Volume 5 Number 2
Accuracy of pulse oximetry using the Garmin fēnix® 6 Pro watch
Patrick Weinrauch
Keywords Garmin, oximetry, austere, fēnix®, watch
For referencing Weinrauch P. Accuracy of pulse oximetry using the Garmin fēnix® 6 Pro watch. JHTAM 2023; 5(2):3-6. DOI https://doi.org/10.33235/JHTAM.5.2.3-6
DOI
https://doi.org/10.33235/JHTAM.5.2.3-6
Submitted 14 June 2023
Accepted 22 July 2023
Abstract
Introduction Multifunctional wearable technologies can provide practical solutions to assist with the delivery of healthcare and research conducted within austere environments. The current study was developed to assess the accuracy of peripheral oxygen saturation (SpO2) measurements using the Garmin fēnix® 6 Pro (GF6) wristwatch.
Methods A total of 25 healthy volunteer subjects were tested under normobaric normoxic conditions breathing room air at sea level. SpO2 and heart rate (HR) measurements were obtained using a 51mm GF6 watch in various wrist positions with comparison of results to an approved clinical reference healthcare device (GE Transport Pro Monitor).
Results Of the total 100 test SpO2 measurements obtained by the GF6 watch, 53 failures were recorded; 38 failures were due to measurement inaccuracy and 15 failures were due to an inability to provide SpO2 measurement after two attempts in the same test location. Watch positioning dorsally and proximal to the wrist and hair shaving was associated with greater accuracy or a trend to more accurate SpO2 results. HR data obtained by the GF6 were more accurate than SpO2 measurements for all test conditions for the purposes of healthcare management.
Conclusion The results of this study do not support the use of the GF6 wristwatch for the purposes of pulse oximetry evaluation in clinical healthcare or research.
Introduction
Physiologic monitoring in austere field settings for the delivery of medical care, expedition management or scientific experimentation requires fit for purpose equipment suitable to environmental demands. Field monitoring equipment therefore not only requires the essential qualities of reliably and accuracy but also the desirable qualities of compact design, physical robustness, energy efficiency and multi-functionality. Wearable physiologic monitoring technologies such as watches and trackers traditionally used for athletic evaluation and performance coaching are gaining traction for applications within austere and remote healthcare. Wearable technologies are relatively cheap and commonly used in outdoor pursuits as they typically also provide users with multiple other functions such as time keeping, navigation, pacing and communication. It is estimated that 30% of the population within the United States of America use wearable healthcare technology, with almost half accessing the information produced at least daily1. Product manufacturer Garmin continues to observe strong growth, with 16.6 million unit sales globally in 2021, 31% being fitness devices2.
The application of wearable technologies within field healthcare therefore represents a logical extension of their use. Whilst accurate heart rate (HR) monitoring using watch and chest band devices has been available for some time, pulse oximetry monitoring of peripheral oxygen saturation (SpO2) is a relatively recent additional feature within wearable technologies. The Garmin fēnix® 6 Pro (GF6) (Garmin International, Kansas, USA) is a Global Positioning System (GPS), Advanced and Adaptive Network Technology (ANT+) and Bluetooth-enabled wristwatch that offers a variety of physiologic monitoring capabilities (HR, SpO2, respiratory rate, near infrared spectroscopy compatibility) in addition to multiple other features such as navigation and mapping, climate monitoring and timing functions. The Garmin fēnix® watch series utilises reflective pulse oximetry at the level of the wrist to monitor SpO2.
Lauterbach et al.3 evaluated the Garmin fēnix® 5X Plus watch (GF5) (Garmin International, Kansas, USA) pulse oximetry function in subjects within a normobaric hypoxia chamber simulating variable altitudes from 900ft (274m) to a maximum of 12,000ft (3,657m). Compared to medical grade Nonin finger pulse oximetry (Nonin Medical Inc; Plymouth, MN, USA) the GF5 watch consistently overestimated SpO2 and underestimated subject HR, particularly at higher simulated altitudes with lower inspired oxygen partial pressures. Lauterbach et al.3, however, concluded that clinically satisfactory correlation between watch oximetry readings compared to medical grade finger pulse oximetry had been observed and recommended the GF5 to be a reasonable tool for physiologic measurement in environmental conditions up to 10,000ft (3,048m).
Schiefer et al.4 evaluated the GF5 in comparison to medical grade Covidien Nellcor finger pulse oximetry (Medtronic, MN, USA) and arterial blood gas analysis (ABG) in healthy subjects after rapid ascent to 4,559m. Schieffer et al.4 found the GF5 failed to meet acceptable validity for clinical use with poor correlation of SpO2 measurements to reference ABG and systematic over estimation of haemoglobin saturation. The authors recommended against the use of the GF5 watch for predictive health monitoring or acclimatisation management, however conceded that future firmware upgrades to the device may be associated with improved accuracy.
To date, the accuracy of the SpO2 function of the GF6 has not been evaluated in the literature and device performance details have not been disclosed by the product manufacturer. The current study was developed to assess the clinical utility of SpO2 and HR measurements using the GF6 watch.
Methods
A total of 25 healthy adult volunteer subjects over 18 years of age (11 male, 14 female) were tested under normobaric normoxic conditions breathing room air at sea level (under 50m altitude). The evaluation test device was a size 51mm GF6 wristwatch. Simultaneous reference standard SpO2 and HR reference measurements were obtained using an approved healthcare assessment device (GE Transport Pro Monitor; GE Healthcare Australia, Springfield, QLD, Australia). All testing was performed in the same room with identical lighting conditions. People with coloured fingernail polish were excluded from participation in consideration of reference measurements being obtained by a finger probe device.
Manufacturer recommendations for SpO2 evaluation using the Garmin GF6 are for the watch to be worn snug and positioned above the wrist (proximal to radial and ulnar styloid) with a minimum of motion during measurement acquisition5. Subjects were provided with these instructions verbally and required to self-apply the device upon their self-selected preferred side for wearing a wristwatch prior to testing. To assess the potential impacts of watch location upon the wrist and the effect of forearm hair on SpO2 measurements taken by the GF6, four test conditions were evaluated in a randomised sequence – DAW: dorsal above wrist; DBW: dorsal below wrist; VAW: volar above wrist; DAWS: dorsal above wrist hair shaved.
SpO2 measurements taken by the GF6 are discontinuous and take up to 60 seconds to acquire. When SpO2 measurement is unable to be obtained within 60 seconds from test initiation, the GF6 delivers notification of an unreadable result. For the conduct of this study, where an unreadable SpO2 result was obtained the wearer was instructed to remove the watch and reapply prior to retesting. Where two consecutive unreadable SpO2 results were obtained from the same location, the test was recorded as a failure.
For the purposes of interpretive data analysis a clinically acceptable error rate for SpO2 measurement using the GF6 was considered within 3 percentage points or less in comparison to the reference device. For example, if the reference device measured SpO2 98%, a simultaneous GF6 SpO2 measurement of 95% was considered clinically acceptable and was recorded as a pass. A failure result was recorded if the SpO2 was greater than 3 percentage points of variance or if two consecutive unreadable results were obtained in the same testing conditions. Similarly, a clinically acceptable error rate for HR analysis using the GF6 was considered five beats per minute (BPM) or less in comparison to the reference device. Chi-squared or Fischer’s Exact testing was used for categorical group comparison where applicable.
Approval for the conduct of this study was obtained from the UnitingCare Health Human Research Ethics Committee (reference 2023.10.388).
Results
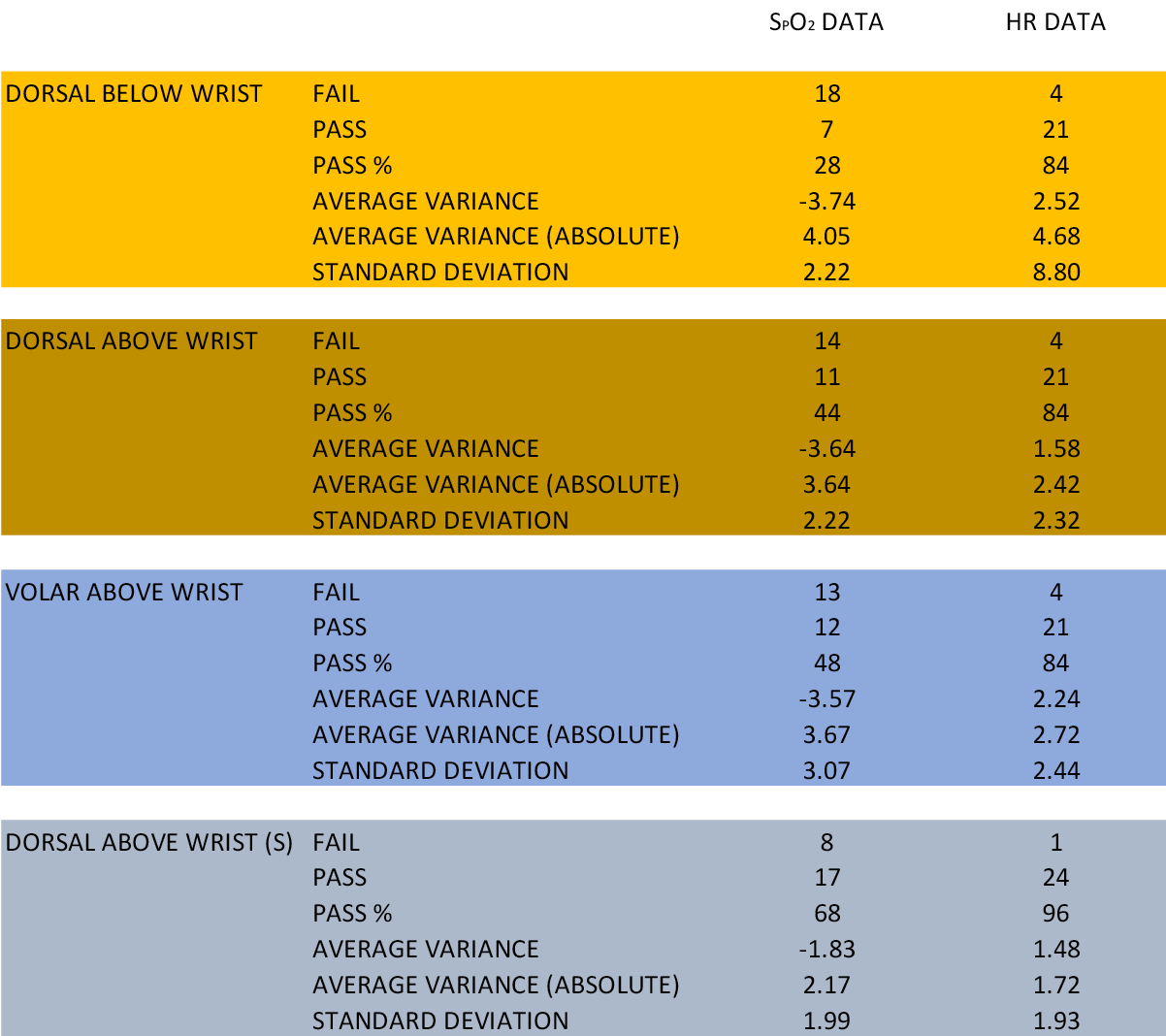
A summary of results obtained is provided in Table 1. Of the total 100 test SpO2 measurements obtained by the GF6 watch in 25 subjects, there were 53 failures; 38 failures were due to measurement inaccuracy greater than 3 percentage points and 15 failures were due to an inability to provide a SpO2 measurement after repeated attempts in the same location. The most accurate SpO2 test results were obtained with dorsal positioning of the GF6 watch proximal to the ulnar and radial styloids with a shaved wrist (DAWS test condition). SpO2 measurements taken distal to the level of the ulnar and radial styloids (DBW) were less accurate than those taken above (p<0.03). Whilst shaving the wrist resulted in a trend towards less failures, the effect of shaving was not statistically significant when compared to results obtained when positioning the watch in an equivalent position unshaved (DAR; p=0.08). The GF6 SpO2 measurements provided lower readings in comparison to the reference device in all test conditions, with lowest absolute variance in observations and standard deviation of measurements observed with DAWS condition testing.
HR data obtained by the GF6 were more consistently observed to be within clinically acceptable limits of accuracy compared to SpO2 measurements for all test conditions (p<0.01). Of the 13 recorded failures of 100 HR measurements obtained, 13 were due to measurement inaccuracy greater than five BPM and one failure was due to an inability to provide a measurement despite repeated attempts. Watch position and shaving were not associated with significant variation in HR results (p>0.3).
Table 1. Summary results of normobaric normoxia testing of SpO2 and HR monitoring accuracy using Garmin fēnix 6 Pro watch
Discussion
The findings of this study demonstrate the GF6 as unsuitable for SpO2 monitoring in clinical healthcare management. Whilst greater accuracy of SpO2 measurement may be obtained with optimised positioning and shaving of forearm hair, the overall accuracy of the device prohibits valid application for austere medical care or research purposes. Furthermore, the discontinuous nature, slow reading time and high number of read failures reduces the utility of the GF6 device to clinically useful SpO2 evaluation. HR data obtained by the GF6 watch, being of continuous real time nature and demonstrating a higher degree of accuracy, may, however, still be of some clinical utility.
The findings of this study reinforce the employment of medically validated pulse oximetry devices in austere environments. Whilst portable medical use pulse oximeters may not provide users with the degree of multifunctionality offered by modern smartwatch devices, they are, however, better suited for the purpose of clinically accurate SpO2 measurement. As clinically validated medical grade finger pulse oximetry devices are generally lightweight and inexpensive, their substitution for less accurate multifunctional devices is therefore not recommended unless appropriate accuracy has been established6.
Limitations of this study include that only a single GF6 device was evaluated. The GF6 is available in three face sizes and, whilst the SpO2 sensor is centrally located on the reverse side of the watch face, it is possible that an altered fit may be experienced with variations in wrist size that may potentially impact on the accuracy of the device. In addition, this study was undertaken only in healthy subjects in normobaric normoxic conditions. Whilst this study is therefore unable to comment upon the performance of the GF6 watch in the evaluation of hypoxia due to disease states or altitude exposure, the results observed within normoxic conditions would still suggest limited utility in any clinical application.
Given the results obtained within this study, further research to include GF6 SpO2 accuracy testing within hypoxic environments is not considered necessary. As the device has been demonstrated as unsuitable for SpO2 measurement in normobaric normoxic conditions, it follows that using the device for any clinical or research activities, including when the subjects are under physiologic stress, would also be inappropriate. Data obtained from hypoxic environmental and stress testing of GF6 SpO2 accuracy would therefore be of no practical value.
Conclusion
The results of this study do not support the use of the GF6 wristwatch for SpO2 evaluation in clinical healthcare or research due to insufficient accuracy and high rates of measurement failure. The use of validated medical grade pulse oximetry devices is recommended in austere environments.
Disclaimer
The views expressed in this manuscript are those of the author and do not reflect official Australian Defence Force policy or endorsement.
Conflict of interest
The author declares no conflicting interests in relation to this manuscript.
Funding
No funding was received for the conduct of this research.
Author(s)
Patrick Weinrauch*
2nd Health Battalion, Australian Army, QLD, Australia
School of Medicine, Griffith University, QLD, Australia
*Corresponding author email pweinrauch@brisbanehipclinic.com.au
References
- Chandrasekaran R, Katthula V, Moustakas E. Patterns of use and key predictors for the use of wearable health care devices by US adults: insights from a national survey. J Med Internet Res 2020;Oct(22),10.
- GlobalData Plc. Number of units sold by Garmin (FY2017 – FY2021); 2023. Available from: https://www.globaldata.com
- Lauterbach CJ, Romano PA, Greisler LA, Brindle RA, Ford KR, Kuennen MR. Accuracy and reliability of commercial wrist-worn pulse oximeter during normobaric hypoxia exposure under resting conditions. Res Q Exerc Sport 2020;1–10.
- Schiefer LM, Treff G, Treff F, Schmidt P, Schafer L, Niebauer J, et al. Validity of peripheral oxygen saturation measurements with the Garmin fēnix® 5X Plus wearable device at 4559m. Sensors (Basel) 2021;21(19).
- Garmin Ltd. fēnix® 6 Pro owner’s manual; 2019.
- Kirszenblat R, Edouard P. Validation of the Withings ScanWatch as a wrist-worn reflective pulse oximeter: prospective interventional clinical study. J Med Internet Res 2021;23(4):e27503.







