Ahead of Print
Management of remdesivir extravasation in patients with COVID: a case report and systematic review
Niall O’Hara, Valdone Kolaityte, Pouya Mafi
Keywords skin, extravasation, wound management, tissue damage, remdesivir
For referencing O’Hara N, Kolaityte V, Mafi P. Management of remdesivir extravasation in patients with COVID: a case report and systematic review. Journal of Wound Management 2024;25(2):To be assigned.
DOI
10.35279/jowm2024.25.02.04
Submitted 29 February 2024
Accepted 22 April 2024
Abstract
Extravasation is a common and potentially severe complication of intravenous drug administration. The COVID-19 pandemic has resulted in a rapid upsurge in the use of remdesivir. Data about complications experienced during use of the drug are limited. The authors describe here the first case report of a patient with remdesivir extravasation in the UK, and a systematic review of all known cases worldwide, as well as their management and long term sequelae. Oedema was reported in all cases, erythema in five cases and pain in four cases. The one case with persistent sequelae had delayed diagnosis which may have caused potential for more severe injury. The two cases which had the fastest resolution of symptoms were those where the extravasation was noticed part-way through the infusion, hinting at a dose-dependent relationship. The acidic pH of remdesivir and high infiltration volumes are the factors which most likely explain the mechanism of tissue injury and should be the targets on which to base a treatment strategy. The clearest recommendation from our review is the importance of awareness to recognise injury early and prevent the most severe sequelae. The authors propose a recommendation for aspiration and hyaluronidase injection to “disperse and dilute” remdesivir.
Key messages
- Remdesivir is being used globally on a large scale and we have highlighted here all known reported cases of its extravasation in the literature.
- The authors propose a recommendation in favour of aspiration and hyaluronidase injection, as well as general conservative measures from the “disperse and dilute” strategy.
- Increased awareness is needed among clinical staff of the possibility and importance of extravasation in order to recognise injury early and prevent the most severe sequelae.
Introduction – extravasation
Extravasation is a common and potentially severe complication of intravenous drug administration and can be defined as the inadvertent administration of a vesicant pharmacological or chemical agent that causes severe skin or tissue reaction.1 Extravasation injury exists at one end of a spectrum of injuries. All patients undergoing intravenous therapy are at risk of extravasation injury and the phenomenon remains under-recognised and under-treated. The incidence of extravasation in adults is estimated to be up to 6.9%.2 The potential sequelae of extravasation injury are severe and include desquamation and local tissue necrosis, compartment syndrome, ulceration, infection, scarring, need for reconstructive surgery and loss of function with impact on activities of daily living and occupation.3
The severity of the soft tissue injury is thought to depend on several factors. Most factors are drug-related, such as whether the medication is vesicant, irritant or cytotoxic. The infiltrated volume, drug dose, concentration or pH and the duration of drug exposure are all important drug-related factors.4 Patient-related factors include administration site local vein quality, as well as factors relating to a patient’s general health, healing capacity, nutrition and level of alertness to detect the injury at an earlier stage. Although infusion sites are checked regularly by nursing staff for signs of extravasation it is often the patient who first notices signs and symptoms of injury. This, however, may not be the case in all patients unwell with COVID-19 on intensive care units, where there may be concurrent sedation and ventilatory support.
Introduction – remdesivir
Remdesivir, developed and manufactured by biopharmaceutical company Gilead Sciences, is an antiviral drug that is administered intravenously.5 It was originally developed to treat hepatitis C before being investigated as a treatment for Ebola and subsequently licensed in the United Kingdom as a treatment for COVID-19. Remdesivir is an adenosine nucleotide prodrug that is metabolised intracellularly to form remdesivir triphosphate. This acts as a ribonucleotide analogue inhibitor of viral RNA polymerase, causing a decrease in coronavirus RNA production.
Remdesivir is currently licensed in the UK for use in hospitalised adults and younger patients over the age of 12 years and 40kg weight.6 Several trials have demonstrated that remdesivir improves outcomes in patients with COVID-19. Beigel et al showed in the ACTT-1 trial that remdesivir shortened recovery time when compared to placebo, from 15 days to 11 days.7 The PINETREE trial showed that in certain high risk patients with COVID-19 remdesivir caused a reduction in risk of hospitalisation and mortality.8
In the UK the recommended dosage of remdesivir is a single loading dose of 200mg intravenously on day one, followed by a once daily maintenance dose of 100mg for the remainder of the treatment course, which should not exceed five days. To prepare remdesivir for intravenous administration it should be diluted in either a 250ml or 100ml bag of 0.9% sodium chloride solution and infused over a minimum of 30 minutes.5
The COVID-19 pandemic has resulted in a rapid upsurge in the use of remdesivir in hospitals in the UK and worldwide. Therefore, data about complications experienced during use of the drug are, as yet, limited. All suspected adverse drug reactions for patients receiving remdesivir in the UK are recommended to be reported to the Medicines and Healthcare products Regulatory Agency (MHRA) via a dedicated COVID-19 Yellow Card reporting site.
Previous works have described case reports of extravasation of remdesivir. It is difficult to estimate the true incidence of extravasation injury from this medication due to the rapid roll out of remdesivir globally and as extravasation injuries are thought to be grossly under-recognised and under-reported. The correct management of remdesivir extravasation is, therefore, still under debate. Although the product label for remdesivir reports hypersensitivity or infusion-related reaction as a potential side effect, there were no reports of extravasation in remdesivir clinical trials and, therefore, no manufacturer’s instructions on the correct management of a remdesivir extravasation injury.9 There have been, to the authors’ knowledge, no reports of extravasation injury with remdesivir in the UK and no consensus regarding the management of these injuries or the predicted outcomes. The authors ,therefore, describe here a case report of a patient with successful management of remdesivir extravasation, and a systematic review of all known reports in the literature. Written informed consent was obtained by the patient in our case report.
Case report
A 66-year-old woman was admitted to the intensive care unit of our hospital with COVID-19 pneumonia. As per hospital guidelines she was commenced on intravenous remdesivir. The course was initially without complications, however the plastic surgery on-call team were contacted regarding extravasation after the third dose of remdesivir administration.
Remdesivir had been administered via a peripheral cannula (18 gauge) into a vein on the dorsum of the left hand. The medication had been prepared as per hospital policy as 100mg of remdesivir diluted in 250ml 0.9% sodium chloride and run through an infusion pump over 30 minutes. The cannula had been checked prior to starting the infusion and documented as patent and it was only noticed at the end of the infusion that extravasation had occurred.
The patient did not initially complain of any discomfort but a 2x2cm area of erythema was noted on a background of global hand swelling. Compartments were soft and there was no tenderness. 5ml of fluid was able to be aspirated from the cannula prior to removal. Warm compresses were advised and the hand elevated in a Bradford sling. It is policy in our hospital to stock emergency extravasation kits in intensive care, theatres and pharmacy. An extravasation kit was sourced and hyaluronidase was administered as per local hospital policy. 1500 units Wockhardt UK Ltd brand hyaluronidase were diluted in 1ml sterile water and administered subcutaneously as four separate injections around the periphery and one injection into the middle of the extravasation site). This dosage and method of administration are recommended by NHS England, as well as the European Oncological Nursing Society.10 (It must be noted that this is in contrast to the use of hyaluronidase in aesthetic practice to dissolve fillers which is currently an off-licence use in the UK and USA and for which there is no current standard agreed-upon dosage). Other brands of hyaluronidase are available outside of the UK and clinicians should follow the manufacturer’s data sheet for brand-specific instructions.
Our patient underwent serial review by the plastic surgery team. Although the patient did not initially complain of pain, the area became tender. At the 12 hour review blisters were noted and were present until day four review. Erythema increased in size to a 3x3cm area and subsequently decreased until resolution at day four. Oedema peaked at day two and had greatly diminished by day four. There was still some swelling present two weeks post-injury but this had resolved by week four. The patient reported no residual skin changes or loss of hand function. The remaining remdesivir doses were administered without complication through a new peripheral cannula at another anatomical site.
Systematic review methods
A systematic review was conducted in adherence to PRISMA guidelines to collate all known reported cases of remdesivir extravasation. A literature search was conducted in the online databases Pubmed, Scopus, Embase and ISI Web of Science for all known articles on the topic from inception up to and including February 2023. The search strategy was constructed by two meta-analysis researchers and included the following search terms: “extravasation”, “infiltration”, “complication”, “adverse event”, “iatrogenic”, “soft tissue”, “skin”, “tissue” and “remdesivir”.
We included studies that met the following criteria:
- Population: adult and paediatric patients with remdesivir extravasation,
- Study design: all study designs were considered including case reports, case series and observational comparative studies.
The titles and abstracts of retrieved records were assessed independently for eligibility by two reviewers and all disagreements were resolved by discussion with a third reviewer.
The following data was extracted from the full-length articles that met our eligibility criteria:
- patient characteristics (age and gender),
- extravasation injury details (site, volume, symptoms, clinical findings), and
- management of extravasation injury and outcome.
Systematic review results
A total of 748 unique records were identified using the search criteria defined above. After examination of titles and abstracts and exclusion of duplicate records, a total of four articles were selected for in-depth analysis (see Figure 1). Publication dates were, perhaps unsurprisingly, all from the years 2021/ 2022. Studies were conducted in India11, Taiwan11, Spain13 and the Netherlands.14 One study was excluded as it was simply a summary article of adverse events from multiple COVID-19 medications, without any data that was not included in the other studies. Therefore a total of three studies, reporting data on five patients, were eligible for data extraction. Table 1 outlines a summary of the findings in these five patients in addition to the patient described in our case report.
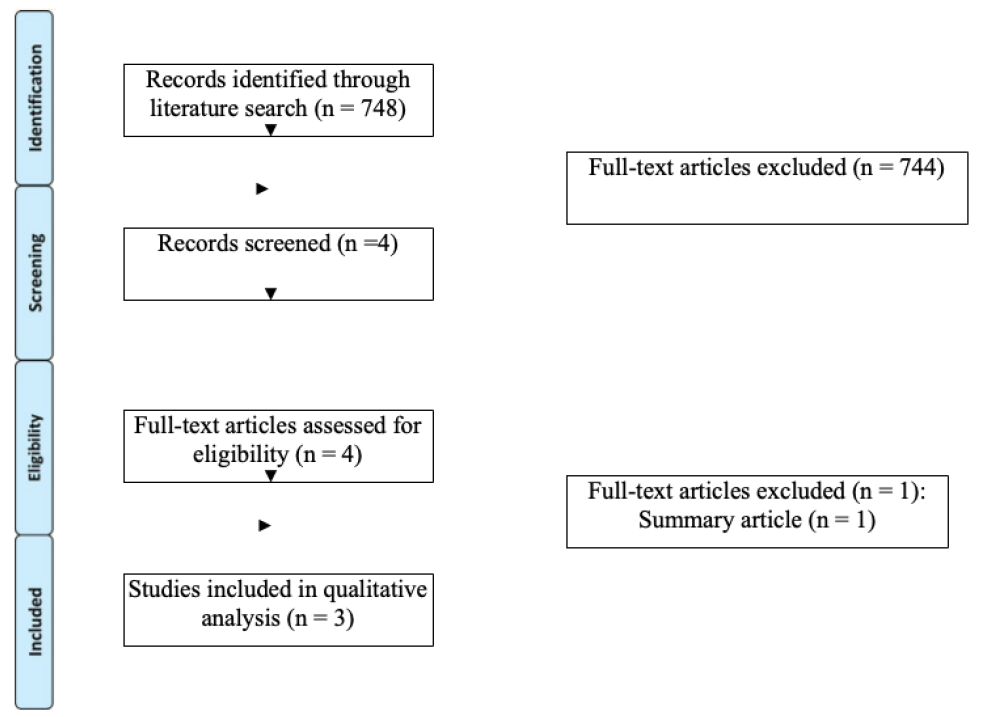
Figure 1. PRISMA flow diagram
Table 1. Summary of all known published cases of remdesivir extravasation
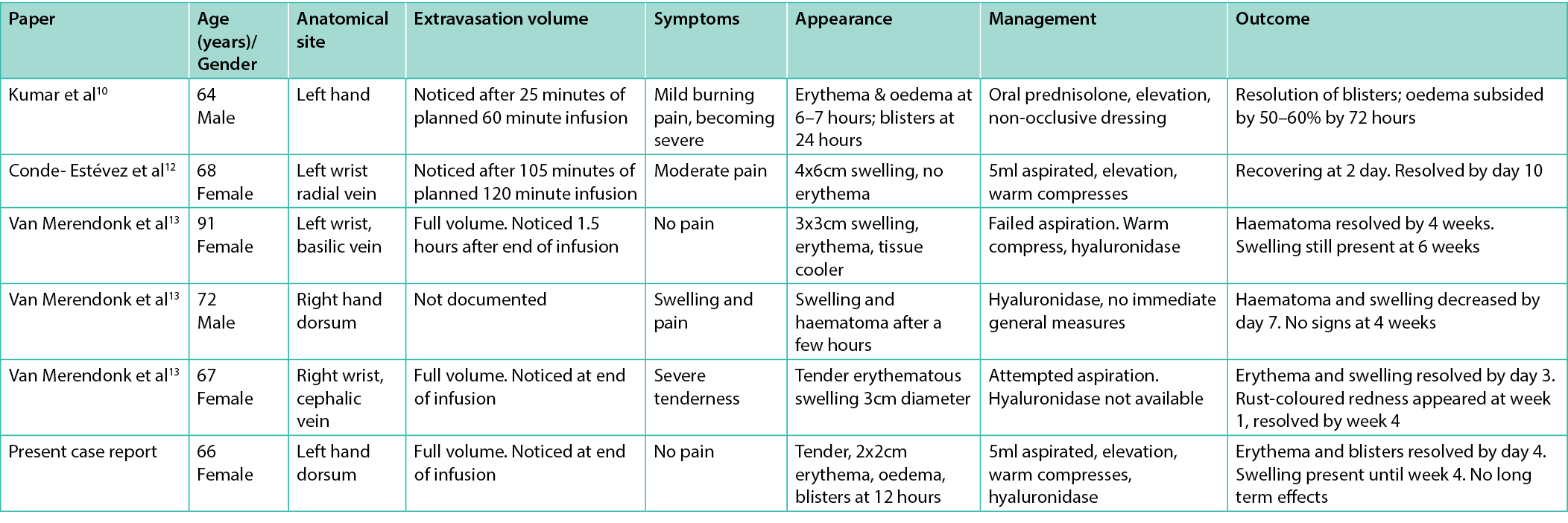
The clinical picture varied between cases in the literature. The only consistent sign in all cases was oedema. Erythema was documented in five of six case reports. Pain was noted in just four of the six cases. It is difficult to draw definitive conclusions from a small sample size but in half of cases hyaluronidase was used with no noticeable effect on long term sequalae compared to patients who did not have hyaluronidase. In five of the six cases there were no long term sequelae past four weeks. In the one case where symptoms were still present past six weeks, the injury had been noticed 1.5 hours after extravasation. This delay may have caused potential for more severe injury than in the other cases, highlighting the need for heightened awareness and rapid diagnosis of remdesivir extravasation. This case was also the only one with a failed aspiration of the remdesivir solution. The two cases which had the fastest resolution of symptoms were those where the extravasation was noticed part-way through the infusion, hinting at a dose-dependent relationship between remdesivir volume and tissue injury. Interpretation of these results is limited by the small sample size of cases as extravasation injury frequently goes unreported and remdesivir is a relatively novel drug.
Discussion – extravasation management
Extravasation injury management can largely be divided into two approaches: ‘disperse and dilute’ vs ‘concentrate and condensate’. The chemical properties of a medication, such as pH, osmolarity, and drug concentration determine the most appropriate strategy.9
‘Disperse and dilute’ is employed when a medication causes local tissue damage through its high concentration or extremes of pH or osmolarity. The infiltrated solution is rendered less harmful by dilution and diffusion across a wider area and the pH is rendered more neutral. This dispersion is achieved by the use of warm compresses to cause vasodilation and enhanced absorption. Flushing saline through the intravenous device can aid dilution if the intravenous access has been left in place. Alternatively stab incisions can be made and washout of the subcutaneous tissues can be attempted at the bedside. All of these techniques can be aided by the use of hyaluronidase injections which hydrolyse hyaluronic acid in local tissue and therefore increase the available area for absorption of the extravasated medication.9
The ‘concentrate and condensate’ method is used for vesicant and highly irritant medications which have the potential to cause highly severe tissue injury. Dispersing these agents may lead to a larger area of inflammation, necrosis and tissue injury. Cold compresses can be employed in order to achieve vasoconstriction and therefore limit the spread and absorption of the agent.9
Discussion – mechanism of tissue damage and management in remdesivir extravasation
Remdesivir has a protein-binding capacity of 88% to 93.6% in human plasma, and its volume of distribution varies from 56.3 to 73.4L depending on the dose administered.9 These properties mean that remdesivir has a tendency to easily enter the extravascular compartments of the body. Data on the pharmacological effect of remdesivir on subcutaneous tissue are limited. However, extravasation of other antiviral nucleotide and nucleoside analogues have been reported previously and the tissue damage thought to be caused by the pH of the solutions.15
Remdesivir formulation for injection contains multiple excipients (sulfobutylether-β-cyclodextrin, hydrochloric acid and sodium hydroxide), any of which might be the potential cause of tissue damage in remdesivir extravasation.5 Sulfobutylether-β-cyclodextrin is added to remdesivir formulation to increase solubility and stability but its effect on subcutaneous tissue is unknown.5 Hydrochloric acid and sodium hydroxide together bring the pH of remdesivir formulation to between 3.0 and 4.0.5 An acidic pH at this level has been shown to cause tissue oedema, precipitation of proteins, vasoconstriction, cellular desiccation, ulceration, coagulative necrosis, and eschar formation.
Damage related to the formulation’s osmolality was not expected due to the osmolality being 278–320 mOsm/kg, which does not exceed the threshold for harmful hyperosmolality.5 Infusion-specific factors, such as the high infiltration volume (between 100ml–250ml) and the high infusion rate (up to 500 mL/h), are risk factors for mechanistic damage resulting in tissue injury.
Conclusion
It appears likely that the acidic pH of the remdesivir solution and high infiltration volumes are the most likely factors which explain the tissue damage caused by remdesivir extravasation and the most pertinent targets on which to base the treatment strategy. It has been previously reported that signs of tissue injury from remdesivir extravasation appeared to resolve after hyaluronidase injection, which was also our experience in this case, although causality remains unclear.
Remdesivir is being used globally and on a large scale and we have highlighted here all known reported cases of its extravasation in the literature. We have laid out the spectrum of known long term sequelae and suggestions for both the mechanism of tissue injury and appropriate management.
Implications for clinical practice and future research
The clearest recommendation from our review is the importance of awareness among clinical staff to the possibility and importance of extravasation of this widely used drug, in order to recognise injury early and prevent the most severe sequelae. The evidence supports conservative measures from the ‘disperse and dilute’ strategy, aspiration and hyaluronidase injection. The role of local or systemic corticosteroid treatment to suppress inflammation from remdesivir extravasation injury could be explored in future work. With raised awareness future research can be carried out with larger study sizes.
Abbreviations
UK – United Kingdom
COVID-19 – coronavirus disease 2019
RNA – ribonucleic acid
MHRA – Medicines and Healthcare products Regulatory Agency
Authors’ contributions
All authors contributed to the study conception and design. The systematic review search strategy was devised by NO and VK. Retrieved records were independently assessed for eligibility by NO and VK and all disagreements resolved by discussion with PM. Analysis was performed by all authors. The first draft of the manuscript was written by NO and all authors edited and approved the final manuscript.
Acknowledgements
None
Conflicts of interest
The authors declare that they have no competing interests
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author(s)
Niall O’Hara*1, Valdone Kolaityte2, Pouya Mafi3
1Plastic Surgery Department, University Hospitals Birmingham, Birmingham, UK
2Plastic Surgery Department, University Hospitals Coventry and Warwickshire, Coventry, UK
3Plastic Surgery Department, University Hospitals of North Midlands, Stoke-on-Trent, UK
*Corresponding author email niall.o’hara@nhs.net
References
- Royal College of Nursing. Standards for Infusion Therapy 4th edition. 2008. Royal College of Nursing, London
- Schulmeister L. Extravasation management: clinical update. Semin Oncol Nurs. 2011 Feb;27(1):82–90. DOI:10.1016/j.soncn.2010.11.010.
- David V, Christou N, Etienne P, Almeida M, Roux A, Taibi A, Mathonnet M. Extravasation of Noncytotoxic Drugs. Ann Pharmacother. 2020 Aug;54(8):804–814. DOI: 10.1177/1060028020903406.
- Reynolds PM, MacLaren R, Mueller SW, Fish DN, Kiser TH. Management of extravasation injuries: A focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014 Jun;34(6):617–32. DOI: 10.1002/phar.1396.
- Gilead Sciences. Veklury (remdesivir) summary of product characteristics. 2023. https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Accessed April 2023
- The National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing COVID-19. 2023. https://www.nice.org.uk/guidance/ng191/resources/covid19-rapid-guideline-managing-covid19-pdf-51035553326. Accessed April 2023
- Beigel JH, Tomashek KM, Dodd L, et al. Remdesivir for the treatment of Covid-19 – Final Report. N Engl J Med. 2020 Nov 5;383(19):1813–1826. DOI: 10.1056/NEJMoa2007764
- Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022 Jan 27;386(4):305–315. DOI: 10.1056/NEJMoa2116846.
- Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020 Sep;13(5):896–906. DOI: 10.1111/cts.12840
- West Midlands Expert Advisory Group for Chemotherapy. Network Guidelines for the Management of Extravasation of a Systemic Anti-Cancer Therapy Including Cytotoxic Agents. 2017. West Midlands Expert Advisory Group for Systemic Anti-Cancer Therapy (SACT) NHS England. https://www.england.nhs.uk/midlands/wp-content/uploads/sites/46/2019/05/management-extravasation-of-a-systemic-anti-cancer-therapy-including-cytotoxic-agents.pdf. Accessed April 2023
- Kumar N, Kumar A, Pradhan S, Kumar A, Singh K. Painful blisters of left hand following extravasation of remdesivir infusion in COVID-19. Indian J Crit Care Med. 2021 Feb;25(2):240–241. DOI: 10.5005/jp-journals-10071-23732.
- Hsu JY, Mao YC, Liu PY, Lai KL. Pharmacology and adverse events of emergency-use authorized medication in moderate to severe COVID-19. Pharmaceuticals (Basel). 2021 Sep 23;14(10):955. DOI:10.3390/ph14100955
- Conde-Estévez D, Barrantes-González M, Cotrina Soliz MR, Grau S. Successful management of remdesivir extravasation. Rev Esp Quimioter. 2022 Apr;35(2):229–230. DOI:10.37201/req/147.2021.
- Van Merendonk LN, Leeuwerik AF, den Brok MWJ,, et al. Peripheral infiltration of remdesivir in 3 patients with COVID-19: Case series and discussion. Am J Health Syst Pharm. 2021 Oct 25;78(21):1944–1951. DOI: 10.1093/ajhp/zxab197.
- Neocleous C, Andonopoulou E, Adramerina A, et al. Tissue necrosis following extravasation of acyclovir in an adolescent: A case report. Acta Med Acad. 2017 May;46(1):55–58. DOI: 10.5644/ama2006-124.187.
