Volume 25 Number 1
Acidified nitrite foam anti-microbial action in an ex vivo porcine dermal model
C Michael Miller, Ellen Lantz, Aaron Strickland, David Bell, Greg Schultz
Keywords wound healing, nitric oxide, antimicrobial, pig explant, acidified nitrite, foam
For referencing Miller CM et al. Acidified nitrite foam anti-microbial action in an ex vivo porcine dermal model. Journal of Wound Management 2024;25(1):11-15.
DOI
10.35279/jowm2024.25.01.02
Submitted 14 June 2023
Accepted 19 September 2023
Abstract
Background Nitric oxide (NO) plays a critical role in wound healing, including stimulating vasodilation, angiogenesis and broad antimicrobial activity.
Aim To determine if acidified nitrite foam (ANF) is effective at killing microbial pathogens.
Methods A novel method to generate and deliver NO gas at the point of care was developed using acidified nitrite in a bubble foam. Using an ex vivo porcine dermal tissue model, the ANF was tested against six common microbial wound pathogens – Acinetobacter baumannii, Candida albicans, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus aureus and Staphylococcus epidermidis. The prevention study tested the ANF against pathogens in the planktonic phenotype. The eradication study tested ANF against pathogens in the mature biofilm phenotype.
Results In the prevention study, a single 5-minute exposure of ANF prevented 4.5-log10 to 8.6-log10 growth of biofilms among the six tested pathogens. In the eradication study, a single 5-minute exposure of ANF generated a 1.2-log10 to 2.5-log10 reduction of mature biofilms among the six tested pathogens. In the same eradication study, two 5-minute topical exposures of the ANF separated by 10 minutes generated a 2.2-log10 to 4.5-log10 reduction.
Conclusions These study results suggest that a single treatment with ANF may be able to prevent biofilm reformation in chronic wounds and may eradicate some mature biofilms following two exposures.
Implications for clinical practice ANF is an effective antimicrobial agent against several tested biofilms and has the potential to become a preferred treatment in the fight against wound infection.
Key messages
- This paper reports the results of an ex vivo porcine dermal tissue study designed to test whether a novel acidified nitrite foam (ANF) could prevent biofilm reformation and eradicate mature biofilms among six common microbial pathogens – Acinetobacter baumannii, Candida albicans, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus aureus and Staphylococcus epidermidis.
- The goal of the manuscript is to communicate the effectiveness of ANF against biofilm, offering a new solution for managing infection in chronic wounds.
- In the prevention study, a single 5-minute exposure of ANF prevented 4.5-log10 to 8.6-log10 growth of biofilms among the six tested pathogens.
- In the eradication study, a single 5-minute exposure of ANF generated a 1.2-log10 to 2.5-log10 reduction of mature biofilms, but two 5-minute topical exposures of the ANF separated by 10 minutes generated a 2.2-log10 to 4.5-log10 reduction among the six tested pathogens.
Introduction
Wound healing is hindered by excessive bioburden. Nitric oxide (NO) plays a critical role in host defence and immune response, acting as a cytotoxic agent against pathogens1 as well as inducing inflammation2. In addition, NO plays key roles in maintaining vascular homeostasis, regulating inflammation, and stimulating antimicrobial action3.
As an antimicrobial molecule, NO has both nitrosative and oxidative mechanisms which eventually results in the production of dinitrogen trioxide (N2O3) and peroxynitrite (ONOO–)4. Dinitrogen trioxide induces DNA deamination, while peroxynitrite causes membrane lipid peroxidation5. NO-mediated inhibition of metabolic enzymes may also constitute an important mechanism of NO-induced cytosis6. These harmful processes are specific only to bacteria because eukaryotic cells have mechanisms to scavenge these reactive species to prevent damage7.
Exogeneous NO can be used to reduce wound bioburden and speed wound healing. Local administration of NO gas has been found to be especially challenging due to its highly reactive nature when applied to the skin and simultaneously exposed to ambient air. Even so, external applications have been attempted using acidified nitrite following the pathway shown in Equations 1 and 2.
![]()
Sodium nitrite, as used above, is easily incorporated into a lotion or gel for topical application. The basal acidic environment in the wound can generate the NO species; however, the addition of acidic co-reactants, such as citric acid, better drives the conversion of nitrite to NO gas8.
Clinically, an acidified nitrite/citric acid gel producing NO gas was shown to improve healing of various types of chronic wounds with major bacterial bioburdens. Treatment of Buruli ulcers, which is a chronic ulcer with contributing mycobacterial infection, stimulated a 56% decrease in average ulcer size compared to a placebo group9. In wounds with substantial infection by methicillin resistant S. aureus (MRSA), NO gas treatment resulted in full eradication of MRSA within 5 days in 15 wounds across eight patients10.
Certain advantages can be realised by applying the acidified nitrite as a foam. In this context, a foam is comprised of small bubbles of gas surrounded by thin films of liquid. One solution of acid, surfactant and water can be added to a hand pump that creates a foam when depressed. Another solution of nitrite salt, surfactant and water can be added to another hand pump that creates a foam when depressed. When the two foams are mixed, gaseous NO is created in the liquid film. Similar to the case of the acidified nitrite gels and creams, the NO must move to the liquid/gas boundary. The thin film and the low viscosity of the film allows the NO gas to move quickly to the gas phase. Once the NO is in the gas phase, the bubbles of the foam keep the NO gas from escaping.
By reducing the mass transfer barrier that is present in the gel or cream formulations, another independent factor affecting the NO release profile can be manipulated. Specifically, the release rate of NO gas is directly related to the amount of mixing between the acid containing foam and the nitrite containing foam. By removing the rate-limiting step of NO diffusing through a high viscosity gel or cream, the mixing of the two foams along with the concentration of acid and nitrite ions will directly affect the release profile of the NO gas.
The mixed NO producing foam will fill the full geometry of a wound and ensure intimate contact with any complicated surface topology due to the presence of the surfactant in the formulation. As the bubbles in the foam contact a surface, they will rupture due to temperature gradients and surface interactions, thus releasing the NO against the surface (Figure 1).
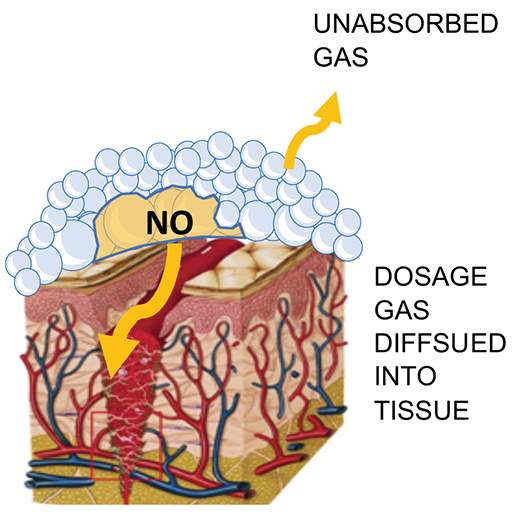
Figure 1. Illustration of ANF delivery of NO gas
To determine if ANF is as effective as gels and creams at killing microbial pathogens, a series of experiments were conducted by the Medical Biofilms Laboratory (MBL) of the Center for Biofilm Engineering (CBE) at Montana State University, USA. ANF was applied to gram-negative and gram-positive biofilms created by six different microbes. The ANF was effective at disrupting mature biofilm and reducing the remaining microbes. To investigate the effect of dermal tissue on the efficacy of acidified nitrite in disrupting biofilms and reducing the number of remaining pathogens, a prevention and eradication experiment was conducted at iFyber (Ithaca, NY) using an ex vivo porcine dermal model of mature biofilm.
Methods
Porcine dermal explants 0.5 inches in diameter were processed for testing of both biofilm prevention and biofilm eradication to investigate the effects of the ANF on preventing biofilm growth and eradication of 72-hour-old mature biofilm.
For the prevention arm testing, ANF was used to investigate the effects of the formulation on microbial colonisation of the porcine dermis and biofilm formation over time (e.g., one timepoint at 24 hours). Porcine explants were inoculated with 106 Colony Forming Units (CFU) of a given bacterial strain (between 105 and 106 for C. albicans) and allowed to incubate with the tissue for 1 hour at 37˚C on soft agar under aerobic conditions. After this initial period, explants were transferred to a well plate and treated with ANF for 5 minutes then washed in Phosphate-Buffered Saline (PBS) to end the treatment. Next, explants were incubated at 37˚C on soft agar for a total of 24 hours followed by recovery of viable organisms from the explants in recovery media (Dey-Engley (D/E) neutralising broth) using a bath sonicator. Enumeration was done by standard 10-fold serial dilution and plating on tryptic soy agar plates. Results were compared to untreated control samples.
For the eradication arm of the study, sterilised explants were inoculated with 106 CFU of a given microbial strain and allowed to incubate with the tissue for 72 hours at 37˚C on soft agar. This soft agar was changed daily and contained broth and antibiotic/antimycotic as needed to prevent overgrowth of the biofilm. All explants were washed twice in PBS for 2 minutes each to remove planktonic bacteria. Explants to be treated were then transferred to a well plate and treated with either one dose for 5 minutes or two doses for 5 minutes separated by 10 minutes of the ANF (ADVANOX™, NOxy Health Products, San Mateo, CA, USA). Single and double treatments were each concluded by two 20-second PBS washes. After the treatment regimen, viable organisms were recovered from the explants in D/E neuralisation broth via sonication and enumerated by standard plate counts.
Pathogens
Testing was performed on biofilms of P. aeruginosa (ATCC BAA-47), multi-drug-resistant A. baumannii (ATCC BAA-1797), methicillin-resistant S. aureus (USA300-0114), C. albicans (ATCC 10231), S. epidermidis (ATCC 35984) and P. mirabilis (ATCC 12453).
Treatments
NOxy Health Products supplied the NO producing foam system that was manufactured at Wasatch Labs (Draper, UT, USA). The foam was supplied through two components (a solution A and a solution B) contained in foam pumps. Solution A comprised a 9.7% w/w solution of citric acid. Solution B comprised a 16% w/w solution of sodium nitrite.
The steps to apply a NO foam treatment are listed below.
- Dispense an amount of Solution A foam into an appropriate container by depressing the Solution A pump head consistently and briskly.
- Dispense an approximately equal amount of Solution B foam into the same container by depressing the Solution B pump head consistently and briskly.
- Using a wooden tongue depressor, stir the two foams for 5 seconds to mix the two foams thoroughly.
- Apply the mixed NO foam by pouring the foam out of the container on the required site or, scoop out the required volume of foam using a spatula/spoon for more precise placement of the foam.
Results
For each test organism, tests from two arms were conducted. For the prevention arm, viable plate counts in CFU/explant were measured for an untreated control and 24 hours after a 5-minute ANF treatment. For the eradication arm, viable plate counts in CFU/explant were measured for an untreated control, after a 5-minute ANF treatment, and after two 5-minute ANF treatments 10 minutes apart. Each experiment was conducted twice, and each test was performed with five replicates (i.e., five replicate explants treated similarly for each experimental replicate).
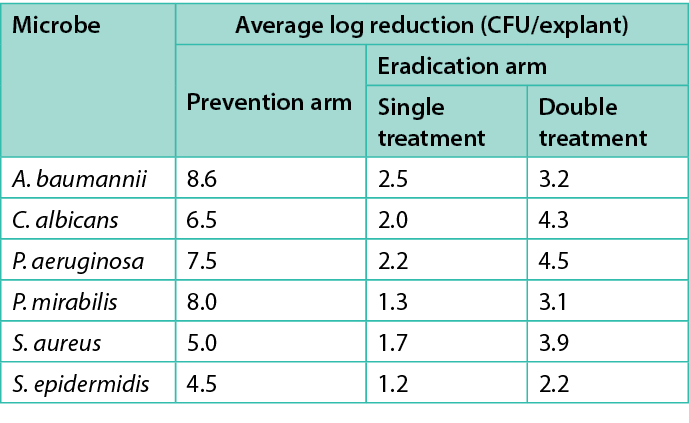
Table 1 shows the average log reduction per microbe for each treatment in the prevention and eradication arms. Figures 2–7 show the same information, with error bars representing one standard deviation from the average.
Table 1. Average log prevention and average log reduction for the prevention and eradication arms respectively
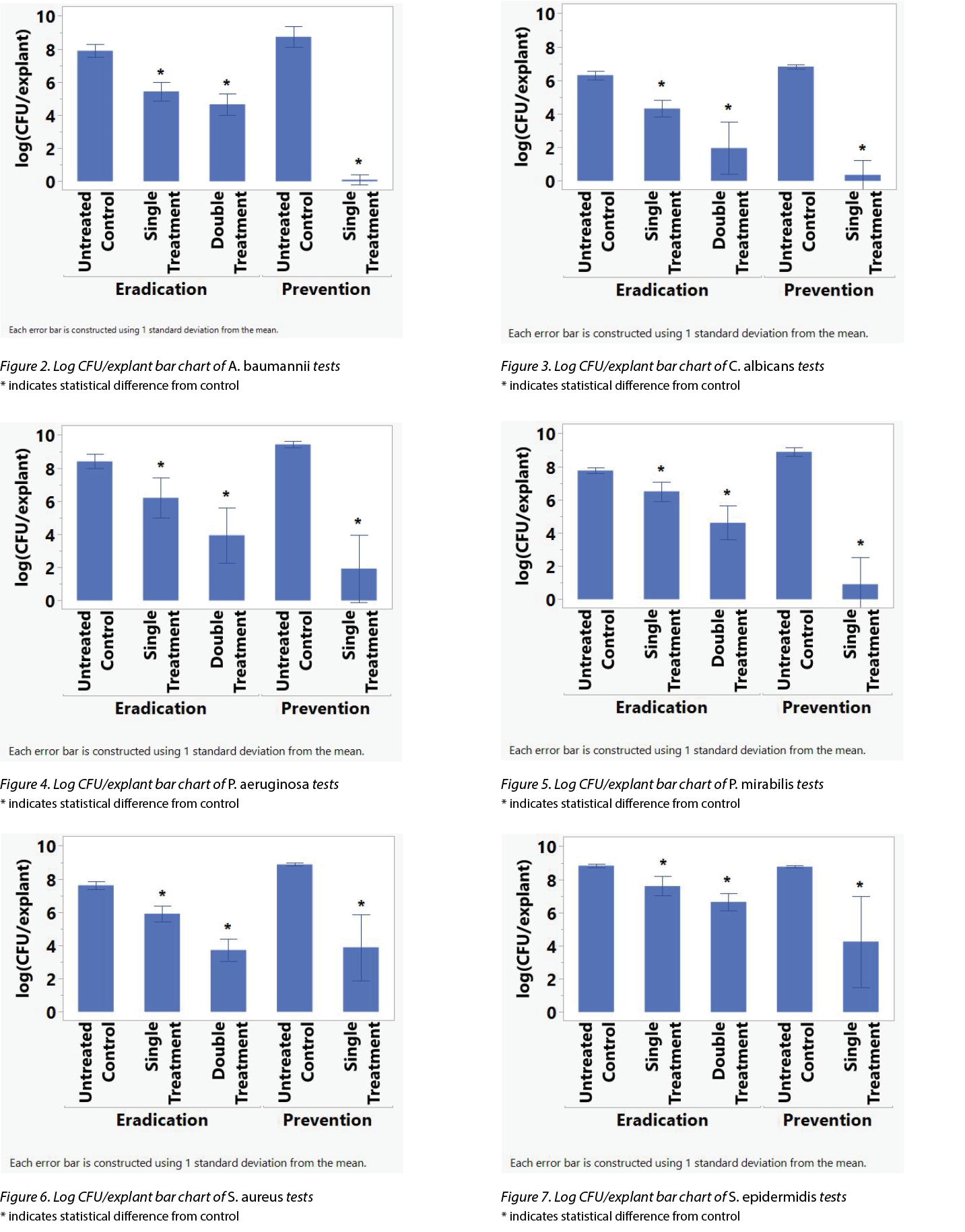
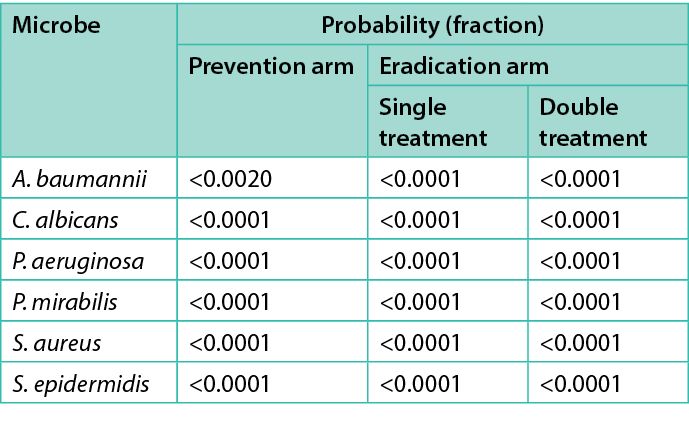
To determine if the differences in the CFU/explant before and after the various treatments were statistically significant, an analysis of variance (ANOVA) investigation was conducted using the Student’s t-test on JMP 16.1 (SAS Institute Inc.). An asterisk (*) in Figures 2–7 indicate the treatment’s difference from the control was statistically significant. Table 2 shows the same information, namely the probability for each treatment that the difference from the control is due to random effects.
Table 2. Microbes tested and average log reduction of CFU/explant as well the probability that the difference is not significant.
For all six pathogens, the log reduction after the first 5-minute treatment was compared to the log reduction after the second 5-minute treatment. The difference between the two treatments in all six cases were not significantly different. The inability of the Student’s t-test to detect a statistically significant difference is possibly due to the viable plate count method. Another experiment is planned to determine if the two treatments are statistically different.
Discussion
ANF significantly reduced the CFU/explant of each microbe tested after a 5-minute treatment in the prevention and eradication arms as well as two 5-minute treatments in the eradication arm. The P. aeruginosa, A. baumannii, and C. albicans seem particularly susceptible to NO antimicrobial actions.
ANF is an effective antimicrobial agent against several tested biofilms. To date, P. aeruginosa, A. baumannii, S. aureus, C. albicans, P. mirabilis and S. epidermidis biofilms have been tested. The ANF treatment was effective at reducing biofilm formation by at least 4 logs in the prevention assay. In biofilm eradication studies, the two-dose combined treatment was effective at reducing viable microbes by 2 to 4 logs depending on the microbe and was more effective than a single combined treatment.
The average log reduction of the number of microbes of the 5-minute treatment in the eradication arm is less than the average log reduction of the number of microbes in a previously reported in vitro study. This suggests that the presence of the porcine skin does negatively affect the ANF treatment effectiveness. However, the ANF is still effective in both disrupting the biofilm and killing microbes.
Conclusions
Historically, some antimicrobial treatments have performed well in a laboratory setting but perform less well against biofilms in vivo. It is thought that the biofilm penetrates the substrate on which it grows, giving the biofilm extra protection from the antimicrobial agent. In other cases, the in vivo environment may interact with the antimicrobial agent, reducing its availability or effectiveness against the biofilm pathogen. This study suggests that this hypothesis might be correct. The 72-hour-old biofilm within the natural tissue environment presents a significant challenge for antimicrobial agents.
In summary, this foundational study highlights the potent antimicrobial and anti-biofilm properties of the ANF under development. The ex vivo model of mature biofilm represents a significant test for the prototype foam product and positions the technology well for advanced development in preclinical animal studies and eventual clinical trials. Given the advantages of the ANF system and its effectiveness detailed here, ANF has the potential to become a treatment in the clinician’s toolbox to fight infection in wounds.
Implications for Clinical Practice
- ANF significantly reduced the CFU/explant of each microbe tested after a 5-minute treatment in the prevention and eradication arms.
- P. aeruginosa, A. baumannii and C. albicans seem particularly susceptible to NO antimicrobial actions.
- ANF is an effective antimicrobial agent against several tested biofilms.
- ANF has the potential to become a preferred treatment in the clinician’s toolbox to fight infection in wounds.
Further Research
Future research is needed to determine if the two treatments are statistically different.
There is also a need to see if ex vivo results can be replicated in humans. A first-in-human trial is planned to study the effect of ANF in humans.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
C Michael Miller*1 MS, PE, Ellen Lantz2 PhD, Aaron Strickland2 PhD, David Bell1 PhD, Greg Schultz3 PhD
1NOxy Health Products, Inc., 118 E. 7th St., Suite 2M, Anaconda, MT 59711, USA
2iFyber, 950 Danby Road, Suite 198, Ithaca, NY 14850, USA
3University of Florida, MD OBGYN Wound Research Center, Gainesville, FL 32610-0294, USA
*Corresponding author email miller@noxyhp.com
References
- Nathan CF, Hibbs JB Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 1991;3(1):65–70. doi:10.1016/0952-7915(91)90079-g
- Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol 1993;54(2):171–178.
- Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Health Mater 2019;8(12):e1801210. doi:10.1002/adhm.201801210
- Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 1998;25(4–5):434–456. doi:10.1016/s0891-5849(98)00092-6
- Möller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem 2005;280(10):8850–8854. doi:10.1074/jbc.M413699200
- Jones ML, Ganopolsky JG, Labbé A, Wahl C, Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl Microbiol Biotechnol 2010;88(2):401–407. doi:10.1007/s00253-010-2733-x
- Fang FC. Antimicrobial actions of reactive oxygen species. mBio 2011;2(5):e00141–11. doi:10.1128/mBio.00141-11
- Dave RN, Joshi HM, Venugopalan VP. Biomedical evaluation of a novel nitrogen oxides releasing wound dressing. J Mater Sci Mater Med 2012;23(12):3097–3106. doi:10.1007/s10856-012-4766-4 Phillips
- Phillips R, Adjei O, Lucas S, Benjamin N, Wansbrough-Jones M. Pilot randomized double-blind trial of treatment of Mycobacterium ulcerans disease (Buruli ulcer) with topical nitrogen oxides. Antimicrob Agents Chemother 2004;48(8):2866–2870. doi:10.1128/AAC.48.8.2866-2870.2004
- Ormerod AD, Shah AA, Li H, Benjamin NB, Ferguson GP, Leifert C. An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Res Notes 2011;4:458. doi:10.1186/1756-0500-4-458
