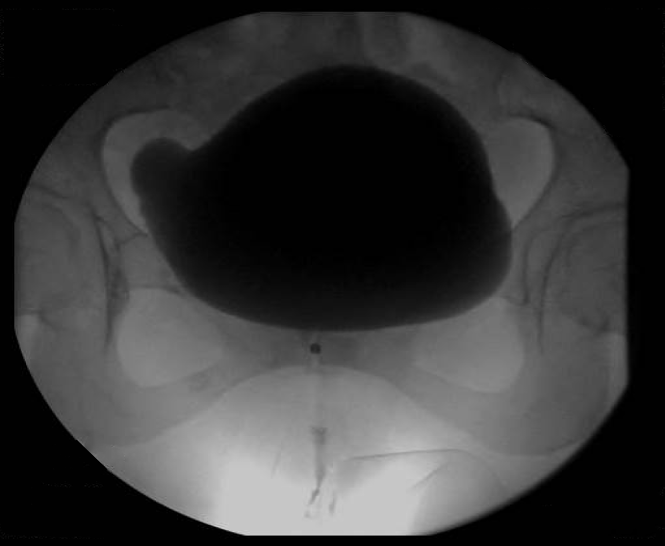
Figure 1. PBNO in a female patient shown on VUDS: note the failure of the bladder neck to open during voiding phase
Clinico-pathological data collected included: gender, age, pre-operative urinary retention status, history of medical management of PBNO, history of surgical management of PBNO, dates of BoNTA injections, dose of BoNTA used at each operation, and progression to BNI. Patients who had previously had a sub-urethral sling procedure were excluded. The primary outcome was subjective improvement in symptoms based on clinical notes on follow-up. Complications were identified from examination of medical notes and a significant complication was defined as Clavien-Dindo Class 3 or above.
A total of 13 patients were included in this study. The median age was 40 years (IQR=30–45). There were five men (38.5%) and eight women (61.5%). Five patients had urinary retention pre-operatively (38.5%), four patients were managed with CISC and one managed with suprapubic catheterisation, six patients were not in retention. Nine patients (69.2%) tried and failed medical management with an alpha-blocker prior to bladder neck BoNTA injection. Two of the patients did not trial medical management pre-operatively and there was no data on medical management on the remaining two patients. None of the patients had previous surgery for treatment of their PBNO prior to bladder neck BoNTA injection. On VUDS pre-operatively, two patients were unable to void. Of the remaining 11 patients, the median maximum flow rate (Qmax) was 14ml/s (IQR=5–15) (Table 1).
Table 1. Characteristics of patients with PBNO
Bladder neck BoNTA injection technique
A rigid cystoscope was gently inserted into the bladder with the patient in a lithotomy position under general or spinal anaesthesia and prophylactic antibiotic cover. 100 units of BoNTA (Botox®, Allergan PLC, Irvine, CA, USA) made up in 4ml of normal saline (25U/mL) was injected transurethrally at four sites of the bladder neck at 3, 6, 9 and 12 o’clock using a 23-gauge Cystoscopic Williams injection needle (Cook Medical® LLC, Bloomington, IN, USA) or a semi-rigid 4-french, 23-gauge single-use transurethral injection needle (Karl Storz®, Tuttlingen, Germany) through a 20-french operating sheath and working element (Karl Storz®, Tuttlingen, Germany) (Figure 2). The bladder was emptied at the end of the operation. Dose adjustments were made on subsequent injections based on patient response and clinical judgement.
Statistical analyses were performed using Stata Statistical Software: Release 16 (Stata®Corp LLC, Texas, USA). Proportions between groups were compared using Fisher’s exact test, with p<0.05 being considered statistically significant.
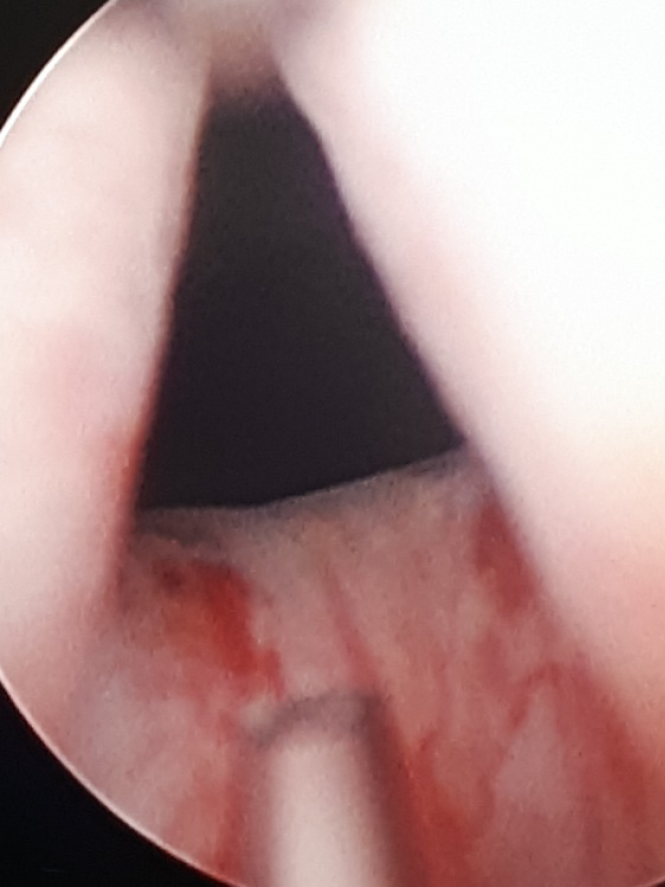
Figure 2. Injection needle at the bladder neck in a male patient
Results
Of the 13 patients, 12 attended follow-up clinic at 4–8 weeks postoperatively. Of these patients, nine (75%) reported improvement in symptoms and three (25%) did not report any improvement. Of the nine patients that reported improvement in symptoms, six provided a subjective improvement rating based on a Likert scale of 1 to 10 (1=no improvement, 10=complete improvement). The median score for subjective improvement in urinary flow symptoms was 8 (range=6–10). Subjective symptom response following bladder neck BoNTA injection for PBNO was significantly higher in men compared with women (100% vs 57.1%, p=0.034, Fisher’s exact test) (Table 2).
Table 2. Subjective response after first BoNTA injection

Of the nine patients who responded to bladder neck BoNTA injection, one female patient elected to undergo definitive surgery with BNI, six patients (four men and two women) underwent a second treatment of bladder neck BoNTA injection, and two patients were lost to follow-up (Figure 3). Of the six patients who proceeded to the second treatment of BoNTA, the same dose of 100 units were used in five patients and one patient had a dose escalation to 150 units. The median time between the first and second treatment of BoNTA was 9.4 months.
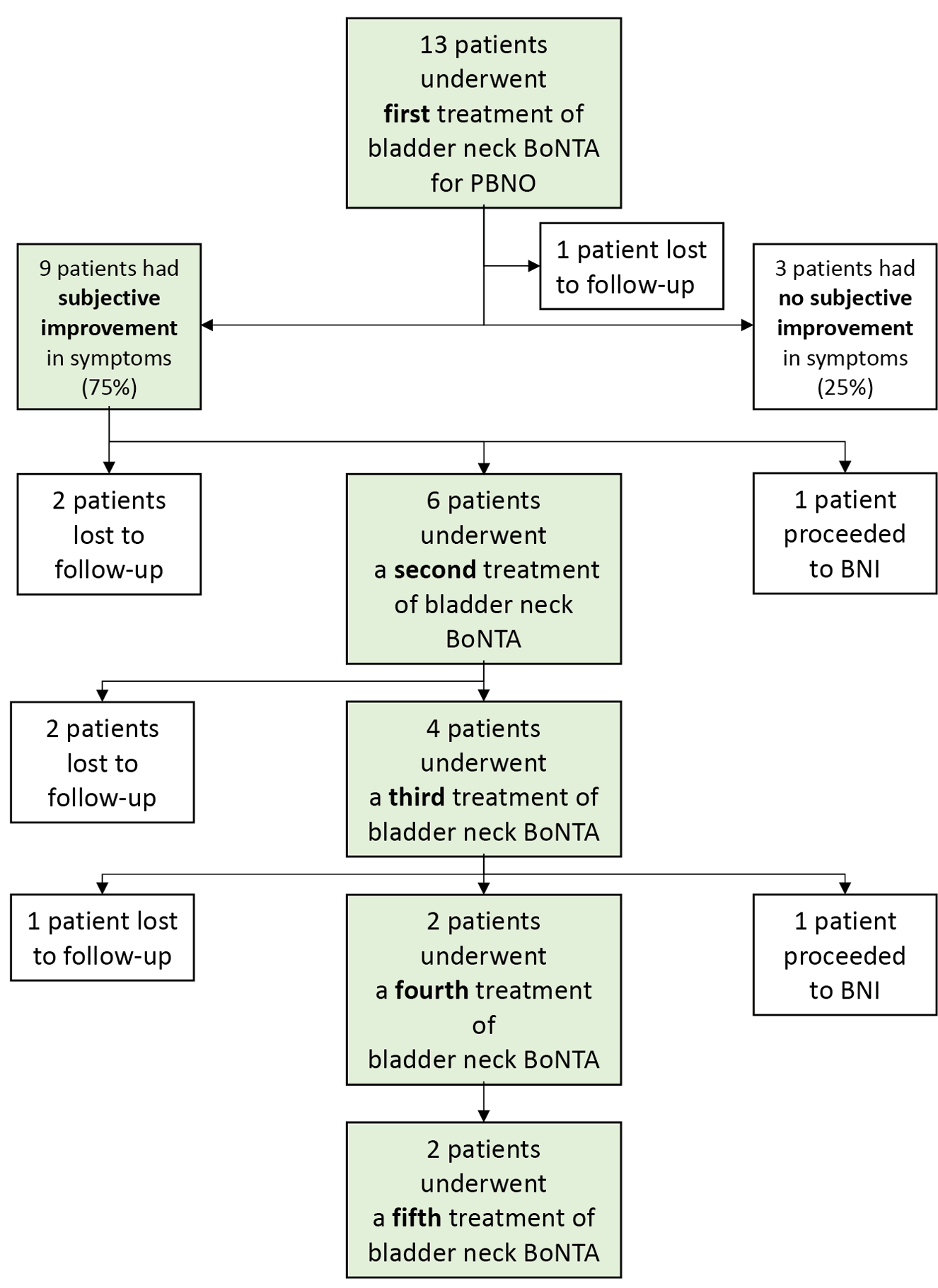
Figure 3. Outcomes of bladder neck injection of BoNTA for treatment of PBNO
Of these six patients, four proceeded to the third treatment of BoNTA and two were lost to follow-up. The dose of BoNTA used was 100 units in three patients and the dose was escalated from 150 to 200 units in the remaining patient. The median time between the second and third treatment of BoNTA was 9.2 months.
Of these four patients, one male patient elected to undergo definitive surgery with BNI, two patients proceeded to a fourth treatment of BoNTA after 12 and 17.7 months, and one patient was lost to follow-up. Of the two patients who proceeded to a fourth treatment, there was no dose escalation for either patients, who received 200 unit and 100 units, respectively and both patients underwent a fifth treatment of BoNTA after 9.7 and 10.3 months, respectively.
There were no significant documented complications reported on follow-up. One male patient developed non-bothersome transient retrograde ejaculation which lasted 3–5 days following each injection of BoNTA treatment. Another patient developed a lower urinary tract infection following the procedure which resolved with appropriate antibiotic therapy.
Discussion
This case series indicates that bladder neck injection of BoNTA may have a useful role in the management of PBNO and is well tolerated, with minimal complications. An improvement in symptoms was observed in 75% of cases treated with bladder neck BoNTA injection. The effect of BoNTA wears off after several months and patients may undergo repeated treatments on follow-up with the expectation of a similar response. The median interval between each BoNTA injection was approximately nine months and this is comparable to the 8–11 month duration of action reported in most studies using BoNTA to treat lower urinary tract dysfunction8,13,15.
We found that bladder neck BoNTA has minimal complications. The most significant complication found in this case series was ejaculatory dysfunction. One patient experienced this for approximately three days that spontaneously resolved.
Bladder neck BoNTA in men
The only previous study of young men with PBNO treated with 200 units bladder neck BoNTA injection reported subjective satisfaction in 80% of men at two months13. A total of 80% of men desired repeated treatment, but the study period was too short to include data for repeat interventions13. In this study, all five men with PBNO treated with 100 units of BoNTA injection had improvement of symptoms. Subsequently, one patient was lost to follow-up and the remaining four patients underwent at least two additional rounds of BoNTA injection. Two men went on to have five rounds of bladder BoNTA injection, and one eventually decided to undergo definitive treatment with BNI.
Bladder neck BoNTA in women
There is minimal published literature on women with PBNO treated with bladder neck BoNTA injection. Pradhan et al. previously reported an average 12 point reduction in IPSS with a mean duration of symptom improvement of 16.8 weeks in seven women with functional BOO that was treated with bladder neck BoNTA injection14. They assayed a heterogenous group of women with dysfunctional voiding, Fowler’s syndrome and PBNO14. Importantly, it only included a single patient with PBNO. With seven women included in this study, we present the largest series of women with PBNO treated with bladder neck BoNTA injection to date.
PBNO had a subjective symptom response rate of 57.1% to bladder neck BoNTA injection. Of these women who responded, 50% proceeded to a second round of bladder neck BoNTA injection. Female patients with PBNO were found to have a poorer subjective response rate following BoNTA injection compared with males (inadequately powered for statistical significance). The reason for this is unclear. Perhaps it signifies that the aetiology of PBNO in females is more complex than isolated overactivity of bladder neck muscle fibres. This has practice implications as it could inform targeted BNI for patients whose PBNO is relieved by bladder neck BoNTA. As BNI in females carries higher operative risks, a 5% risk of permanent stress urinary incontinence or vesicovaginal fistula11, a trial of bladder neck BoNTA improve risk benefit calculations of definitive BNI.
Bladder neck BoNTA as initial interventional management for PBNO
Trial of bladder neck BoNTA prior to a BNI offers an opportunity to stratify patients into separate cohorts based on likely benefit. As BNI is a definitive step that carries significant risk, this could avoid unnecessary patient harm. It ensures that the risk of retrograde ejaculation and sterility in males or stress incontinence from sphincter damage is only taken if maximal benefit is possible. Thus, bladder neck BoNTA offers an exciting initial interventional step and could serve to minimise harm to patients who are unlikely to benefit from a BNI.
Management algorithm for PBNO
From this experience we have developed a management algorithm for intuitive diagnosis and management of PBNO (Figure 4). In patients diagnosed with BOO, an initial clinical assessment including history, examination, flexible cystoscopy and VUDS is to be performed. If anatomical obstruction is identified, it should be promptly managed. To subcategorise functional BOO, we assess voiding pressures, urethral dilatation and bladder neck opening at voiding under VUDS. PBNO is defined by high voiding pressures, normal urethral relaxation and absence or delay of bladder neck opening at voiding. If diagnosed with PBNO, we recommend starting with conservative measures such as appropriate fluid intake, bladder retraining exercises such as timed voiding and double voiding, and avoiding triggers. If these are unsuccessful, devices such as indwelling catheters (IDC), CISC or intra-urethral stents may be trialled. If patients poorly tolerate or fatigue from device use, pharmacological agents such as alpha-blockers may be added. If symptoms are persistent despite this, we recommend bladder neck BoNTA. If there is improvement of symptoms and flow after this, we recommend patient consultation for shared decision making as to whether to pursue repeated bladder neck BoNTA of definitive surgical management. Importantly, if bladder neck BoNTA is unsuccessful, we would recommend avoiding BNI. However, further work needs to be done to validate the significance of this.
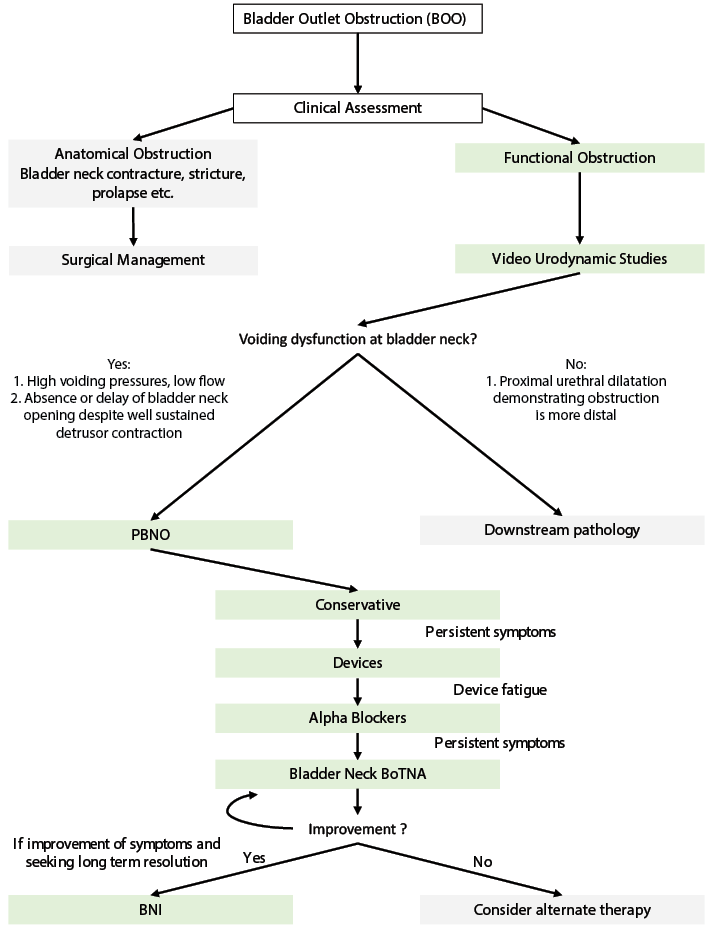
Figure 4. Diagnostic and management algorithm for PBNO
Limitations
There are several limitations of this study. Firstly, due to the retrospective nature of the study, it relies on the accuracy and completeness of medical records. Secondly, this study has a small number of patients. As current clinical practice generally dictates that patients who fail medical management receive BNI, this is expected13,14. Thus, small pilot projects like this must be published to allow recruitment of larger patient cohorts. Thirdly, there is no comparison arm and it is difficult to comment on the efficacy of this procedure over other treatment options such as medications or BNI.
Conclusion
Bladder neck injection of BoNTA for the treatment of PBNO has a favourable response with minimal risk of complications. For patients who fail or are unable to tolerate alpha-blockers for the treatment of PBNO, bladder neck BoNTA injection is a reasonable off-label alternative to BNI even if they choose to undergo repeat injections. It has a role in patients wishing to avoid the side effects of BNI or as a trial of treatment to provide evidence in support of bladder neck surgery. Further larger, prospective, comparative studies are required to further delineate the role of bladder neck BoNTA injection for treatment of PBNO.
Data availability statement
Data used in this publication is available from the corresponding author upon request. These are not publicly available due to privacy and ethical reasons.
Acknowledgement statement
Author(s)
David C T Homewood†
Department of Urology, Western Health, Melbourne, VIC, Australia
University of Sydney
Andrew W Silagy†
Department of Urology, Western Health, Melbourne, VIC, Australia
Christopher C K Ip
Department of Urology, Western Health, Melbourne, VIC, Australia
Henry H Yao
Department of Urology, Western Health, Melbourne, VIC, Australia
Sophie Plagakis
Department of Urology, Concord Repatriation Hospital, Sydney, NSW, Australia
Vincent Tse
Department of Urology, Concord Repatriation Hospital, Sydney, NSW, Australia
Department of Urology, Macquarie University Hospital, Sydney, NSW, Australia
Lewis Chan
Department of Urology, Concord Repatriation Hospital, Sydney, NSW, Australia
Johan Gani
Department of Urology, Western Health, Melbourne, VIC, Australia
University of Melbourne, Melbourne, VIC, Australia
Helen E O’Connell
Professor, University of Melbourne, Department of Surgery
Email helenoc@bigpond.net.au
*Corresponding author †Co first authors
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
References
- Nitti VW. Primary bladder neck obstruction in men and women. Rev Urol 2005;7(Suppl 8):S12.
- Padmanabhan P, Nitti VW. Primary bladder neck obstruction in men, women, and children. Current Urol Rep 2007;8(5):379–84.
- Sussman RD, Drain A, Brucker BM. Primary bladder neck obstruction. Rev Urol 2019;21(2–3):53.
- Aggarwal H, Lemack GE. Primary bladder neck obstruction in men and women: an update on diagnosis and management. Curr Bladder Dysfunct Rep 2015;10(3):288–94.
- Yang SS, Wang CC, Cheng HH, Chen YT. α1-Adrenergic blockers in young men with primary bladder neck obstruction. J Urol 2002;168(2):571–4.
- Kaplan SA, Ikeguchi EF, Santarosa RP, D’alisera PM, Hendricks J, Te AE, et al. Etiology of voiding dysfunction in men less than 50 years of age. Urol 1996;47(6):836–9.
- Nitti VW, Tu LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol 1999;161(5):1535–40.
- Kuo HC. Videourodynamic characteristics and lower urinary tract symptoms of female bladder outlet obstruction. Urol 2005;66(5):1005–9.
- Trockman BA, Gerspach J, Dmochowski R, Haab F, Zimmern PE, Leach GE. Primary bladder neck obstruction: urodynamic findings and treatment results in 36 men. J Urol 1996;156(4):1418–20.
- Kumar A, Mandhani A, Gogoi S, Srivastava A. Management of functional bladder neck obstruction in women: use of α-blockers and pediatric resectoscope for bladder neck incision. J Urol 1999;162(6):2061–5.
- Zhang P, Wu Z-J, Xu L, Yang Y, Zhang N, Zhang X-D. Bladder neck incision for female bladder neck obstruction: long-term outcomes. Urol 2014;83(4):762–7.
- Sahai A, Khan M, Fowler CJ, Dasgupta P. Botulinum toxin for the treatment of lower urinary tract symptoms: a review. Neurourol Urodyn 2005;24(1):2–12.
- Sacco E, Tienforti D, Bientinesi R, D’Addessi A, Racioppi M, Pinto F, et al. Onabotulinumtoxin A injection therapy in men with LUTS due to primary bladder-neck dysfunction: objective and patient-reported outcomes. Neurourol Urodyn 2014;33(1):142–6.
- Pradhan AA. Botulinum toxin: an emerging therapy in female bladder outlet obstruction. Indian J Urol 2009;25(3):318–20.
- Lim SK, Quek PL. Intraprostatic and bladder-neck injection of botulinum A toxin in treatment of males with bladder-neck dyssynergia: a pilot study. Eur Urol 2008;53(3):620–5.