Ahead of Print
Clinical characteristics and healing outcomes of patients with pressure injuries: insights from Singapore’s first chronic wounds registry
Fazila Aloweni, Priya Bishnoi, Nanthakumahrie Gunasegaran, Ju Xia Zhang, Yi Zhen Ng, Tze Tec Chong,
Shin Yuh Ang
Keywords nursing, pressure injury, Chronic wounds, Healthcare Utilisation, Tertiary Hospital
For referencing Aloweni F, Bishnoi P, Gunasegaran N, et al. Clinical characteristics and healing outcomes of patients with pressure injuries: insights from Singapore’s first chronic wounds registry. Journal of Wound Management. 2024;25(2):To be assigned.
DOI
10.35279/jowm2024.25.02.03
Submitted 27 October 2023
Accepted 22 April 2024
Abstract
Aim To evaluate and provide insights on the clinical characteristics and healing outcomes of patients with pressure injuries (PIs) over six months using data from the Chronic Wounds Registry (CWR) database.
Method This was a retrospective database review study where data were extracted from Singapore’s CWR. The patient’s basic demographic, co-morbidities, wound-related clinical data, and wound images were analysed. The study outcomes were on clinical characteristics and healing outcomes over six months.
Results A total of 63 patients with 63 PIs were included in the study; 46.0% of the PIs were community-acquired, and the rest were hospital-acquired. The mean age was 67.3 years, and 56% were males. The most common ethnicity was Chinese (66.7%), followed by Malays (20.6%) and Indians (7.9%). At baseline, 30.2% of patients had deep tissue injury (DTI), 44.8% had stage I/II, and 13.8% had stage III/IV PIs. Twenty-nine (46.0%) of the PIs healed within six months, while 12 (19%) still had ongoing PIs at month 6. Wounds that failed to heal at six months exhibited a higher proportion of wound infection (33.3% vs 6.9%, p=0.02), wound bed slough (46.7% vs 10.3%, p=0.02), maceration of peri-wound skin (46.7% vs 3.5%, p=0.001)), and exudate (75.0% vs 38.0%, p=0.03).
Conclusion While nearly half of the PIs healed within six months, ongoing cases were more likely to exhibit infection, sloughing, skin maceration, and excess exudate, indicating these factors as critical targets for improving healing rates in PIs care.
Key messages
- The prevalence of pressure injuries is expected to increase with the ageing population.
- It is expensive to treat and manage pressure injuries, and it also poses significant economic challenges to the healthcare system.
- It is a global challenge for healthcare professionals to achieve closure of pressure injuries.
Introduction
Chronic wounds are hard-to-heal wounds/ulcers, which can take four weeks to more than three months to heal.1 Apart from venous leg ulcers, diabetic foot ulcers and pressure injuries (PI) are also common types of chronic wounds.2–5 In a systematic review comprising 39 studies, the overall global prevalence of PIs using a point and period prevalence was 14.8% and 11.6% in the hospital setting, respectively.6 The overall mean incidence of PIs was 6.3%.6 In Singapore, the prevalence of PIs was 18.1%, and the incidence was 8.1% during a survey conducted in 2005.7 Notably, there has been a two-fold increase in the PIs incidence rates among hospitalised patients over the last decade in Singapore.4, 8
The cost of managing PIs increases with the severity, as more extensive injury requires a longer time to heal and is associated with a higher incidence of complications; therefore, more healthcare resources are needed to care for this group of patients.6 Admission type, surgical interventions, medications, the length of hospital stay, and hospital environments such as nurse staffing, unit type, and nursing workload have all been identified to impact PIs development.9 In Singapore, a local tertiary hospital reported that the average gross charge per PIs episode was SGD 16,636 (USD 12,291) over five years (2013-2017). In 2017, the estimated PIs gross healthcare costs for hospitalised patients in primary care was SGD 21,394 (USD 15,789).10 Both studies reported similar findings and consistent cost expenditure over the years.
Beyond the substantial financial burden, PIs have a negative impact on all domains of quality of life.11 Individuals with PIs experience constant discomfort that affects their sleep and social well-being.12 A decrease in health-related quality of life (HRQoL) is also associated with the severity of the PIs.13 Extended periods of immobility can lead to the development of PIs due to muscle atrophy and joint contractures, making it challenging for patients to perform daily activities or maintain independence. A recent systematic review reported that PIs adversely influence the patient’s QOL, particularly on a psychological level. These individuals experience a significant disruption in their lives, relying heavily on their supportive surroundings and healthcare resources for their well-being.14 In a case-control study, it was reported that having PIs were significantly associated with an increased risk of not being discharged home (OR, 5.55; 95% CI, 4.35–7.08), along with increased risks of readmission to the hospitals (OR, 1.30; 95% CI, 1.05–1.62) and emergency department visits after discharge (OR, 1.70; 95% CI, 1.29–2.23).15
In Singapore, clinical data exists as isolated pockets within each institution’s databases. There is a lack of harmonised and consolidated source of data on wounds at the national level. To address this gap, a chronic wound registry (CWR) was established to collect prospective clinical and outcome data on various chronic wounds, such as neuro-ischemic ulcers (NIU), venous leg ulcers (VLU), and PIs. The registry collected information on patient demographics, comorbidities, wound characteristics, interventions, management, healthcare cost, and quality of life of patients with chronic wounds. In this study, we specifically extracted and examined only the PIs wound data and the healing outcomes.
Aims
To evaluate and provide insights on the clinical characteristics and healing outcomes of patients with PIs over six months using data from the CWR database.
Method
Study design, sample, and setting
This was a retrospective database review study. The dataset was obtained from CWR in Singapore. This registry was established in November 2019 and is maintained by the Skin Research Institute (SRIS). The registry database stores wound data specifically on diabetic foot ulcers, venous wounds, neuro-ischemic wounds, and PIs (all stages). Three tertiary hospitals of the three healthcare clusters of Singapore – SingHealth, National University Health System, and Nation Healthcare Group contribute to the registry data. Each healthcare cluster is affiliated with primary and community care centers, providing a full complement of healthcare services. A dedicated study coordinator collects information from admitted patients with their consent purely for storing wound data in the registry database. A hard-copy clinical research form (CRF) is completed by the study coordinator, and this is manually transcribed into the REDCap database. Hardcopy documents are stored under lock-and-key, and electronic data is stored on an encrypted, secured, and password-protected hard disk in the hospitals. Individual datasets are de-identified, harmonised at source, and uploaded to the CWR database housed at SRIS. The database is securely maintained and password-protected behind the SRIS security system, with limited access granted only to study-specific personnel.
PIs cases were selected for this study to explore the healing outcomes further. As part of the study, the index (largest) PIs wound data from inpatient and outpatient services were collected, and patients were followed for six months (1,3 and 6 months) or until the wound healed. The PIs staging were performed as defined in the International PIs guidelines.16 The standard PI care management involves referral and management by medical specialists and wound care specialty nurses. The scope of the care included weekly wound assessment, selection of the appropriate dressing, treatment of infection (if any), off-loading, optimisation of medical therapy, and bedside wound debridement if needed.
Ethical considerations
This study was granted ethical approval by the National Healthcare Group Domain Specific Review Board (Ref No: 2019/00971). Further consent was waived as patients had already consented to storage and use of their data in an anonymised format as previously described.
Data extraction, study variables, and outcomes
We extracted and analysed PIs (all stages) wound data that were available. Study variables, including age, sex, ethnicity, body mass index (BMI), smoking status, presence of co-morbidities, stage of PIs, wound size, site, healing status, mortality, and wound images, were also available for validation where a wound care specialist nurse verified the PIs stages and images.
Data analysis
All statistical analyses were performed with R software (R-4.2.1, R Foundational Statistical Computing, Vienna, Austria). An independent study team member conducted cross-checks to ensure data accuracy before analysis. Patients were divided into two groups – those whose PIs healed within the six-month study period and those who failed to heal. The demographics, comorbidities, and wound characteristics of the two groups were compared. Categorical variables were expressed as n (%), and the chi-square test was used for comparison. Normally distributed continuous variables were described as mean accompanied by standard deviation (SD), and skewed continuous variables were summarised as median (1st quartile – 3rd quartile). Two sample T test and Mann Whitney U test were used for comparison, respectively. The level of significance was set at p < 0.05.
Results
Patient demographics
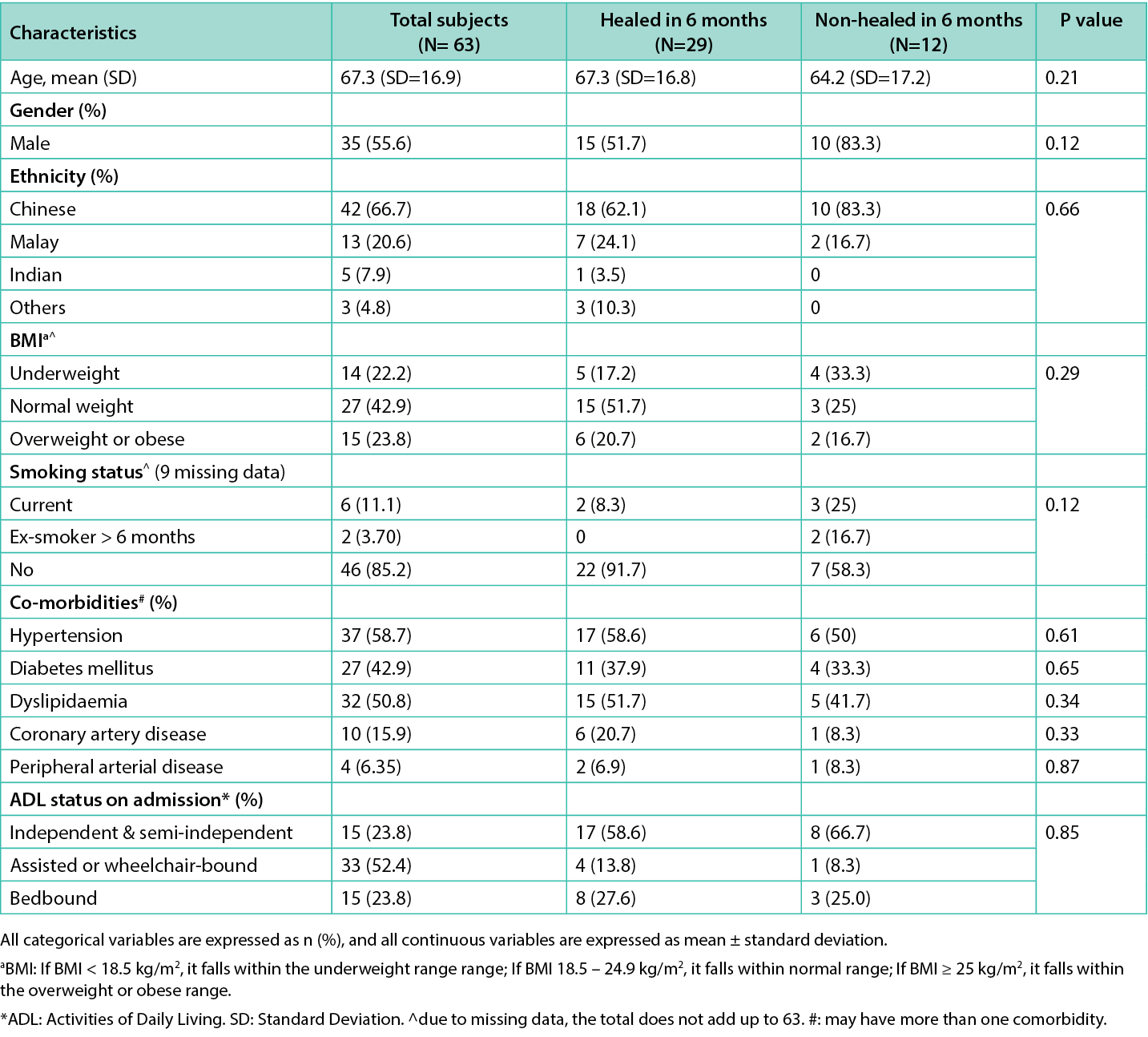
A total of 63 patients were included in the study; 46.0% of the PIs were community-acquired, and the rest were hospital-acquired (Table 1). Of 63 patients, 29 (46%) of the PIs healed within six months, while 12 (19%) still had ongoing PIs at month six. There were 19 (30.2%) patients deceased with non-Index wound-related mortality (Table 3). The mean age was 67.3, SD=16.9 years, and 56% were males. The most common ethnicity was Chinese (66.7%), followed by Malay (20.6%) and Indian (7.9%). Twenty-four per cent of patients had a BMI above 25kg/m2, and 22.2% had a BMI below 18.5kg/m2. Half of the study population had hypertension (58.7%), dyslipidemia (50.8%), and diabetes (42.9%). The demographics and co-morbidities between the two groups (healed vs non-healed) were comparable, with no statistically significant difference. Table 1 summarises the demographics of all included patients.
Table 1. Comparison of patient demographics, co-morbidities between healed and non-healed groups of patients with Pressure Injuries.
Wound characteristics
Almost half of the patients had a prior history of PIs, which was community-acquired. Sacrum (44.4%) was the most common location of PIs, followed by the heels (19%). At baseline assessment, the wound size was smaller in the healed group than in the non-healed group (median size: 6cm2 vs 10cm2. Patients in the non-healed group had a higher proportion of infection compared to the healed group (33.3% vs 6.9%, p=0.02). Similarly, more patients in the non-healed group had the presence of slough in the wound bed (46.7% vs 10.3%, p=0.02), maceration of the peri-wound skin (46.7% vs 3.5%, p=0.001) and presence of exudate (75% vs 38%, p=0.03). Dry and scaly peri-wound skin was seen more in the healed group of patients (37.9%, p=0.03). At baseline, 30.2% of patients had deep tissue injury (DTI), 44.8% with stage I/II, and 13.8% with stage III/IV. The proportion of stage III/IV PIs was higher in the non-healed group (58.3% vs. 10.3%, p=0.02). Refer to Table 2 for the PIs wound characteristics.
Table 2. Comparison of wound characteristics of patients with pressure injuries between the healed and non-healed groups.
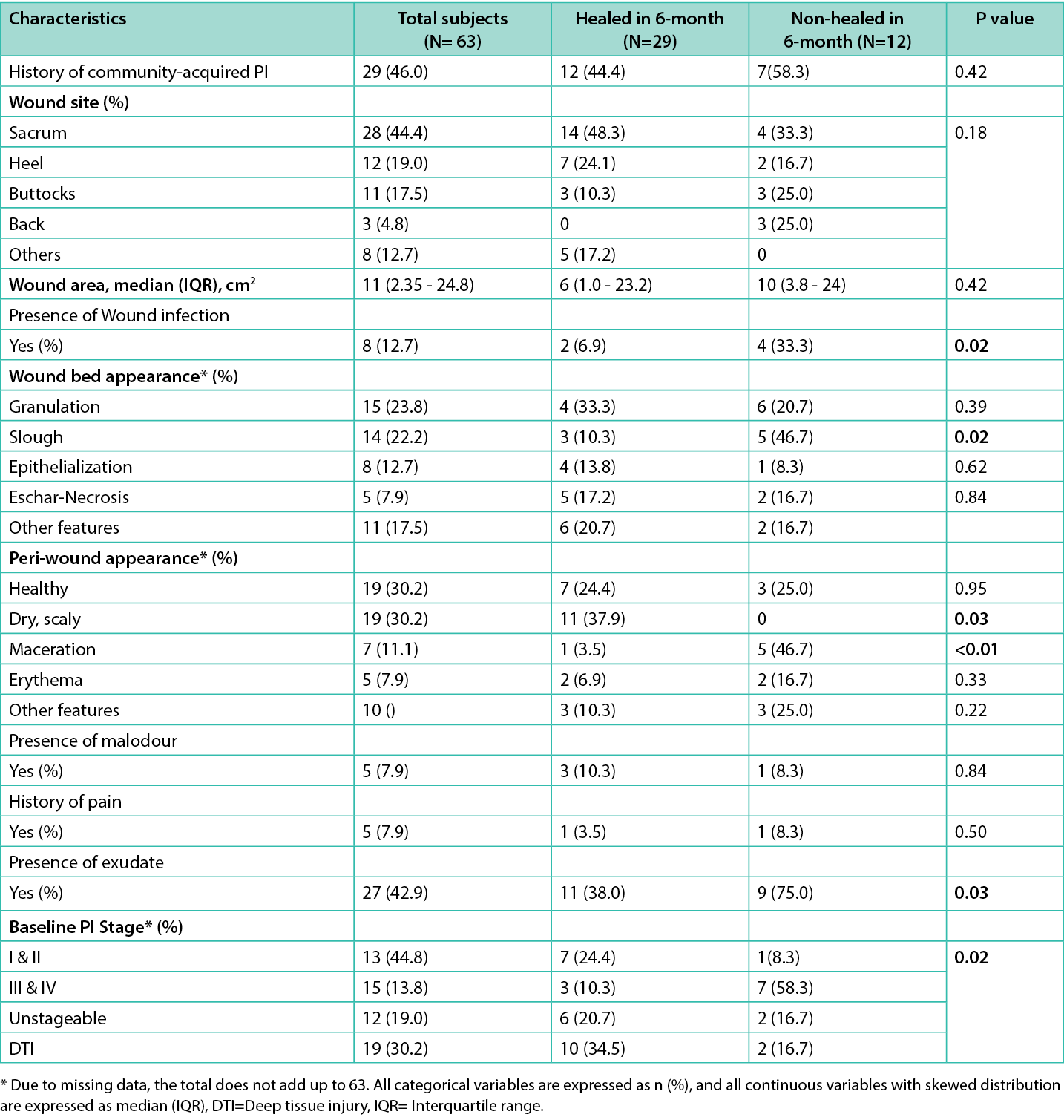
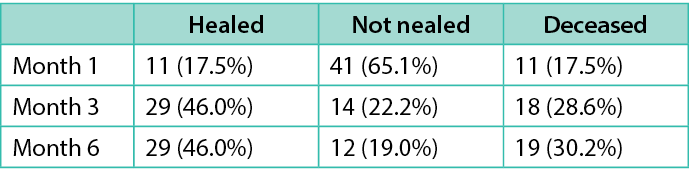
Table 3. Healing outcomes over six months
Discussion
This study examined the PIs characteristics, healing outcomes, and factors associated with healing. In our study, the average age of patients with PIs was younger than commonly reported.17 However, this aligns with other studies identifying younger populations with PIs,8, 18 suggesting age as a variable with inconsistent association to PIs.
Some studies have investigated the effects of body weight and BMI, and increased rates of PIs have been frequently documented among underweight and overweight people.19–21 Patients with higher BMI rates are susceptible to developing PIs due to poor circulation, reduced vascularity of adipose tissue, and excess weight compressed on areas such as the sacrum, coccyx, and heels. However, in this study, BMI was not associated with the healing outcome.
A study in Singapore reported that the prevalence rate of community-acquired PIs was 6–29% in an acute care hospital.8 The previous research also documented that 75% of PIs were community-acquired.8 Likewise, our study found that half of the recruited patients (n=29; 46%) had a history of community-acquired PIs.
In a recent systematic review, 16 studies (132,530 patients with 12,041 PIs) reported on PIs staging, and the most commonly occurring stages were Stage I, 43.5%, and Stage II, 28.0%.22 Stage III and IV PIs occurred less frequently, accounting for 12.8% (95% CI 10.9–14.9%) and 9.9% (95% CI 7.5–12.5%). However, in our study, we found DTI was the most common stage (30.2%). This could be due to our patients, who required more assistance to move, as only 23.8% were independent in their activity of daily living status while the rest were assisted and bedbound.
Multiple chronic medical conditions can worsen the prognosis of patients with PIs.17 Cardiovascular-related diseases are often cited as a common risk factor for PIs.23, 24 The changes include differences in blood pressure results, decreased perfusion, and peripheral ischemia, contributing to the occurrence and development of PIs.23,25 Likewise, hypertension was most prevalent among our patients (n=37; 59%), followed by dyslipidemia (n=32; 50.79%) and diabetes (n=27; 43%) (Table 1). Diabetes is known to have micro or macrovascular complications, such as sensory perception, which impacts wound healing and causes re-infections.23 However, in this study, diabetes is not significantly associated with the healing outcomes. This could be due to the small sample size.
In the non-healed group, the presence of infection, slough, and exudate were statistically significant, supporting established knowledge and aligning with wound healing principles. These factors, particularly slough, are well-documented to impede the inflammatory phase by creating a barrier to tissue repair and fostering a hospitable environment for bacterial growth. This, in turn, can contribute to infection, increased exudate, and potentially, malodor.26, 27 We also found that peri-wound maceration was prevalent in this group. Wound exudate is known for its role in promoting healing by preventing the wound bed from becoming dry and facilitating cell movements leading to tissue repair. However, an increased amount of exudates can lead to peri-wound skin maceration.28 The epithelium becomes soft due to extended exposure to increased fluid, making it more vulnerable to bacteria and fungi invasion causing increased wound size and peri-wound complications.29 The literature also recognises that maceration impedes the movement of keratinocytes from the edges to the base of the wound, leading to a delay in the overall healing process.30, 31
Another finding was that patients in the healed group had a dry/scaly peri-wound appearance (p=0.03). Dry, scaly, calloused, or hyperkeratotic peri-wound conditions can impede wound healing and need to be addressed before the wound can progress towards closure. There is a need for such peri-wound conditions to be removed via sharp debridement, thus allowing epithelialisation. Debriding helps the wound to regenerate during the healing process. This disturbance stimulates the release of growth factors, initiating the development of new, healthy skin.32 We were unable to determine if the PIs were debrided to remove dry/scaly skin, which aided in healing, due to the study design.
In addition to evaluating the peri-wound and wound bed appearance, the baseline PI stage emerges as a crucial determinant of the wound’s healing trajectory over a six-month period. Notably, baseline PIs stages I/II exhibited a rapid healing rate in 44.8% of cases (n=13), with only 8.3% (n=1) failing to heal within six months. In contrast, stages III and IV demonstrated a slower healing pace. Among the unhealed group, 58.3% of patients with stage III/IV did not have their PIs healed.
The underlying rationale lies in the complexity associated with Stage III/IV PIs, characterised by deeper involvement of underlying tissues and extensive damage to surrounding structures, encompassing subcutaneous tissue, muscle, tendon, ligament, cartilage, and/or bone.16 The potential presence of undermining and/or tunnelling further magnifies the true size of the wound, leading to a delay in the healing process. Likewise, a cohort study reported that the mean time to healing was eight months for stage III/IV.33 Contrastingly, a retrospective study highlights Stage II as the most prevalent PIs type, with a propensity for faster healing.34 These collective findings reinforce the significance of considering the initial PIs stage as a pivotal factor influencing the healing dynamics.
Our analysis notably revealed a slow healing rate for PIs, with only 17.5% (n=11) healed at one month and 46% (n=29) healed at six months. Notably, 18% (n=11) of patients passed away within the first month, followed by 29% (n=18) at three months. While prior research indicates higher mortality rates associated with PIs (e.g., 23.6% at one month in a study of n=89) 35, it did not distinguish PI-related deaths. Similarly, in our study, although the mortality was high, the cause of death was not due to PIs. However, the small sample study limits our ability to definitively assess the mortality risk associated with PIs in this population.
Limitations and Recommendations
This study has limitations due to the way the data were collected. Firstly, only the largest PIs per patient was recorded, despite the potential presence of multiple PIs at recruitment. This limits our understanding of complex wound healing dynamics associated with multiple PIs. Future studies should collect comprehensive data on all PIs present at recruitment, potentially using standardised and validated tools for accurate identification and staging. Additionally, manual PI measurements could introduce measurement bias. Implementing wound imaging technology with automated PI detection and quantification systems could improve accuracy and efficiency compared to manual measurements by trained nurses.
Beyond data capture, some incomplete records were identified within the database. Establishing data entry protocols for research staff and healthcare professionals, including mandatory fields and timely validation checks, could minimise missing data in future studies. Furthermore, integration with hospital electronic health records could streamline data entry and reduce incompleteness.
Finally, excluding vulnerable patients who could not provide consent might introduce selection bias, potentially underrepresenting patients with severe PIs (Stages III and IV) who often face communication challenges. Future studies could explore alternative consent procedures or consider collaboration with ethics committees to address this challenge.
Conclusion
This is a snapshot of the number of managed PIs within our institutions over six months. While nearly half of the PIs healed within six months, ongoing cases were more likely to exhibit infection, sloughing, skin maceration, and excess exudate, indicating these factors as critical targets for improving healing rates in pressure injury care.
Acknowledgments
We acknowledge the support of the entire team of the Wound Registry project and especially our clinical coordinators, Corrina Kee Pei Yin, Amilia Foo Ai Jun and Li Xiao Mei, for the recruitment of patients.
Author contributions
All authors reviewed and extensively edited the manuscript and approved the final version of this manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
This research was supported through grant funding from the Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund – Pre-Positioning Programme (IAF-PP) grant number H19/01/a0/0Y9 as part of the Wound Care Innovation for the Tropics (WCIT) Programme.
Author(s)
Fazila Aloweni†1MSc, Priya Bishnoi†2,3 MD Dermatology, Nanthakumahrie Gunasegaran*4 MSc, Ju Xia Zhang5 MSc, Yi Zhen Ng2,3 PhD, Tze Tec Chong6 MBBS (Hons 1st Class), FACS (General & Vascular Surgery), Shin Yuh Ang7 MBA
1Clinical Asst Prof (Ms), Nursing Division, Singapore General Hospital, Singapore
2A*STAR Skin Research Labs, Singapore
3Skin Research Institute of Singapore, Singapore
4Nursing Division, Singapore General Hospital, Singapore
5Nursing Division, National Heart Centre, Singapore
6A/Prof, Department of Vascular Surgery, Singapore General Hospital, Singapore
7Adj A/Prof (Ms) Nursing Division, Singapore General Hospital, Singapore
†Joint 1st authorship *Corresponding author email nanthakumahrie.gunasegaran@sgh.com.sg
References
- Järbrink K, Ni G, Sönnergren H, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Systematic Reviews. 2017; 6. DOI: 10.1186/s13643-016-0400-8.
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4:560–582. DOI: 10.1089/wound.2015.0635.
- Graves N, Zheng H. The prevalence and incidence of chronic wounds: a literature review. Wound Pract Res. 2014; 22 (1):4–19.
- Goh OQ, Ganesan G, Graves N, et al. Incidence of chronic wounds in Singapore, a multiethnic Asian country, between 2000 and 2017: a retrospective cohort study using a nationwide claims database. BMJ Open 2020; 10: e039411. DOI: 10.1136/bmjopen-2020-039411.
- Graves N, Ganesan G, Tan KB, et al. Chronic wounds in a multiethnic Asian population: a cost of illness study. BMJ Open 2023; 13: e065692. DOI: 10.1136/bmjopen-2022-065692.
- Demarré L, Verhaeghe S, Annemans L, et al. The cost of pressure ulcer prevention and treatment in hospitals and nursing homes in Flanders: A cost-of-illness study. Int J Nurs Stud. 2015;52:1166–1179. DOI: 10.1016/j.ijnurstu.2015.03.005.
- Chan EY, Tan SL, Lee CK, et al. Prevalence, incidence and predictors of pressure ulcers in a tertiary hospital in Singapore. J Wound Care. 2005;14:383–388. DOI: 10.12968/jowc.2005.14.8.26820.
- Graves N, Maiti R, Aloweni FAB, et al. Pressure injuries among admissions to a hospital in the tropics. Int Wound J. 2020;17:1659–1668. DOI: 10.1111/iwj.13448.
- Team V, Jones A, Teede H, et al. Pressure injury surveillance and prevention in Australia: Monash Partners Capacity Building Framework. Front Public Health. 2021;9:634669. DOI: 10.3389/fpubh.2021.634669.
- Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5-year institutional population health review. Int Wound J. 2020; 17: 790–803. DOI: 10.1111/iwj.13333.
- Fox C. Living with a pressure ulcer: a descriptive study of patients’ experiences. Br Jf Community Nurs. 2002;7:10–22.
- Young T, Furtado K and Alves P. Health related quality of life (HRQOL) implications for people with pressure ulcers. In Science and practice of pressure ulcer management. Springer, 2018, pp.79–87.
- Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019; 27: 114–125. DOI: 10.1111/wrr.12683.
- Roussou E, Fasoi G, Stavropoulou A, et al. Quality of life of patients with pressure ulcers: a systematic review. Med Pharm Rep. 2023; 96: 123–130. DOI: 10.15386/mpr-2531.
- Han Y, Jin Y, Jin T, et al. Impact of Pressure Injuries on Patient Outcomes in a Korean Hospital: A case-control study. J Wound, Ostomy Continence Nurs. 2019;46:194–200. DOI: 10.1097/WON.0000000000000528.
- European Pressure Ulcer Advisory Panel NPIAP, Pan Pacific Pressure Injury Alliance Prevention and Treatment of Pressure Ulcers/Injuries: Quick Reference Guide. 2019.
- Song YP, Shen HW, Cai JY, et al. The relationship between pressure injury complication and mortality risk of older patients in follow‐up: a systematic review and meta‐analysis. Int Wound J. 2019;16: 1533–1544.
- Aghazadeh A, Lotfi M, Asgarpour H, et al. Frequency and risk factors of pressure injuries in clinical settings of affiliated to Tabriz University of Medical Sciences. Nursing Open. 2021;8:808–814. DOI: 10.1002/nop2.685.
- Kottner J, Gefen A, Lahmann N. Weight and pressure ulcer occurrence: a secondary data analysis. Int Journal Nurs Stud .2011; 48: 1339–1348.
- Pokorny ME, Rose MA, Watkins F, et al. The relationship between pressure ulcer prevalence, body mass index, and braden scales and subscales: a further analysis. Adv Skin Wound Care. 2014; 27: 26–30.
- Ness SJ, Hickling DF, Bell JJ, et al. The pressures of obesity: The relationship between obesity, malnutrition and pressure injuries in hospital inpatients. Clinical Nutrition. 2018; 37: 1569–1574. DOI: https://doi.org/10.1016/j.clnu.2017.08.014
- Li Z, Lin F, Thalib L, et al. Global prevalence and incidence of pressure injuries in hospitalised adult patients: A systematic review and meta-analysis. Int J Nurs Stud. 2020; 105: 103546. DOI: https://doi.org/10.1016/j.ijnurstu.2020.103546.
- Jaul E, Barron J, Rosenzweig JP, et al. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr 2018; 18: 305. DOI: 10.1186/s12877-018-0997-7.
- Lee HJ, Han MY, Hwang JH, et al. Risk factors for heel pressure injury in cardiovascular intensive care unit patients. International Wound Journal 2022; 19: 1158–1164. DOI: 10.1111/iwj.13711.
- Alderden J, Rondinelli J, Pepper G, et al. Risk factors for pressure injuries among critical care patients: A systematic review. Int J Nurs Stud 2017; 71: 97–114. DOI: 10.1016/j.ijnurstu.2017.03.012.
- Percival SL, Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care. 2015; 24(11): 498–410. DOI: 10.12968/jowc.2015.24.11.498.
- Harries RL, Bosanquet DC, Harding KG. Wound bed preparation: TIME for an update. Int Wound J. 2016;13(S)3:8–14. DOI: 10.1111/iwj.12662
- Chhabra S, Chhabra N, Kaur A, et al. Wound healing concepts in clinical practice of OMFS. J Maxillofac Oral Surg. 2017; 16: 403–423.DOI: 10.1007/s12663-016-0880-z
- Woo KY, Beeckman D and Chakravarthy D. Management of moisture-associated skin damage: A scoping review. Adv Skin Wound Care. 2017; 30: 494–501. DOI: 10.1097/01.ASW.0000525627.54569.da
- Rippon MG, Rogers AA, Ousey K, et al. The importance of periwound skin in wound healing: an overview of the evidence. J Wound Care 2022; 31: 648–659. DOI: 10.12968/jowc.2022.31.8.648
- Rippon M, Ousey K, Rogers AA, et al. Wound hydration versus maceration: Understanding the differences. Wounds UK. 2016;12: 62–68.
- Stojadinovic O, Zabielinski M, Tomic-Canic M. Healing competence of the keratinocytes and the chronic wound edge. Advances in Wound Care: Volume 1. New Rochelle: Mary Ann Liebert, Inc., publishers, 2010, pp.171–176.
- Guest JF, Fuller GW, Vowden P, et al. Cohort study evaluating pressure ulcer management in clinical practice in the UK following initial presentation in the community: costs and outcomes. BMJ Open 2018; 8: e021769. DOI: 10.1136/bmjopen-2018-021769.
- Karahan A, Abbasoğlu A, Işık SA, et al. Factors affecting wound healing in individuals with pressure ulcers: A retrospective study. Ostomy Wound Manage. 2018; 64: 32–39.
- Gurun P, Ceylan S, Guner M, et al. Closure of pressure injury and mortality in internal medicine wards. Eur Geriatr Med. 2023;14(2)373–380. DOI: 10.1007/s41999-023-00757-2
