Ahead of Print
Cold Plasma An emerging technology for clinical use in wound healing
Apelqvist J, Robson A, Helmke A, Rousseau A, Boekema B, den Braber E, Szili E, Stuermer E, Boeckmann L,
Gaur N, Short R, Bekeschus S, Emmert S, von Woedtke T, Gerling T
For referencing Apelqvist J, Robson A, Helmke A, Rousseau A, Boekema B, den Braber E, Szili E, Stuermer E, Boeckmann L, Gaur N, Short R, Bekeschus S, Emmert S, von Woedtke T, Gerling T. Cold Plasma: An Emerging Technology for Clinical Use in Wound Healing; J Wound Management, 2024;25(3 Sup1):S1-S84
DOI 10.35279/jowm2024.25.03.sup01
Abbreviations
1. Introduction and aim
Support the nature and extent of current issues facing wound management: from the policy making and healthcare system perspective.
The ongoing controversy regarding high-level evidence in wound care is well known. There is a consensus that clinical practice should be evidence-based, which can be difficult to achieve due to uncertainty about the value of the various approaches to wound management; however, we must rely on the best available evidence.
There is further fundamental confusion over the best way to evaluate the effectiveness of interventions in this complex patient population. This is, for example, illustrated by reviews of the value of various treatment strategies for non-healing wounds, which have highlighted methodological inconsistencies in primary research.1,2
This situation is further complicated by differences in the advice given by the regulatory and reimbursement bodies in various countries regarding both study design and how results are, and should be, interpreted. Despite this, there is an urgent need to review wound strategies and treatments to reduce the burden of care efficiently. If patients at risk of delayed wound healing are identified earlier, and aggressive interventions are taken before the wound deteriorates and complications occur, both patient morbidity and healthcare costs can be significantly reduced.
1.1 Objectives
The European Wound Management Association (EWMA) believes that cold atmospheric plasma (CAP) for wound treatment potentially represents a new, sustainable, advanced therapy, while CAP on the other hand may still have to reach its full potential. The general awareness level about CAP among healthcare professionals (HCPs) is relatively modest, which may impede the dialogue between researchers, clinicians, policy makers and payers.
This document intends to highlight and focus on technological advances in CAP for wound management, which are seen currently to be heading in several directions from a scientific, clinical and patient caregiver perspective.
When reviewing this, various critical non-clinical issues will also be discussed, especially since access to care and the evaluation of the benefits of treatment are becoming more and more a financially-driven critical factor.
The objectives of the document are to:
- review and discuss scientific evidence and clinical experiences;
- review and discuss the potentials and challenges for CAP in wound management;
- review emerging and available CAP therapies;
- discuss safety issues, reimbursement, and the regulatory framework for CAP;
- supply knowledge and support for future discussions with healthcare providers and payers;
- be an inspiration for solution providers;
- call for research and actions in recommended areas if needed.
However, the document has two fundamental demarcations. It does not promote a specific intervention compared to other alternatives as this is beyond the scope for EWMA. For the same reason, non-clinical applications of CAP are not included.


1.2 Methodology
This document originates from expressions of interest by various EWMA stakeholders in an EWMA internal note which focused on the role and use of CAP in wound management. Based on a literature search conducted in PubMed and other sources, a short description of the document’s aim, objectives and scope was developed during H2 2022. As a follow-up, a set of guidelines for this document, outlining General Conditions, Author Conditions and Industry Supporter Conditions was prepared in the fourth quarter of 2022. The guidelines are available upon request from the EWMA Secretariat. These two basic documents were subsequently used to identify the experts who constitute the author group.
Each author has taken responsibility for the elaboration of the first draft of a whole or part of a chapter. It has been the obligation of each author to search and investigate the relevant literature. The opinions stated in this document have been reached by a consensus of the author group, weighing their professional opinions based on their respective research, and that of their peers, as well as their own clinical experience. Several of the key opinion leaders (KOLs) and scientific high-level experts in CAP are among the authors of this document.
Therefore, a uniform search strategy was not defined, since the authors for the most part are thoroughly familiar with the existing literature. Several collaborators are also authors of reviews of various aspects of CAP for wound management.
Where there is a lack of scientific evidence, the document is based on the available literature and experts’ opinions. Before its publication, the document has been reviewed by the EWMA Council, the Industrial Supporters and other stakeholders. The resulting comments have been discussed by the author group, and were either accepted or dismissed based on their scientific validity.
1.3 Structure and content
The chapters of this document were drafted and assembled to provide a logical flow, and contribute to making it accessible to HCPs and other readers, including those who are not experts in the field of CAP, for clinical use in wound management. Hence, the first chapters will present the history of CAP, review findings from the basic research on CAP, followed by chapters that describe the clinical experiences with CAP in wound management, and reflect on additional (potential) emerging CAP therapies. The last chapters will focus on the challenges an innovative wound healing therapy such as CAP can confront, and sum up the opportunities and challenges identified earlier concerning the uptake of CAP into routine clinical use for wound management.
The authors hope the document will be interesting and relevant for HCPs and other stakeholders within wound management such as solution providers, regulatory authorities, payers, procurement officers and society at large.
2. The history of CAP
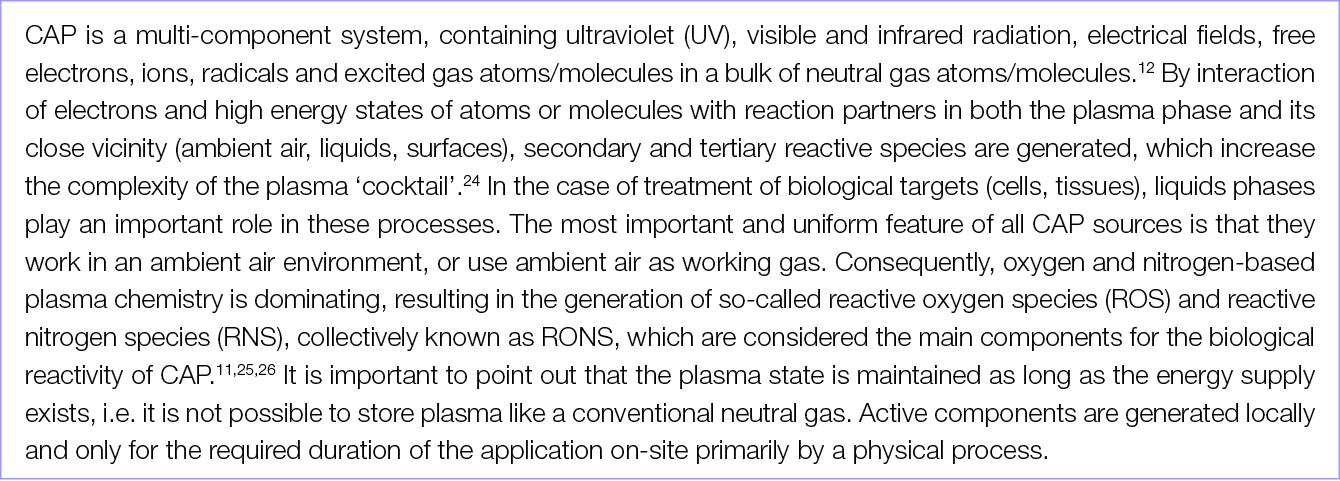
2.1 What is CAP?
Physical plasma is a special gas state where atoms or molecules of a gas are excited and ionised. Plasma is often called the fourth state of matter following solid, liquid and gaseous. Plasma is generated by energy supply to a neutral gas; the application of electric fields or electromagnetic radiation is the most common method (besides chemical processes, heating or compression). Plasma generation can be realised under low pressure, atmospheric pressure and high pressure conditions. Depending on these and several other parameters, particularly the working power, plasma can be generated at low or very high temperatures. For more information and details, see Appendix I: The physics of CAP in this EWMA document.
In general, plasmas for clinical use must work at low temperatures (e.g. ±40˚C at the target site during treatment) as well as in an atmospheric pressure environment. To distinguish physical plasma from the better known blood plasma in the biomedical community, several terms or amendments are in use, e.g. gas plasma, non-thermal plasma, low-temperature plasma, tissue-tolerable plasma, cold physical plasma; all of these refer to the same phenomenon. The most widespread term for plasma for medical use is cold atmospheric plasma or cold atmospheric-pressure plasma, commonly abbreviated as CAP.
Neutral gases, especially under atmospheric conditions, contain some ‘background’ electrons resulting from, for example, cosmic rays or radioactive radiation. By applying an electric field to a neutral gas, energy is transmitted to the electrons, which are the most mobile charged species. From this, electrons are accelerated, and they transmit energy to the neutral species by collisions. These collisions can be either elastic, resulting in no change to the internal energy of the neutral species but in a slight rise of its kinetic energy, or inelastic if electron energy is high enough. In that case, the electronic structure of the neutral species is modified, resulting in excitation or ionisation of the neutral gas atoms or molecules. Excitation means that electrons move into a higher energy state inside the atom or molecule. Most of these excited species are unstable; spontaneous de-excitation results in the emission of photons. Therefore, a plasma is visible because of the emission of light. Other excited species with longer lifetimes are called ‘metastable species’; their decay by emission of radiation is hampered. In the case of ionisation, electrons are ejected from the atomic or molecular structure resulting in an electron avalanche and the generation of ions. Because of this generation of free charge carriers, plasma is electrically conductive.8–12 A more detailed and in-depth description of the principles of CAP can be found in the Appendices of this EWMA document.
2.2 Basic types of CAP devices
For medical purposes, usually two basic types of CAP sources are tested and partially applied in medical devices – dielectric barrier discharges (DBD) and atmospheric-pressure plasma jets (PJ) (Figure 1).13–18 A more detailed and in-depth description of the different available CAP devices can be found in Appendix II.
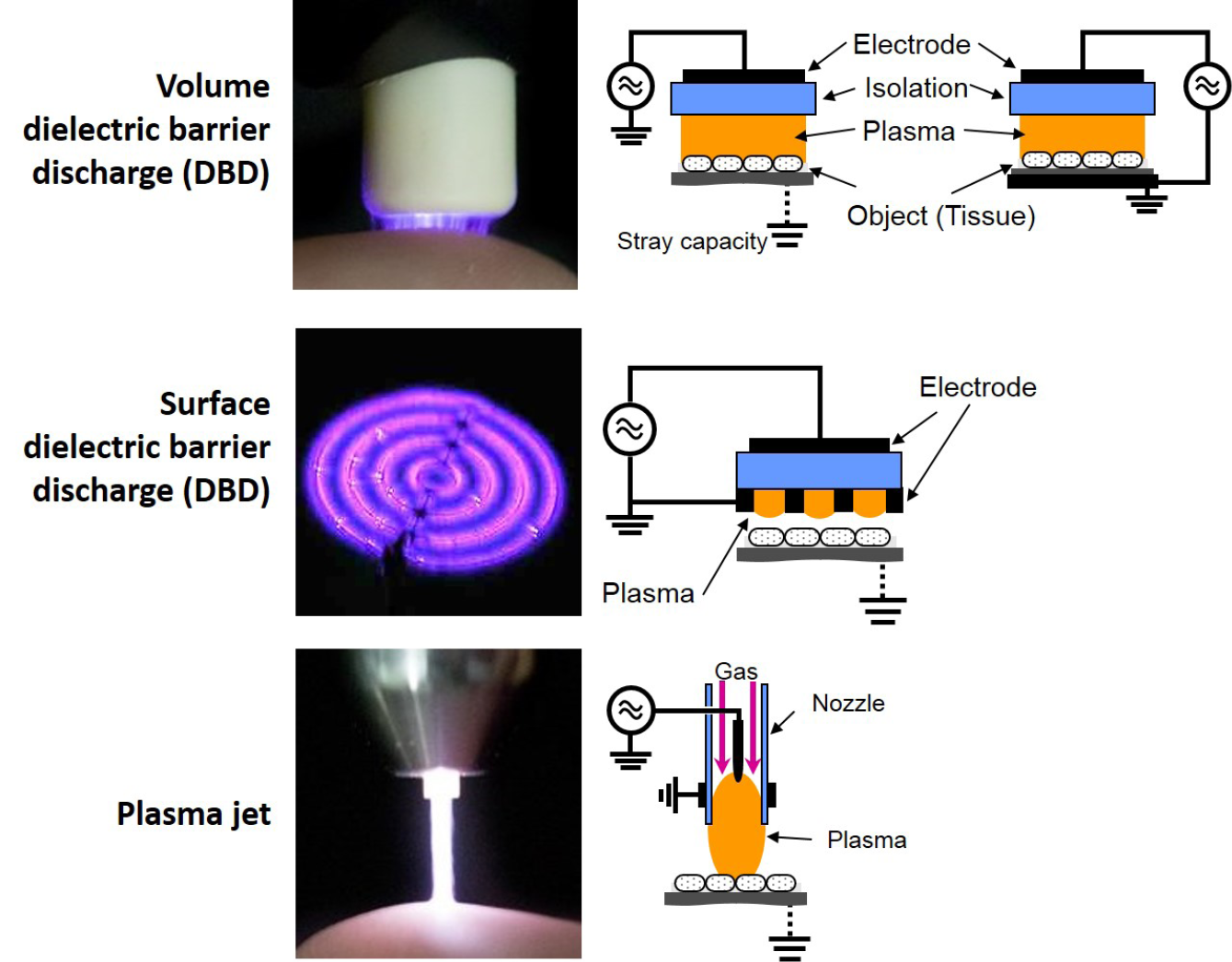
Figure 1: Basic technical principles of CAP sources for biomedical research and medical application
In DBD, plasma is ignited in a gap between a high voltage electrode and a second (counter) electrode. A dielectric (isolating) material covers one of the two or both electrodes. In the so-called volume DBD (VDBD), the target to be treated (e.g. skin wound, etc.) serves as second electrode. In this case, a direct contact between the plasma and target is realised, including a low electric current flow between the plasma device and target to be treated. Alternatively, in a so-called surface DBD (SDBD), plasma is generated around a variably designable electrode structure (e.g. circular or grid-like), which is isolated from the counter electrode which is part of the device. In this case, no direct contact of the active plasma with the target occurs and the plasma must be brought to close vicinity to the target for treatment purposes. In DBD, as working gas for plasma generation atmospheric air is usually used. Both VDBD and SDBD can be designed to generate plasmas over larger areas.12,19
In a so-called PJ, a two-electrode setup of variable configuration (e.g. pin electrodes, ring electrodes, plate electrodes etc.) is mounted in or around a tube-like arrangement, in most cases inside a pen-like device. For plasma generation, a working gas flowing through the tube and the electrode arrangement is used, resulting in a so-called plasma effluent (or afterglow), which is driven out of the tube with the gas flow. In PJ, usually prefabricated gases are used, mostly noble gases – helium (He) or argon (Ar) – often doped with small amounts of molecular gases (nitrogen, oxygen, air). Because the target to be treated is not part of the primary electrode configuration, a direct plasma (effluent) contact can be realised or not, dependent on the distance between the nozzle of the tube and the target.12,20–22 In general, PJ are preferable for localised, spot-like treatments. Large-area treatments are possible by moving the plasma effluent over the surface to be treated. To increase the treatment area, PJ arrays are possible.23
2.3 General applications of CAP
Many plasma applications can be found in a wide variety of industries, mostly cleaning, etching, coating and preparation of surfaces. Here, plasma treatment optimises specific applications. These applications range from the production of integrated circuits for computers and cell phones, additive-free and low-temperature plasma-supported synthesis of chemicals and nano-materials, to the activation and hardening of material surfaces. In addition, CAP-based surface technologies are also useful for medical materials and devices such as implants, diagnostic tools or surfaces in the clinical environment, not only generating improved biocompatibility and cell attachment, but also cell-repellent and anti-bacterial characteristics. Furthermore, anti-bacterial surfaces based on CAP technologies may be also useful in clinical settings and in the immediate patient environment to prevent the transmission of infections.27–31
2.4 Medical applications of CAP
The same is true for another field of plasma application in the medical field – plasma-based decontamination or ‘sterilisation’. A special property of CAP is that it can inactivate microorganisms and viruses without permanently influencing or destroying their surrounding structures. Consequently, this opens the way for gentle decontamination or sterilisation of sensitive and thermo-sensitive goods. Research on plasma techniques for bio-decontamination of medical devices and materials is a field of long-term research. Focused primarily on low pressure non-thermal plasma, the improved availability of CAP technology from the 1990s also led to several studies on its applicability for antibacterial treatment of medical devices, surfaces or materials.32–36 However, sterilisation in accordance with strict regulatory criteria does not seem to be feasible with CAP, or only under special conditions.37–39 This could be a reason why plasma-based technologies have rarely reached the level of established bio-decontamination methods. Nevertheless, there are promising application potentials, not only in medicine, hygiene and infection prevention, but also in food processing and agriculture.40–45 The plasma applications described previously for biomaterial and implant treatment, or for decontamination purposes, can be considered such indirect medical plasma applications – materials or devices are plasma treated and subsequently supplied to a medical application.
With the direct application of physical plasma on, or in, the human body for therapeutic purposes, an innovative and interdisciplinary research field including physics, life sciences and medicine has been established within the past 15 to 20 years called ‘plasma medicine’. In fact, the medical use of physical plasma in the context of so-called electro surgery, for example as argon plasma coagulation (APC), has been established for many years.46,47 Based on the antimicrobial efficacy of CAP without influencing sensitive surfaces negatively, research was initiated to investigate CAP treatment of specific body surfaces, e.g. infected wounds or skin. First laboratory experiments on CAP application on cultivated mammalian cells demonstrated the possibility to selectively manipulate them without killing.48,49 Together with several theoretical considerations, first experimental findings initially led to the research focus of plasma medicine on wound healing with special regard to chronic wounds.50,51 A hypothesis was made very early of a potential dual plasma efficacy, i.e. simultaneously deactivate wound-contaminating microorganisms and stimulate tissue regeneration directly.52 Since its original formulation, many in vitro experiments, animal studies and clinical trials seem to confirm this hypothesis. The common consensus is that these unique properties of CAP are based most likely on effects like increased wound tissue oxygenation and vascularisation, amplified apoptosis of senescent cells, activation and/or modulation of redox signalling cascades, as well as immune cell attraction and stimulation. With continuing research, the molecular mechanisms of these effects are becoming more and more understood.53–58
In parallel to preclinical and clinical research on CAP in wound healing, CAP for cancer treatment has arisen as the second largest research field in plasma medicine (paragraph 5.5). First indications from in vitro studies of a possible increased sensitivity of cancer cells to CAP compared to non-malignant cells – a kind of ‘selectivity’ – led to high expectations of bringing about a “paradigm shift in cancer therapy”.59 Meanwhile, many in vitro experiments as well as experimental animal studies with a great number of cancer cell lines were realised to evaluate the potential of CAP in oncological treatment.60–62 Basic molecular mechanisms of CAP on cancer cells are considered to comprise of both a direct cancer cell killing by apoptosis, and indirect (systemic) effects by initiation of immunogenic cell death (paragraph 5.5).63,64 Despite experimental data, clinical applications are rare, caused by open questions with regard to the safety of CAP application for cancer treatment. In particular, it should be clarified whether stimulating plasma effects, which form the basis of tissue regeneration in wound healing, can lead to metastases in the case of sub-effective treatment of cancer tissue.65 Furthermore, due to the limited penetration depth of CAP, any treatment of bulk tumours is limited by its efficacy (paragraph 5.7).
3. Review of basic research on CAP
3.1 Cold plasma and wound healing in vitro
Cold plasma provides a rich source of ROS and RNS, collectively known as RONS. These are, as the name suggests, highly reactive molecules that can readily oxidise biomolecules. Historically, RONS were thought to be harmful and associated with free radical ageing and disease. Currently, it is widely recognised that RONS are essential for the maintenance of good health through their participation in a plethora of cellular signalling pathways that aid wound healing and help the body fight infections.66 Normally, this occurs through a process called redox homeostasis, where cells balance the generation and consumption of RONS to maintain function of redox-sensitive signalling proteins.67
Redox homeostasis is disturbed usually in slow healing wounds; this contributes to cell death and poor cell function that ultimately delays, or even prevents, healing.68 Since CAP is a rich source of RONS, it can be theoretically used to restore the redox balance in these wounds to promote cellular activity and healing.69 In addition to healing, CAP also has a strong broad-spectrum antibacterial property. This makes CAP suitable for targeting all stages of wound healing, from infection control through to tissue regeneration. The following subsections provide a summary of the in vitro experiments to investigate the antibacterial activity of CAP in wound decontamination in order to elucidate the underlying mechanisms of how CAP regulates cellular activity in wound healing, and how to tailor CAP devices specifically for wound treatment.
3.1.1 Broad-spectrum antibacterial activity of CAP
CAP has been shown to be effective against a broad-spectrum of wound pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli,70–77 and antimicrobial resistant strains such as methicillin-resistant S. aureus (MRSA),78–80 demonstrating a strong antibacterial efficacy of CAP in wound care. Apart from treatment of planktonic and single colony bacteria, CAP has been shown to be effective at reducing growth of bacterial biofilms in vitro.71,81–87 The reason why CAP can target a broad-spectrum of bacteria and mature biofilm infections is attributed to its unique chemical and physical mode-of-action. CAP readily produces nitric oxide (NO), which is known to disrupt quorum sensing.88 This can help break down the biofilm architecture, enabling penetration of other antibacterial agents produced by CAP, such as hydrogen peroxide (H2O2), which is a widely recognised effective disinfectant.89 In addition to its chemical action, the physical components of CAP, such as UV irradiation, further enhance its antimicrobial efficacy.70
3.1.2 Major components of CAP implicated in wound healing
CAP readily generates a heterogeneous mixture of RONS, which are considered beneficial for wound healing. Amongst these RONS, i.e. H2O2 and NO, have arguably been most widely studied due to their well-known role in the regulation of cellular signalling processes, which stimulate wound healing.90–96 Furthermore, H2O2 and NO have also been linked to enhancing cell proliferation in vitro.97–100 In addition to these, CAP produces a variety of other molecules which can also stimulate different processes in wound healing.
3.1.3 Cellular mechanism of CAP in wound healing
CAP has been shown to upregulate growth factor production in cells, including vascular endothelial growth factor (VEGF),101 fibroblast growth factors FGF‑2,102 FGF‑7,103 and alter integrin expression104 to stimulate cell proliferation and migration. Given the heterogeneous mixture of RONS generated by CAP, it is likely that CAP can be used to target many cellular pathways to stimulate growth. Although some insights into the biomolecular mechanisms of CAP in wound healing have been gained through the use of transcriptomics105 and proteomics,106 this research is still in its infancy, and much more research is still required to elucidate the major cellular pathways regulated by CAP in wound healing.
3.1.4 Oxygenation of CAP in wound healing
Oxygen is a key requirement to achieving a good wound healing outcome.107,108 Therefore, methods to enhance blood flow and oxygenation at the wound site can help to stimulate healing, particularly in ischaemic wounds characterised by poor blood flow. CAP has been shown to promote angiogenesis109 and oxygenate hypoxic solutions.110 CAP can oxygenate tissues through the conversion of some of the RONS (e.g. H2O2) into oxygen, while it also generates NO, an important molecule associated with CAP-induced vasodilation, blood flow, and increased tissue oxygenation.111,112 In vitro experiments using simple models of biological tissues constructed from gelatine, agarose and simple mimics of biological fluid such as water, phosphate buffered saline and cell culture media, have shown that CAP treatment directly influences the concentration of dissolved oxygen.110,113–115 However, these results also showed that CAP has the potential to oxygenate or deoxygenate, depending on the start oxygen concentration in the biological target. An explanation for this is that CAP produces molecular oxidants which convert into molecular oxygen side products when the oxidants decay in solution.
Configurations such as PJ are operated typically with an inert gas such as He or Ar. Since only a very small fraction of the gas is ionised (typically 10–4–10–7%),116 the neutral He and Ar gas can readily displace molecular oxygen in a process referred to as sparging.110,117 These in vitro findings could be important for the clinical treatment of wounds, since they suggest that CAP treatment has the potential to both stimulate healing through oxygenation, but also conversely impair healing through deoxygenation, depending on the starting oxygen concentration in the wound.
3.1.5 Tailoring CAP for wound treatment
With wound healing processes, the concentrations of H2O2 and NO are elevated generally at the early inflammatory stages to recruit neutrophils and macrophages to fight infection. Afterwards, these molecules reduce in concentration to allow infiltration of lymphocytes, fibroblasts and keratinocytes to close the wound.25 Chronic wounds with delayed healing, however, typically have an imbalance of RONS that impairs the ability of the body to clear infection or heal the wound. Therefore, restoring the optimal RONS and oxygen balance can potentially be useful to help the body fight infections, and stimulate healing for these wounds.
The dose and chemical composition of RONS produced by CAP can be tailored to change its bacteriocidic or cell stimulating effect. For example, the ratio of ROS:RNS can be tuned through variation of the gas composition interacting with the plasma discharge to optimise CAP treatment for bacterial inactivation118 or cell growth.119 CAP can be humidified, thus moistening wounds while also promoting the production of certain ROS, such as H2O2, to regulate cell growth.120 In addition, the composition of RONS produced by CAP can be controlled through electrical manipulation of the plasma discharge; this is most easily done with CAP sources operated with bipolar pulsed direct current (DC) power supplies. Such power supplies can be used to control the production of ROS:RNS by switching the polarity of the applied voltage.121 Furthermore, RONS and oxygen concentrations can also be regulated in vitro by cycling the CAP treatments under different modes of operation.122
Whilst the potential to influence RONS concentration and composition with CAP sources has been demonstrated, there is still a lot more work to be done to elucidate how the physicochemical properties of CAP are influenced by operational parameters and the surrounding environment. This work needs to be completed to provide knowledge on how the RONS’ composition can be tuned to wounds specifically. More details can be found in Appendices I and II of this EWMA document.
3.2 Cold plasma and wound healing in vivo
The healing potential of CAP has been investigated in vivo in numerous studies using various animal models. The majority of CAP preclinical studies have been conducted in small size rodent models (mice and rats),123,124 although other medium size and large animals, such as rabbits, pigs and sheep, have also been used.56,125 To test the efficacy of CAP in wound healing, various types of PJ devices (e.g. various commercial devices, custom lab-based CAP jets, Ar CAP jets, He CAP jets, air CAP devices and jets using inert gas mixed with O2) and treatment regimes (e.g. direct plasma treatment, using plasma-treated solutions indirectly, plasma plume in contact with the tissue, plasma plume not in contact with the tissue) have been explored (Table 1).
Table 1: Animal studies on CAP wound healing.
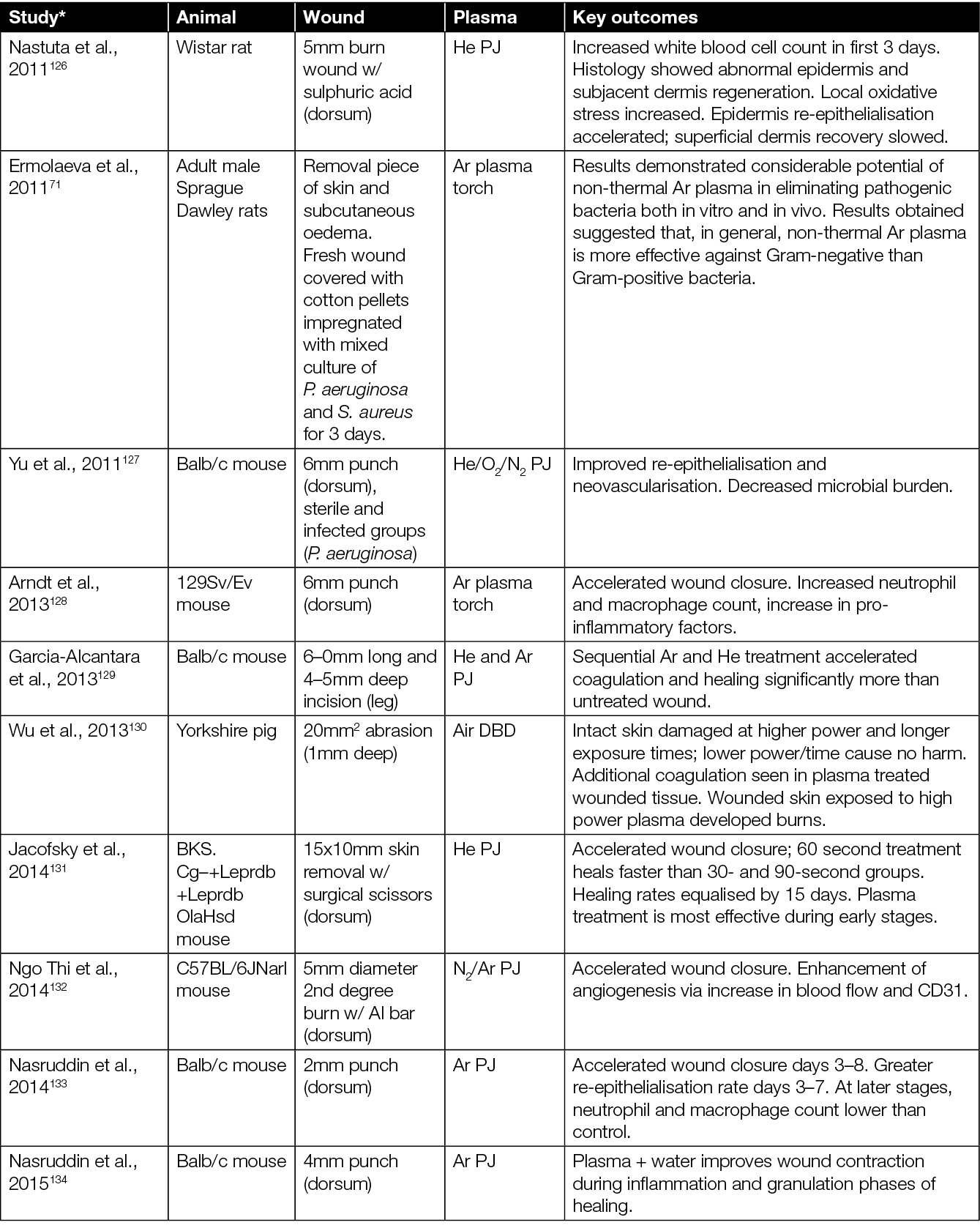
* Articles sorted by year of appearance.
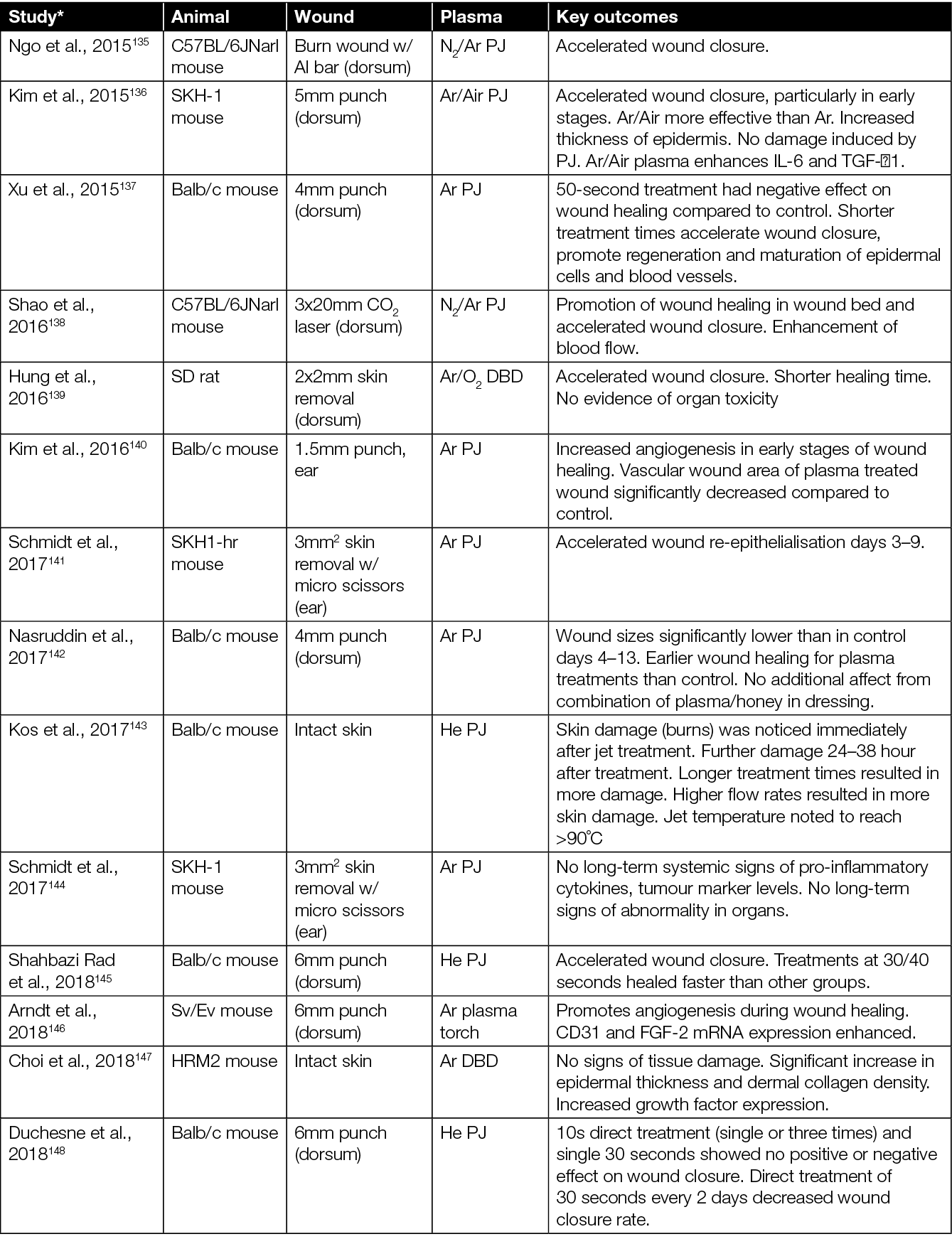
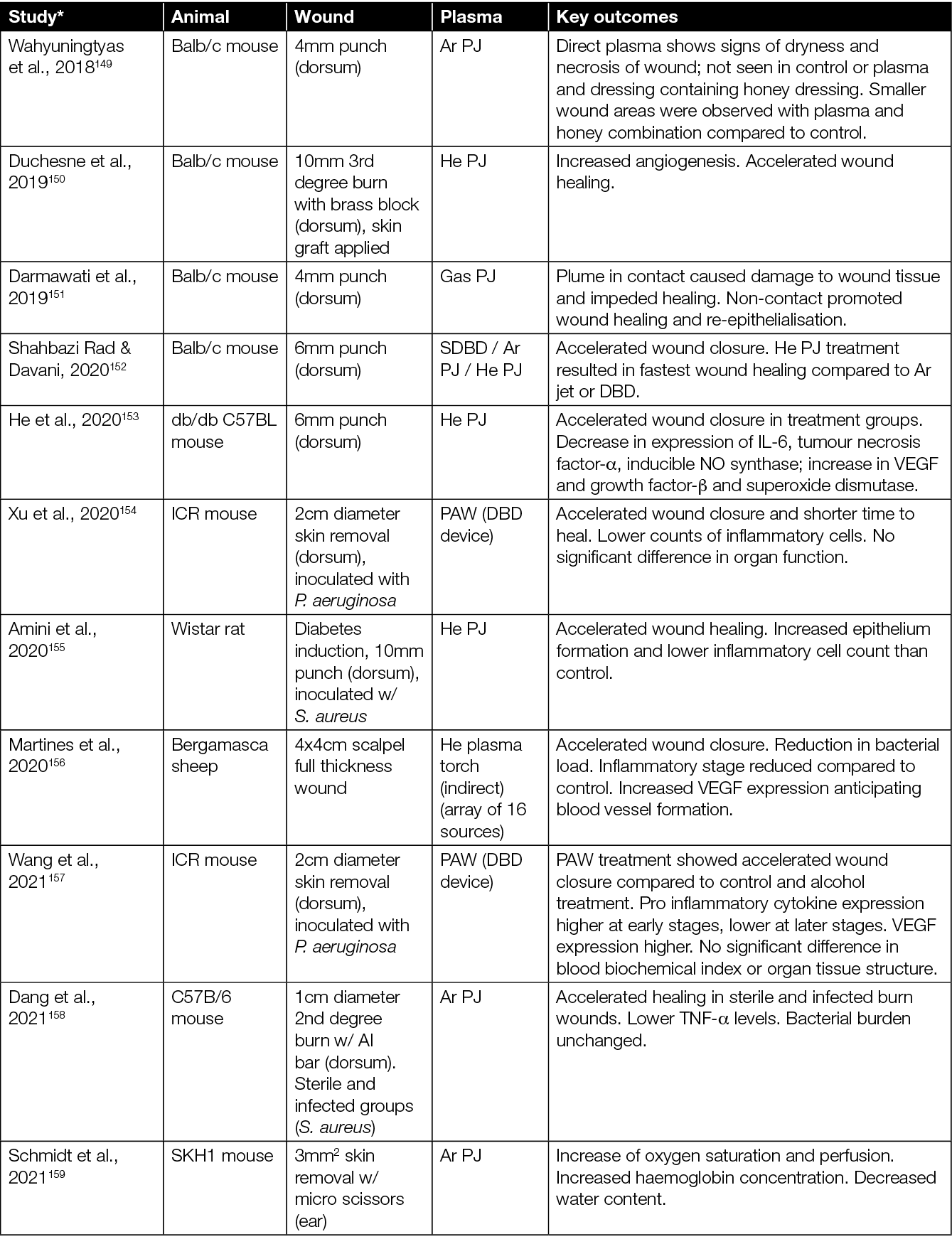
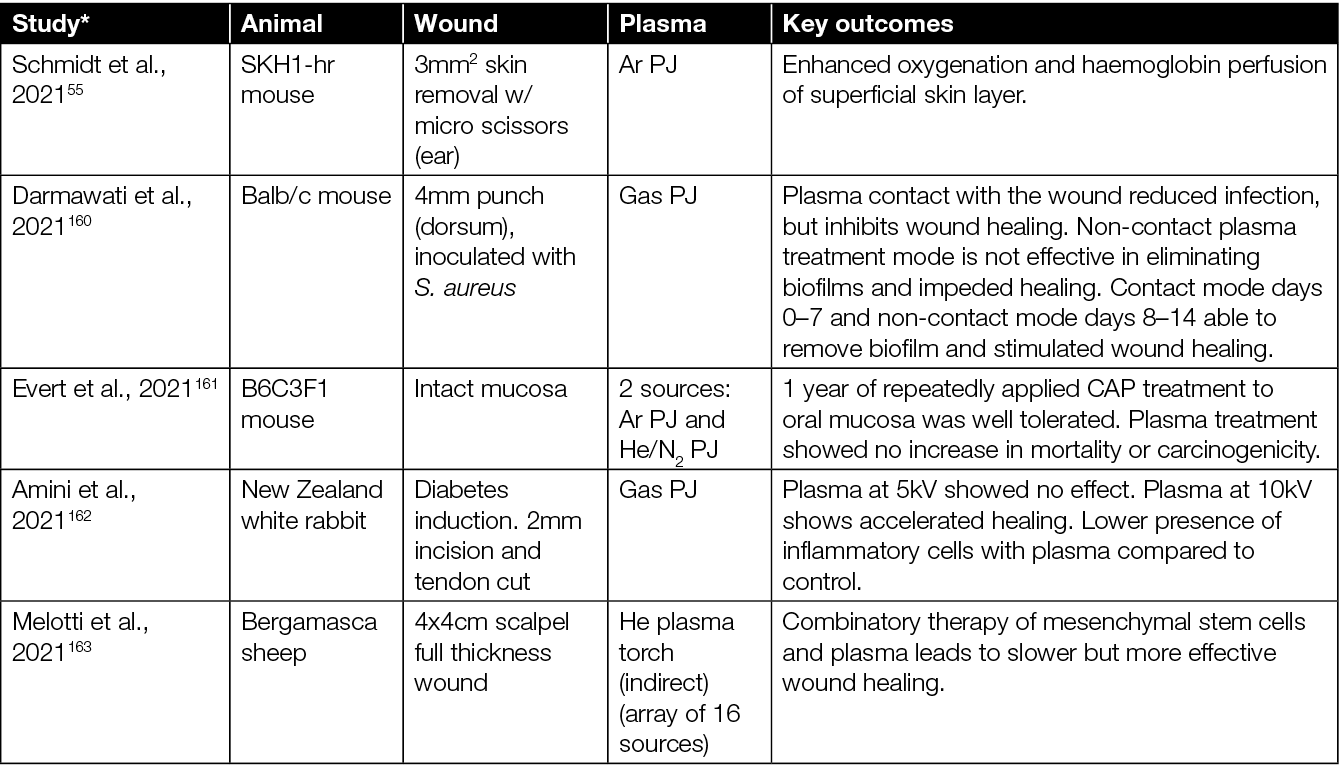
Some studies also assess the effect of CAP treatment in combination with standard care (e.g. hydrocolloid dressings) and other experimental therapies including honey and zinc oxide (Table 1). In terms of wound types, most studies focussed on the effect of CAP in acute (non-diabetic) wounds. There are studies, however, which extend to infected wounds, wounds in diabetic mice, burns, and wounds from x-ray irradiation. In mice studies, the typical sample size ranged from 5–40 mice, assigned mainly into two groups – an experimental group wherein the wound was directly exposed to CAP, CAP-treated liquid, or CAP combined with other therapies; and a control group wherein the wound was left untreated or treated with a therapy without CAP. In some studies, each mouse served as its own control (i.e. each of two wounds at different locations in a mouse served as a control and experimental arm) while, in others, separate mice were used for control and experimental group.
The majority of studies on the effects of CAP in wounded mice point to: accelerated wound closure;128,129,131,134–136,138,139, 141,145,152–154 improved angiogenesis, re-epithelialisation and vascularisation;132,140,142,146 neutrophil and macrophages infiltration;128,132,133 elevated levels of cytokines and growth factors;146,147,155,157,158 oxygenation of tissue;55,159 and reduced microbial burden.126,127,157,157,160 However, it is worth highlighting that, in some studies, a higher rate of wound closure and accelerated re-epithelialisation were observed only in the early phase of wound healing, while in later stages, at the end of the observation period, wounds closed completely similarly in both CAP-treated wounds and untreated wounds.128,133,140,141 A few studies showed that the CAP exposure had no effect on wound healing (Table 1).140,142,148,149
In summary, the healing effects of CAP treatment were more evident using moderate or shorter treatment times,131,137 pointing towards a dose dependence and the possibility to ‘over treat’ a wound. For example, prolonged treatment and/or treatment when the CAP plume is in contact with the skin/wound has been shown to have deleterious effects such as dehydration, hypoxia and skin damage.137,143,149,150 When the CAP plume is in contact with the wound, Darmawati et al. showed an increase in bacterial killing,160 but it is cautionary to note that the plasma plume contact showed significant damage to wound tissue.151 However, both studies demonstrated a faster healing of the wound with non-contact regimes. Investigators observed that optimising each CAP device, and determining an optimum threshold dose of CAP exposure, overcomes undesirable effects of CAP, while still inducing beneficial effects such as disinfection and wound healing.
Other solutions include using CAP-activated liquids154 or introducing hydrogels/dressings along with CAP treatment.149 Various studies to date report considerable variation in results, which most likely is caused by a lack of standardisation in method. Current literature refers to a range of different types of CAP devices and different treatment modalities (i.e. direct, indirect, contact, non-contact, different gases). Nevertheless, so far, no significant adverse side effects of CAP treatments in vivo have been observed (Table 2).
Table 2: Plasma devices used for in vivo studies of wound healing.
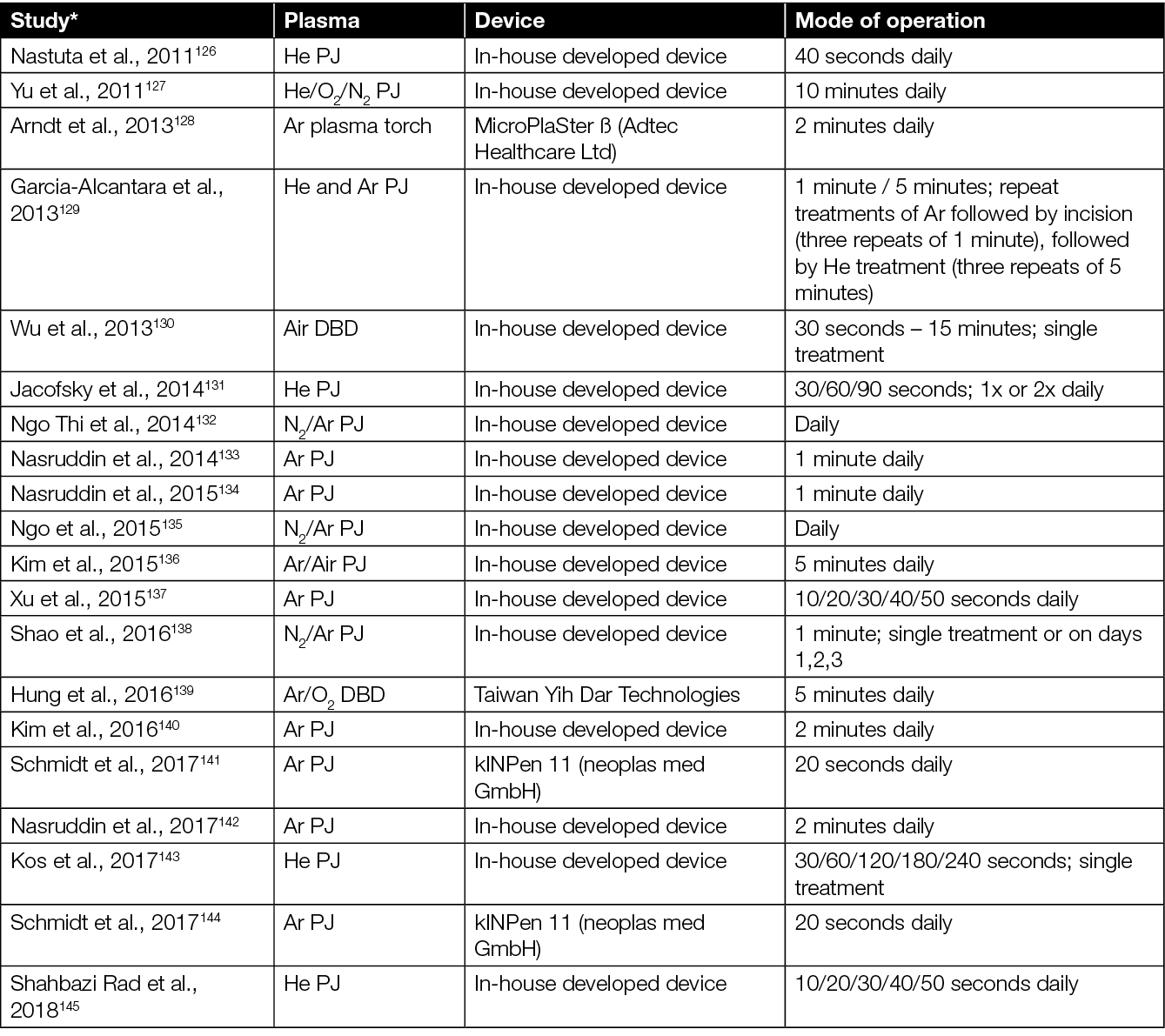
* Articles sorted by year of appearance.
A study by He et al. showed that CAP treatment did not lead to any damage to the mice’s skin, nor to liver and kidney function, days after the CAP treatment.153 Similarly, Evert et al. reported no carcinogenic effects in mice after repeat treatments 3 times per week over a 12 month period,161 while Schmidt et al. observed no inflammatory or carcinogenic effects 350 days after CAP wound treatment,144 highlighting the long-term safety of the used CAP devices.
Besides mice, CAP has also shown positive effects on wound healing in larger animal models. For example, CAP improved neovascularisation and collagen production in rabbits162, wound healing and an increase in proliferation and growth factors in sheep,156,163 and blood coagulation in wounded pigs.130 Similar to the results in mice, extended CAP exposure durations can damage the intact and wounded skin in pigs, while shorter treatment times did not.130
To conclude, there exists ample preclinical evidence to show the potential of CAP devices in wound healing; however, studies indicate that optimisation of the operation and treatment parameters is essential for each plasma device. Whilst most studies to date have been conducted in small murine models, a number of studies were done with larger animals. These models remain relevant as studies progress towards human trials, helping to unravel mechanistic understanding of CAP interventions.
4. The clinical perspectives of CAP in wound healing
4.1 Clinical evidence for the efficacy of CAP to promote healing of chronic wounds
As discussed in previous chapters, CAP is a partially ionised gas, and consists of multiple components including, among others, RONS, electromagnetic fields, as well as UV, visible and infrared radiation.12 This cocktail of different components acts synergistically and has two major properties that are considered to promote tissue regeneration and wound healing. On the one hand, CAP inactivates microorganisms efficiently, including multi-resistant bacteria. On the other hand, it stimulates migration, as well as proliferation of eukaryotic cells, and increases micro-circulation in treated tissues.164–170
The treatment of chronic wounds is only one of many different potential medical applications of CAP.171 However, it is the most studied field, with the most elaborate clinical evidence for the efficacy and safety of CAP. Early pilot studies that led to the certification of the first plasma devices have, in the meantime, been complemented with a series of structured case reports and several randomised controlled trials (RCTs) (summarised in Table 3).
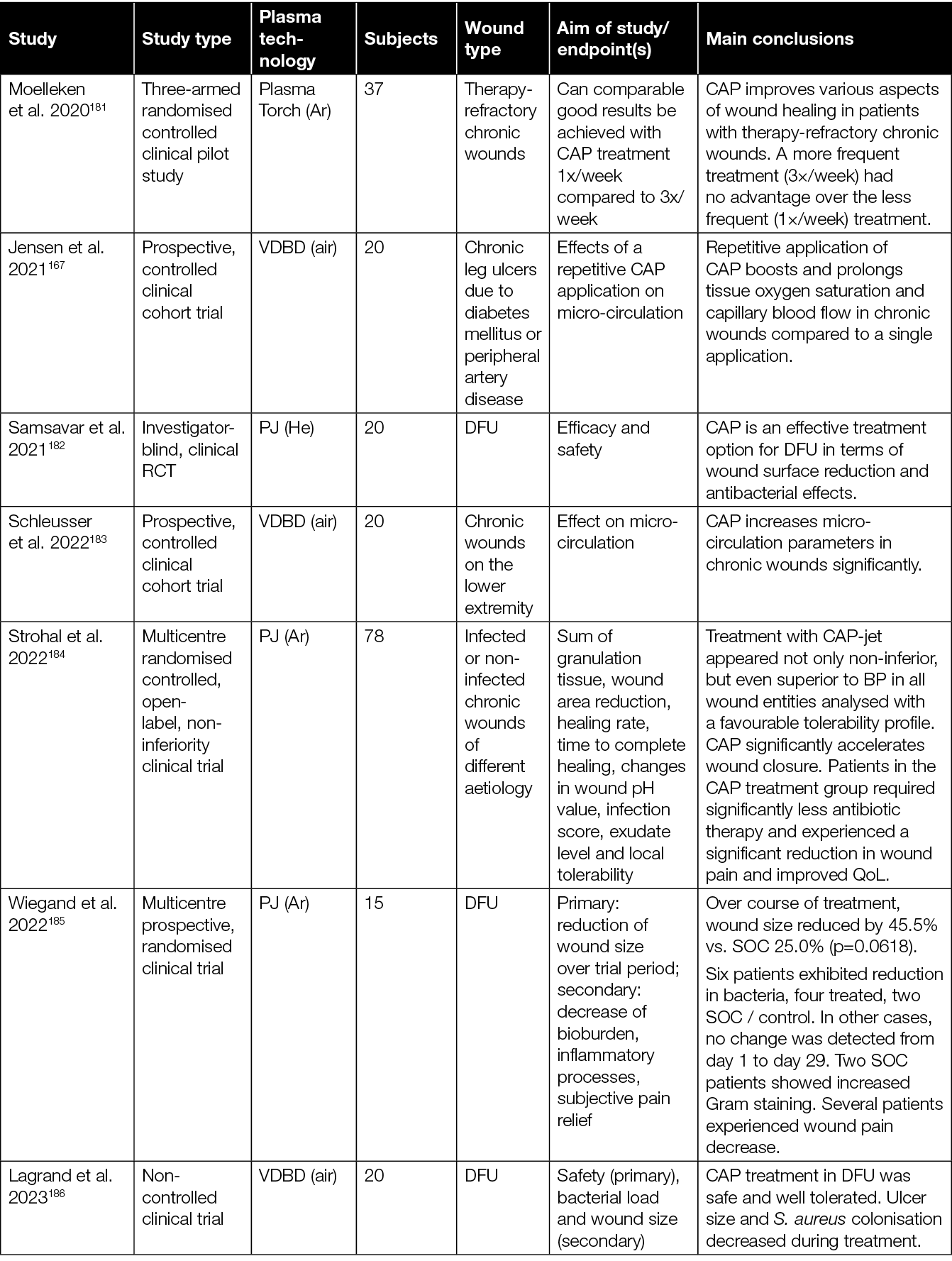
Table 3: Overview of clinical studies assessing the efficacy of CAP in the treatment of chronic wounds.
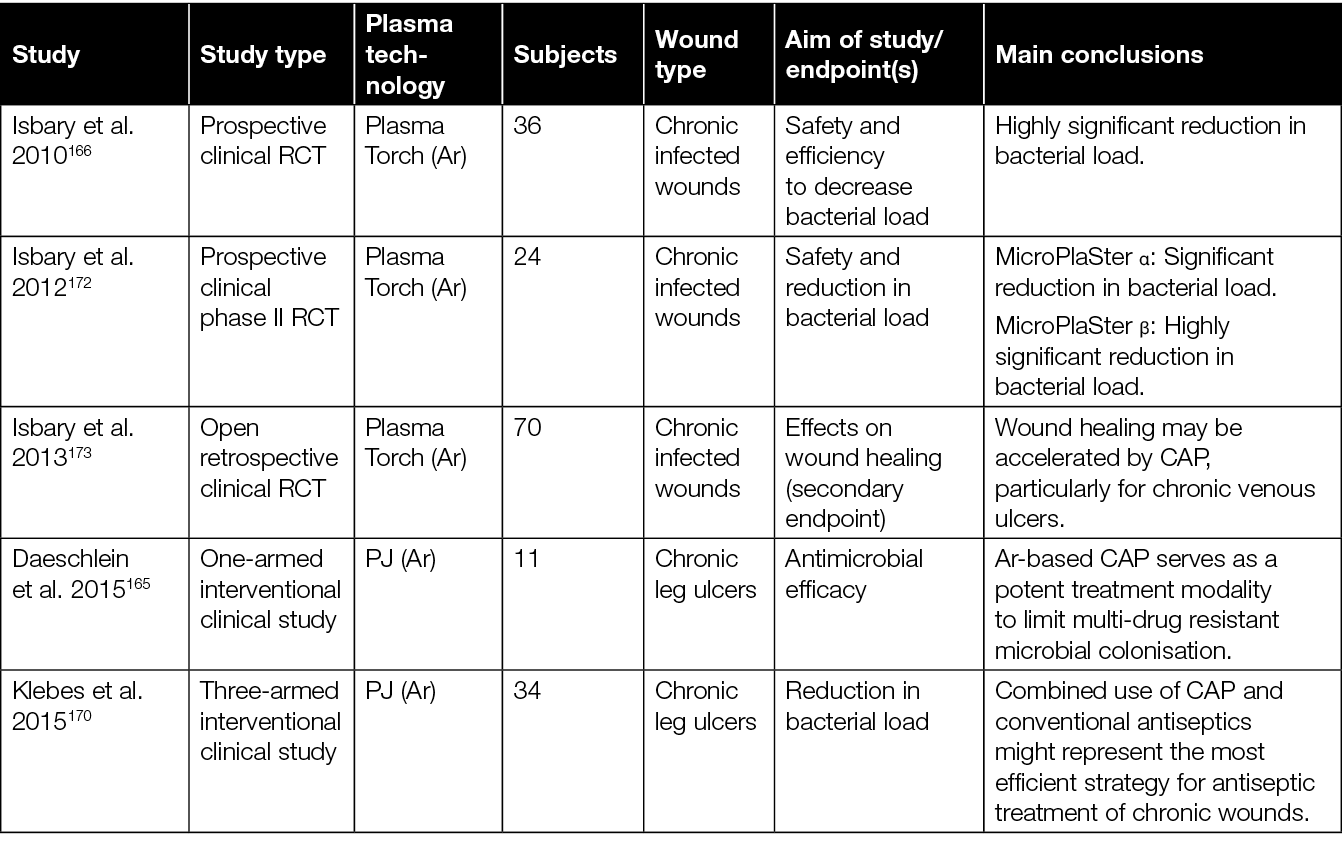
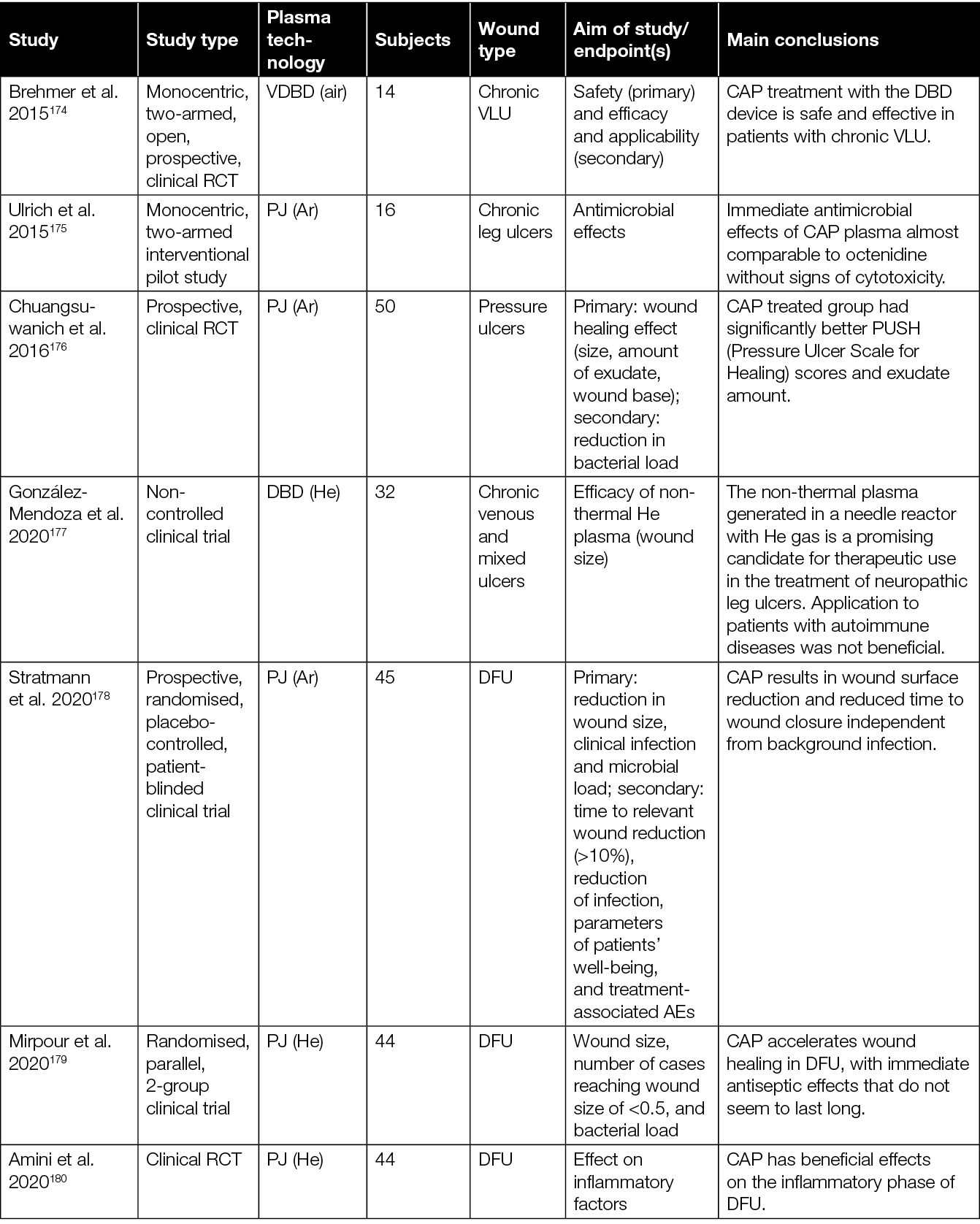
The initial investigations focused primarily on demonstrating the safety of various CAP devices and their ability to decrease bacterial load in persistent wounds.166,170,172,175 For example, two prospective RCTs evaluating the application of a plasma torch revealed a significant reduction of the bacterial load in chronic wounds.166,172 Another case-control study showed a comparable antibacterial outcome after CAP treatment using an Ar PJ device when compared to an octenidine-treated group.175
While the primary endpoint of these feasibility studies was safety and reduction of bacterial load, a subsequent retrospective follow-up study conducted by Isbary and colleagues166,172 provided insights on the potential of CAP to promote healing of chronic wounds.173 A first mono-centric, two-armed, open, prospective, randomised and controlled pilot study using a DBD plasma source for the treatment of chronic leg venous ulcers enrolled 14 patients.174 Fifty percent of the patients received conventional wound care, while the remaining half underwent CAP treatment in addition to standard wound care. In both groups, wound size reduction of approximately 50% was observed; however, the CAP-treated group exhibited a quicker and more substantial decrease in wound size after 3 weeks. Notably, one patient in the CAP-treated group achieved complete healing. Plasma treatment resulted in a significant reduction in lesional bacterial load (P=0.04, Wilcoxon signed-rank test). A more than 50% ulcer size reduction was noted in five of seven and four of seven patients in the standard and plasma groups, respectively. A greater size reduction occurred in the plasma group (plasma –5.3cm2, standard of care (SOC) –3.4cm2, P=0.42, log-rank test). The only ulcer that closed after 7 weeks received a plasma treatment originally. In total, two serious adverse events (SAEs) and 77 adverse events (AEs) were observed, distributed equally among both groups (P=0.77 and P=1.0, Fisher’s exact test).
Following these early studies on the antibacterial efficacy and safety, eight clinical RCTs with focus on the efficacy of CAP to enhance healing of chronic wounds were conducted.176,178–182,184,187 One of the parameters to assess wound healing in these studies was wound size reduction. For example, significantly accelerated wound size reduction in the CAP treated group compared to the control group was observed by Chuangsuwanich and colleagues after treatment of pressure-induced ulcers using an Ar-based PJ.176
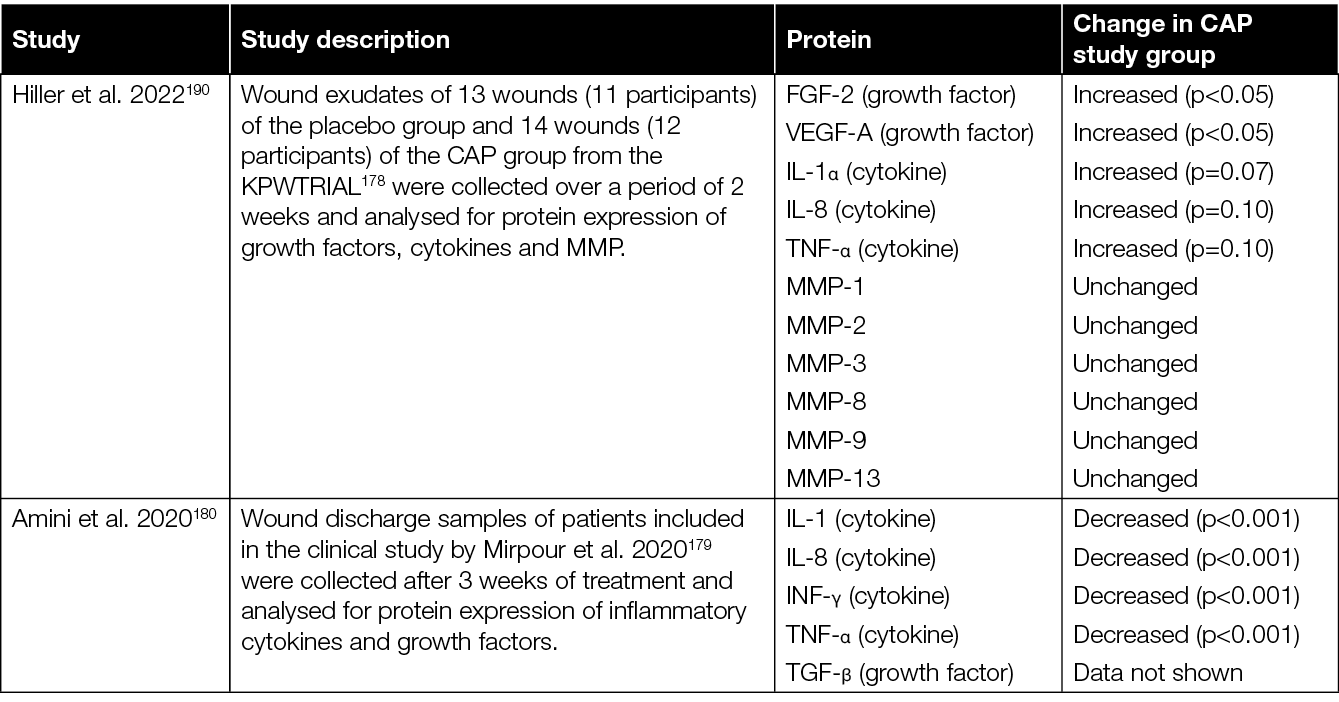
With regard to the various therapeutic effects of CAP, it is relevant to consider the 2020 study of Stratmann and colleagues in more detail (Table 3).178 Results of this prospective, placebo-controlled, blinded, multicentre study showed a significantly more pronounced reduction in wound size, as well as reduced time to relevant wound area reduction with CAP treated subjects. Interestingly, no significant difference in the reduction of infection and microbial load was observed between CAP and placebo treated patients. This could suggest that the observed wound size reduction and time to closure changes might hold a direct causal relation to the CAP treatment received, independent of the (background) infection.178 To further investigate this significant study finding, Hiller et al. analysed wound exudates of a sub-cohort of this study to evaluate the expression of FGF‑2, VEGF‑A, cytokines and matrix metalloproteinases (MMP) (Table 4).190 These analyses revealed an increase of crucial growth factors like FGF‑2, VEGF‑A and interleukins, suggested to be important factors of CAP-mediated enhancement of granulation, vascularisation and renewed epithelialisation with diabetic foot morbidity.
Similar analyses were performed by Amini et al., who investigated cytokines and growth factors in the wound exudates retrieved during the earlier RCT by Mirpour et al., which considered the treatment of diabetic foot ulcers (DFU) with CAP (Table 3).179,180 Where the clinical results of Mirpour and colleagues showed immediate antiseptic effects due to CAP treatment, the analyses of Amini et al. showed a significant reduction of IL‑1, IL‑8, tissue growth factors TGF‑β, TNF‑α, and INF‑γ after 3 weeks (Table 4).180 These results are, however, not corroborated by the later observations by Hiller et al. (Table 4).190
Table 4: Cytokine and growth factor levels in wounds treated with CAP compared to control wounds.
While some of the RCTs mentioned here focused on DFU, multiple other studies investigated the efficacy of CAP on pressure ulcers, therapy-refractory chronic wounds, chronic non-healing arterial or venous wounds on the lower leg, and infected or non-infected chronic wounds of different aetiology. However, at the time of writing, no study has been conducted which compares the efficacy of CAP for the treatment of wounds of different aetiology systematically. Therefore, it is still unclear whether wounds of a certain aetiology might be more prone to benefit from a CAP therapy than wounds of another aetiology.
As illustrated here, results indicating a beneficial efficacy of CAP in promoting healing of chronic wounds have been growing steadily, suggesting that CAP can reduce bacterial load, increase micro-circulation, accelerate wound closure, and reduce pain. Having said this, however, there are many differences between the various studies with respect to patient cohort, devices, treatment times and frequencies, and outcome measures. For example, most RCTs so far have been conducted with PJ devices, introducing the need for more non-jet plasma device studies. Considering the many differences between plasma sources, and the composition of the resulting plasma they produce, it is important that clinical efficacy is investigated for every device before routine clinical use can be suggested. Furthermore, it should be acknowledged that not every patient benefits from the addition of CAP to their standard wound care, despite the often observed significantly improved wound healing. Hence, more clinical research is required to identify patient groups that are most likely to benefit from CAP treatment. Potentially, parameters such as age, smoking, alcohol consumption, body mass index or wound aetiology could play a significant role in the clinical efficacy of CAP treatment.
4.2 Clinical evidence for the efficacy of CAP to promote healing of acute wounds
However, while a fair number of RCTs have shown beneficial effects of CAP on healing of chronic wounds, the evaluation of CAP to improve healing of acute wounds is still sparse (Table 5). In a series of case reports, sterile laser skin lesions were treated with an Ar-based PJ for either 10 seconds, three times 10 seconds, 30 seconds, or left untreated as control.191 When wound healing was evaluated 6 and 12 months after treatment, the best outcomes were observed with subjects treated with the three times 10 seconds and single 30-second regimens. In another study, laser lesions were treated with CAP for 60 seconds and compared to ointment (betamethasone valerate and gentamicin sulfate), basic FGF, or no treatment.192,193 Here, only a significant difference in the redness one day after treatment was observed. However, on day 3, 7, 14 and 28 after treatment, there was no difference between the various treated groups. A study by Vandersee and colleagues compared CAP treatment (Ar PJ) of vacuum-generated acute wounds with either no treatment, octenidine treatment, or a combination of sequential CAP and octenidine treatment.194 Their results showed that CAP treatment led to a more rapid area decline that was statistically significant in comparison to the other treatment groups. Morphologically, the authors reported that wound healing was found to initiate from the edges with the formation of dendritic structures consisting of keratinocytes.194
Table 5: Overview of clinical studies assessing the efficacy of CAP in the treatment of acute wounds.
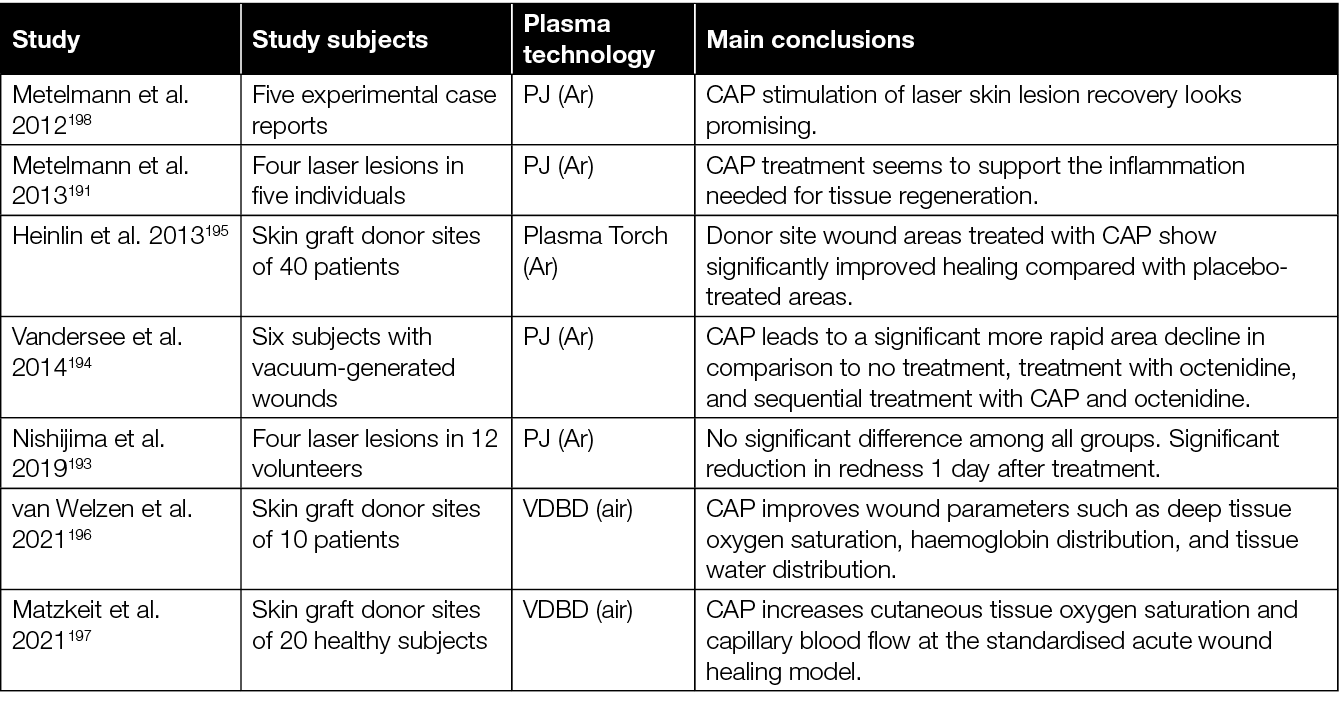
Some studies used skin graft donor sites to investigate the effects of CAP on acute wounds. In one of these studies, half of the skin graft donor wounds were treated with CAP, while the other half received placebo treatment.195 When wound healing was assessed in a blinded fashion by independent experts, it showed that wound healing was improved significantly after CAP treatment using a plasma torch.195 In a pilot study, the efficacy and safety of a novel VDBD plasma dress device has been explored for treatment of skin graft donor sites.196 In this study, the course of wound healing was assessed using hyper-spectral imaging which revealed that CAP improves important wound healing parameters such as deep tissue oxygen saturation, haemoglobin distribution and tissue water distribution. Increased cutaneous tissue oxygen saturation and capillary blood flow have also been observed by Matzkeit and colleagues after treatment of skin graft donor sites with a VDBD device.197 In all, these studies demonstrate a potential of CAP to improve wound healing parameters in acute wounds. There is, however, a clear need for larger RCTs to corroborate these early findings.
4.3 Treatment times and frequencies
To date, no standards for the clinical application of CAP in wound treatment have been established. Besides different plasma technologies and devices, there is a variety of parameters that differed between the clinical studies investigating the efficacy of CAP to promote healing of chronic wounds (Table 6). The studies varied, for example, by treatment time from around 1 second/cm2 up to 7 minutes. In some studies, wounds were treated just once, while treatment was repeated up to five times a day or several times a week over a period of several weeks in other studies. Moelleken and colleagues compared a single treatment per week with three treatments per week, and observed that a single treatment is not inferior to the more frequent treatment.181
Table 6: Summary of plasma technologies and treatment modalities used in clinical studies for CAP treatment of chronic wounds.
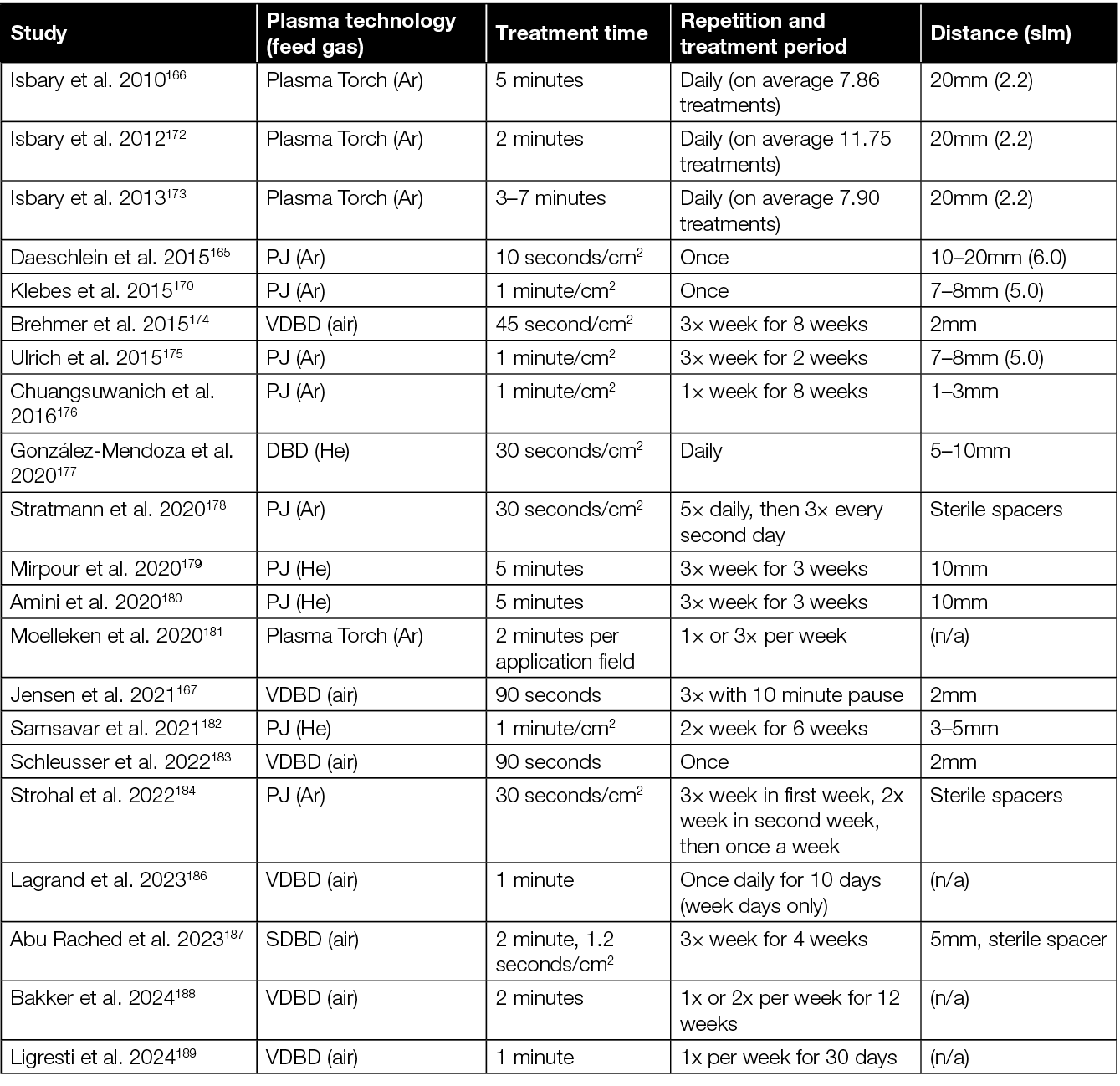
Preclinical studies indicate a treatment efficacy following the principle of hormesis.97 Hormesis is a dose response phenomenon describing a stimulating effect of a treatment at low doses, and an inhibitory effect at higher doses. Against this background, a treatment schedule with few treatments per week, followed by longer treatment pause (2–3 weeks), is suggested for wound healing purposes.199 For a sole antimicrobial and antiseptic treatment, a more frequent treatment, daily for 1 week, is suggested. However, due to the lack of studies comparing different treatment times and frequencies in a clinical setting, the optimal treatment modalities and the therapeutic window still need to be determined. Furthermore, it is also important to note that, because of the different plasma technologies, the optimal treatment modalities and therapeutic window will have to be determined for each device individually.
In general, it is recommended to follow the instructions by the manufacturer of the respective device. Nevertheless, more clinical studies are needed to gain further insights into the optimal treatment modalities. Furthermore, additional clinical observation will help identify profiles and characteristics of patients who will most likely benefit from CAP treatment. To date, clinical observations of CAP wound healing treatment results are accumulating steadily, as illustrated by systemic literature reviews.200 General requirements for plasma sources in medicine are provided by the German Institute for Standardization (DIN SPEC 91315).199 Due to continuous developments, this 2014 document is being revised currently, with an updated version expected to be published in German and English in 2024.
5. Review of emerging CAP therapies: preclinical and clinical perspectives
CAP therapy is an emerging medical technology that has garnered considerable attention due to its potential applications in wound healing and various other medical conditions. Here, a comprehensive review of preclinical and clinical studies exploring the effects of CAP beyond current applications for wound healing is presented. While doing so, some future prospects of CAP therapy that might serve a valuable approach for promoting wound healing in the coming years are highlighted.
5.1 Exploring potential of CAP for wound healing
There are a significant number of papers showing positive effects or an absence of negative effects on cell cultures in vitro. Although these studies have no direct implications for the clinical use of CAP in wound care, they do provide valuable information for fine tuning of existing devices, the development of new CAP applications, and broadening the understanding of the different mechanisms of action; for example, short (<60 seconds) treatment with a CAP PJ of primary keratinocytes, immortalised keratinocytes (N/TERT1, HaCaT), and improved fibroblasts in vitro cell migration.158,201,202 Similarly, short (60 seconds) treatments of primary keratinocytes with DBD enhanced migration, while longer treatments showed a pro-differentiation effect.203 These results were demonstrated to be related to the concentration of H2O2 and were modulated by nitrite/nitrate.
As described earlier in this document (paragraph 3.1), animal studies showed CAP stimulates wound closure through increased cell proliferation and migration, and increased expression of collagens and alpha smooth muscle actin (αSMA). Furthermore, it also shifts the cytokine balance from a pro-inflammatory (mainly IL-1β, TNF-α and IL-6), and induces a switch in the macrophage subtype from a pro-inflammatory phenotype (M1) to a repair-promoting phenotype (M2). In addition, cellular antioxidant stress and deoxyribonucleic acid (DNA) damage repair are suggested to be enhanced by CAP.
In human trials, CAP was also shown to promote faster healing by disinfecting the wound and stimulating tissue regeneration, increasing tissue oxygen pressure, improving overall wound condition, and reducing infection rates compared to conventional therapies (Table 5).
Notably, CAP therapy has shown promising results in patients with hard-to-heal wounds, suggesting its potential as a non-invasive and effective alternative for challenging wound cases. Plasma can stimulate the micro-circulation of healthy skin beyond the treatment time,164,167–169,204,205 which can improve the healing potential, especially of chronic wounds. The enhanced micro-circulation is probably mediated by plasma-produced NO204 which easily penetrates the skin.206,207 The potential role of plasma-generated NO in medicine is discussed in detail elsewhere.208 NO is an important messenger and regulator of blood flow, immune response and wound healing, and NO can act as an antioxidant.
An increased micro-circulation might in part be due to heat produced by CAP. Heating skin to 42˚C also stimulated blood flow,209,210 while fast heating resulted in a higher endothelial activity.210,211 Although referred to as cold or low temperature, CAP transiently increased the skin temperature 164,205,212 or resulted in a sensation of heat score of 0–5.213,214 Furthermore, the effect of CAP on wound healing might in part be related to acidification by locally inducing nitrite and nitrate formation. Acidification of wounds by natural or artificial means plays a role in wound healing and the control of polymicrobial infections.215
It is important to note that changing the dosing or treatment regimens of CAP can affect the desired outcomes and/or side effects. Currently, several clinical trials are being conducted to investigate the application of CAP on various types of wounds, including chronic ulcers, burns and surgical wounds. A comprehensive overview of the status to date of these clinical trials can be found in Table 7.
Table 7: Overview of completed, but not yet published, ongoing or planned clinical trials for testing CAP devices.
Trials testing CAP for anti-tumour effects were not included.
ID: study identifier at clinicaltrials.gov (NCT) or onderzoekmetmensen.nl/en (NL); n: number of participants.
There were no studies found with CAP at https://euclinicaltrials.eu/ or https://www.clinicaltrialsregister.eu/
5.2 Blood coagulation
Blood coagulation is a critical process in the body’s response to injury, playing a crucial role in preventing excessive bleeding. However, modern medical practices often require the ability to control blood coagulation rates. Various methods, like electric cauterisation and medications, have been employed, but may have undesirable side effects.
CAP has emerged as a promising tool for precisely managing blood coagulation with minimal adverse effects, and is studied as a means to control bleeding during surgery and promote haemostasis.216 CAP likely promotes coagulation by enhancing the physiologic coagulation process through direct selective activation of fibrinogen, as well as through platelet activation, leading to aggregation and clotting217,218 without affecting, for example, albumin, pH or Ca2+ concentration in blood. Furthermore, CAP’s ability to control coagulation appears largely unaffected by the presence of anticoagulants, making it a versatile tool for surgical procedures and wound healing. It can be tailored to either accelerate or reduce coagulation, offering flexibility in medical application.
In the context of surgical procedures involving implantation and artificial implants, CAP’s role is significant. Surface hydrophilicity of implants is a critical factor influencing clotting at the implant-bloodstream interface. CAP treatment can increase the hydrophilicity of implant surfaces, reducing clot formation and tissue adherence. Plasma treatment can significantly increase protein adsorption and cell adhesion of murine osteoblasts on different graft materials.219 Moreover, CAP has shown the ability to induce natural coagulation around artificial implants, making it a valuable tool for managing clotting in these situations. The characteristics of CAP, such as power, feed gas and device type, can be adjusted to meet specific requirements for treatment. Overall, CAP could suggest a promising avenue for controlling blood coagulation, both for managing bleeding during surgeries and preventing excessive clotting around medical implants.
5.3 Modulation of implants
Treatment of implants with CAP allows for better integration into tissue or a lower risk of rejection,31,219–221 although this seems more relevant for orthopaedic and dental surgery. The role of CAP application on biocompatibility and surface improvement in implantology is reviewed in an excellent review by Hui and colleagues.222 CAP is capable of ameliorating surgical implants using various strategies of interface biotechnology, such as surface modification,223–225 coating deposition,226 and drug delivery, e.g. silver nano particles.31 CAP modification dramatically increased surface pore size and wettability of a poly vinyl alcohol/poly lactic acid alcohol (PVA/PLA) core-shell scaffold, thereby increasing the loading capacity for medication.227
After transplantation of CAP-treated human acellular dermal matrix, fibroblast infiltration and proliferation was increased, indicating improved biocompatibility and bio-integration. CAP treatment significantly improved hydrophilicity, protein adsorption capacity, biocompatibility and bio-integration efficiency without compromising the structure of the human acellular dermal matrix.509 Depending on the specific implant strategy, CAP enhancement of a surgical implant can be performed pre- or intra-operatively. For example, CAP can be used to maximise the bonding between adhesive and human teeth dentin or implant during a dental implantation surgery. Coating and drug loading are ideally prepared before the surgery, as the deposition process could be relatively time-consuming.
5.4 CAP indication extension, exploring other pathologies
The utilisation of CAP devices designed for skin resurfacing demands cautious handling due to the higher temperatures (>60˚C) and long recovery periods for patients.228–234 Ozone, which is produced by many CAP devices, can also be used as the single active component to treat atopic dermatitis.235 In addition, CAP can modulate immune responses and alleviate symptoms. Currently, a He-based PJ is tested for the reduction of lymphoceles following pelvic lymph node dissection (see NCT02658851 in Table 7). Lymphoceles is lymphatic fluid that forms in a cavity in the body, typically as a result of surgery which disrupts the normal flow of lymphatic fluid. CAP can be used to dissect the lymph nodes and seal the lymphatic channels to prevent lymph leakage. This is slightly similar to the well-known plasma scalpel which has been used since the 1980s for the simultaneous division of tissue and coagulation of blood vessels.236 These plasma scalpels do, however, exhibit higher temperatures to cauterise tissue, and are therefore not referred to as CAP.
CAP has been explored for various other therapies and treatments, such as skin rejuvenation,237,238 actinic keratoses,239 androgenetic alopecia,240 herpes zoster,241 warts,242 dental applications,243,244 bone regeneration,245 rheumatism,246 surgical site infections,247 demodex mites248 and psoriasis.249,250 More recent fields of interest include ophthalmology251,252 and cancer therapy.253,254 An overview of recently completed or ongoing trials can be found in Table 7.
5.5 Cancer treatment
CAP has shown potential in cancer therapy by inducing apoptosis (programmed cell death) in cancer cells while sparing healthy cells. It is being researched as a potential complementary or alternative treatment to traditional cancer therapies, but is currently mainly used in in vitro and animal studies. Early-phase clinical trials have shown encouraging results, with CAP demonstrating potential anti-cancer effects in various tumour types and favourable safety profiles (Figure 2).
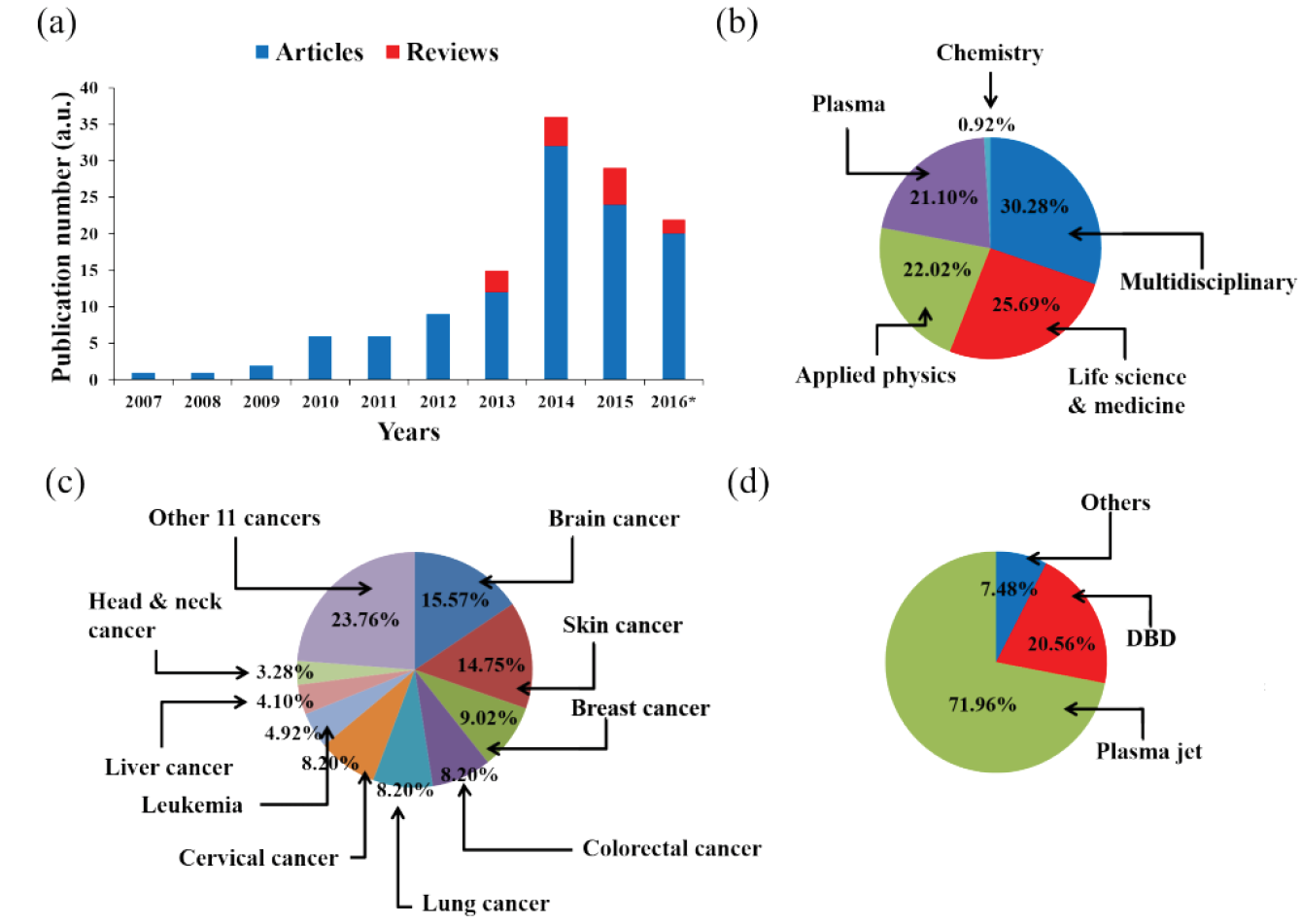
Figure 2: The research status of the application of CAP on cancer treatment by 2016.
a) publication number; *) by the end of September; b) the journal type of articles; c) cancers in articles; d) plasma devices in articles.
Reprinted with kind permission. 258
In 2015, a private company, US Medical Innovation (USMI), carried out a clinical trial on stage IV metastatic colon cancer at Baton Rouge General Medical Center in Baton Rouge, Louisiana, USA. CAP treatment was performed on the post-surgical tissue to kill potential residual cancer cells after a removal surgery. No relapse and progression of cancer occurred in patients.254,255 In Germany, CAP treatment on 12 patients with advanced squamous cell carcinoma of the head and neck resulted in decontamination of infected cancer ulcerations, including a decreased request for pain medication and a reduction of typical fetid odour and microbial load.256 In some cases, superficial partial remission of the tumour and even wound healing of the infected ulcerations have been observed.256 Six patients with local advanced (pT4) squamous cell carcinoma of the oropharynx with open infected ulcerations were treated by an atmospheric pressure PJ (APPJ) in a cycle of three single applications within a week, each followed by an intermittence of another week.257 CAP treatment noticeably improved the therapeutic effect of this locally advanced head and neck cancer (Figure 3). CAP treatment not only improved patients’ social functions, but also caused a reduction in odour and pain medication requirements.
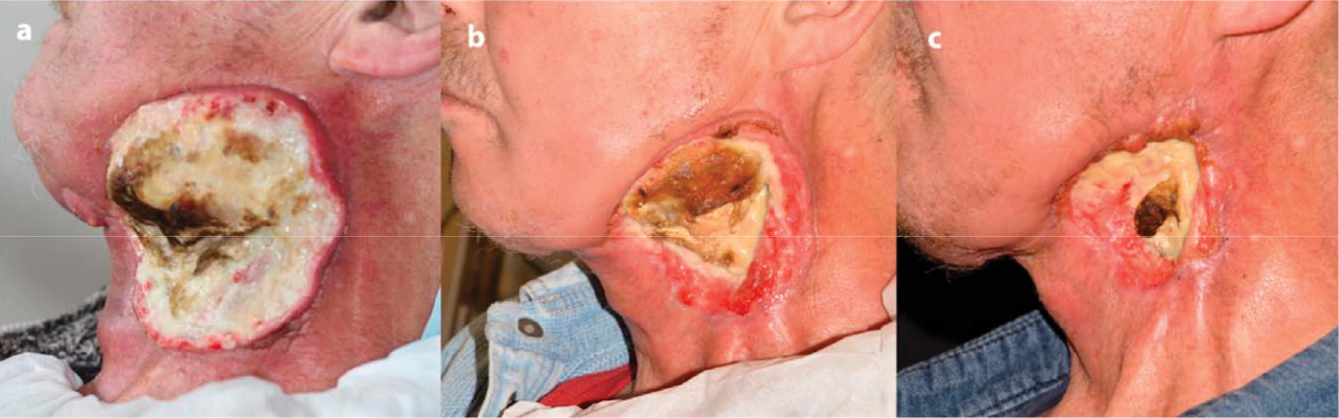
Figure 3: The clinical effect of CAP treatment on a patient with locally advanced head and neck cancer.
The patient’s therapeutic effect was recorded in: (a) April 2016; (b) June 2016; and (c) August 2016.257 © Elsevier GmbH
The mechanism by which CAP eliminates cancer cells is thought to be (in part) due to the higher expression of aquaporins and lower intracellular levels of lactase, which together result in a strong intracellular increase in ROS, leading to cell death (Figure 4).258 CAP may also activate the immune system to attack tumorous tissue by reactive species or other factors. CAP could trigger cancer cells to emit signals known as damage-associated molecular patterns (DAMP), which may attract and stimulate local immune cells.255 This suggests that CAP could possibly be used in combination with immunotherapy or chemotherapy to treat cancer.
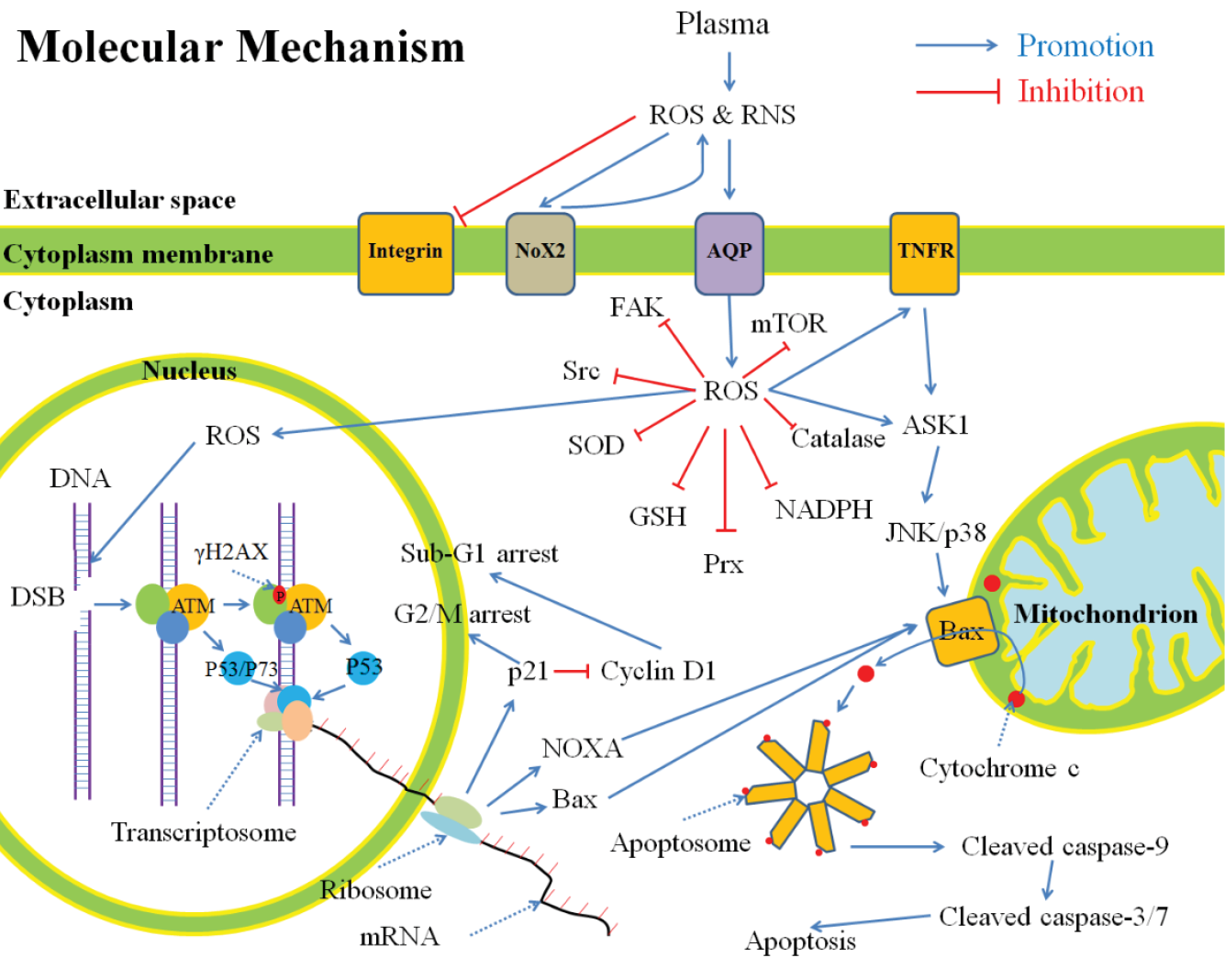
Figure 4: A general summary for the anti-cancer mechanism of CAP in vitro, based on publications.
The CAP-originated reactive species will cause a noticeable rise of intracellular ROS which weakens the intracellular antioxidant system and further causes serious DNA double-strand break (DSB). As a result, cell cycle arrest and apoptosis based on mitochondrion-pathway or tumour necrosis factor receptor-pathway occur.
ROS: reactive oxygen species; RNS: reactive nitrogen species; Nox: NADPH oxidases; AQP: aquaporins; TNFR: tumour necrosis factor receptor; FAK: focal adhesion kinase; Src: Src kinase; SOD: superoxide dismutase; GSH: glutathione; Prx: peroxiredoxin; NADPH: reduced nicotinamide adenine dinucleotide phosphate; mTOR: mechanistic target of rapamycin; DNA: deoxyribonucleic acid; DSB: double-strand break; ATM: ataxia telangiectasia mutated; mRNA: messenger ribonucleic acid; ASK: apoptosis signal-regulating kinase; JNK: c-Jun N-terminal kinase. Reprinted with kind permission.258
5.6 Other uses of CAP in a medical setting
5.6.1 Sterilisation and disinfection
Surface disinfection, especially in a medical setting, also plays an important role in infection prevention.259 Careful cleaning and disinfection of environmental surfaces are essential elements of effective infection prevention programmes. However, traditional manual cleaning and disinfection practices in hospitals often prove to be suboptimal.260 Disinfection of medical instruments, surfaces, rooms and vehicles can be done with CAP due to its ability to inactivate microorganisms and compatibility with all kinds of (sensitive) materials.261 By enhancing clean practice environments through the antimicrobial properties of CAP, it is possible to reduce the risk of wound infections, and thus improve clinical outcomes.
During the COVID-19 pandemic, CAP was shown to be an inexpensive and sustainable technology for disinfection of face masks.262 Based on this experience, the authors suggest that therefore this disinfecting technology could also be applied to other objects and personal protective equipment used in hospitals. Furthermore, CAP can also be used for the sterilisation of implants, as reviewed in reference 222 and paragraph 5.3 of this EWMA document. One of the benefits of the utilisation of CAP is the speed of disinfection. For example, high log reductions were obtained after treating glass plates or silicone hands for 5–10 seconds with a SDBD device.263 In addition, a period for neutralisation or cooling down after autoclaving is not required after CAP treatments. The management of biofilms on or in equipment by conventional methods or CAP is, however, more challenging.264
5.6.2 Air purification
Cold plasma devices are being explored to sanitise the air in hospital rooms and other healthcare facilities to help reduce the risk of healthcare-associated infections by eliminating airborne pathogens.265,266 Plasma air treatment has, for example, shown to rapidly and effectively inactivate aerosol transmitted SARS-CoV-2 in rooms and therefore has great potential preventing the transmission of virus and infections.267 In another study, CAP treatment significantly reduced bacterial counts in the air in hospital blood sampling rooms.268
5.7 Different sources for direct plasma delivery
At present, CAP devices that have been certified and approved for biomedical use in wound care are based on either PJ or DBD (Figure 1 and Table 8). In most studies with PJ, Ar gas is used.200 Other CE-certified devices can be used during surgery for incisions (cauterise), or removal of tissues (ablation). These devices will not be discussed here, as these function at higher temperatures, and therefore cannot be regarded as CAP. Depending on demands, different delivery routes and plasma sources are explored such as biomedical device-assisted plasma delivery like in tubes,269,270 for endoscopy,271 large distances, or in patches212 (paragraphs 5.8 and 5.9).
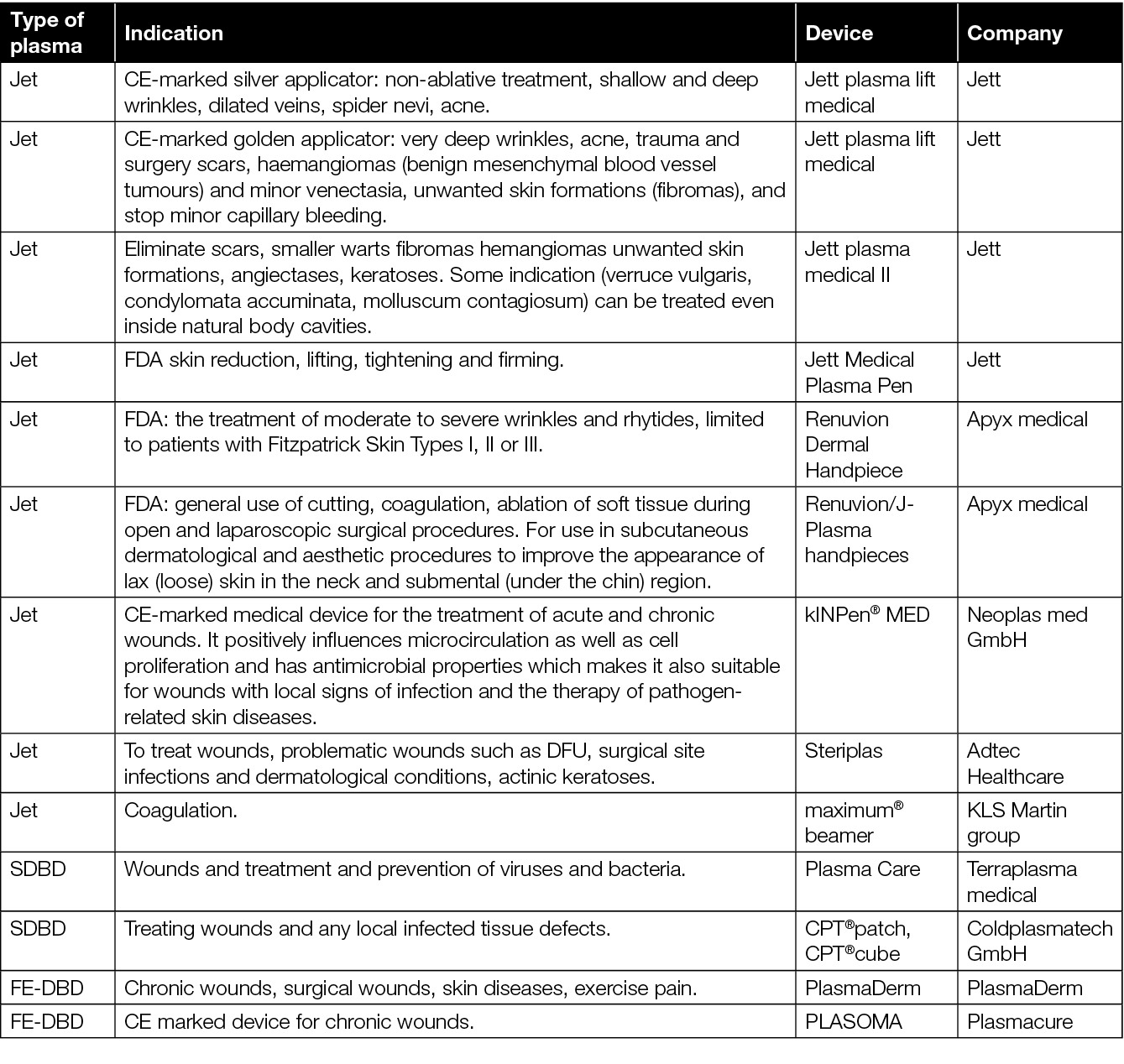
Table 8. Approved plasma devices for wound care and their indications for use.
CAP device type, design and settings greatly affect outcomes and side effects. For example, distribution of reactive species on agarose gel or pig skin has proven to be strongly dependent on the discharge frequency.272 When comparing two jet-based plasma devices in a side-by-side comparison, these differed in their effect on cell proliferation and migration.53 The two devices did show similar effects
on expression of collagen, cytokines and growth factors, activation of immune cells and improved wound healing.
In general, DBD devices can be used for larger surface areas than PJ. For the treatment of larger surfaces with jets, PJ arrays have been invented.23,272–276 Electrosprays and plasma sprays can bring a wide range of potential biomedical applications, especially since the combination of plasma and aerosols is used in situations where direct contact needs to be avoided.266 PJ can treat surfaces with irregular shapes because the reactive species are blown to the target area by the working gas. By adjusting the design of the plasma device, the plasma plume can be modified.277–280 Nevertheless, the small size of the plasma treated area can lead to a low efficiency, or to long treatment times when treating large areas.281,282 Furthermore, although PJ arrays can be used to treat larger areas, they are hindered by the complex interaction between the individual jets and the use of vast amounts of expensive gases.276 Whether the direct plasma application of DBD is more effective than indirect plasma from jets has not been shown yet. As for the first DBD devices, these consisted of rigid plates which were not compatible with the curved human body. To anticipate this, various flexible variants of DBD have been developed and tested for the treatment of large areas with irregular surfaces.212,281,283–289
Floating electrode DBD (FE-DBD) is a DBD-based device which uses the treatment object, i.e. the patient, as a ground electrode,290 and requires additional safety measures. For FE-DBD, the surface to treat should be flat to ensure a uniform discharge.286,291,292 It can be regarded as a VDBD, for which the discharge occurs between the high voltage electrode and the grounded human body.293–295 Contrary to FE-DBD, with SDBD, the plasma is produced on the surface of the dielectric284,296–298 and does not require additional grounding to produce plasma. Extra grounding of the patient might be needed for safety regulations. Compared to SDBD, FE-DBD was more efficient in killing bacteria and produced less ozone, which is beneficial for biomedical applications.212,265,299,300
Thin films can be used as dielectric in DBD, which can be bent to treat different surface shapes, although bending can affect the discharge.287 The development of a flexible plasma source that can be used to treat different shapes is of great significance for plasma medicine. Important issues are optimising the plasma dose, and minimising side effects in the wound and adjacent healthy tissues.301 It might even be possible to use an adaptive plasma approach which is based on the ability to read the cellular response to CAP in real time and modify the composition and power of the plasma via a feedback mechanism.302
A fence-like plasma source might be used to generate a large area FE-DBD288,303,304 which can even be produced as curved variants to match the treatment area.288 These devices demonstrated good antibacterial effects and were operated at safe levels for human contact.288,305 Furthermore, wearable plasma sources might be used to treat large areas with irregular shapes.283,306
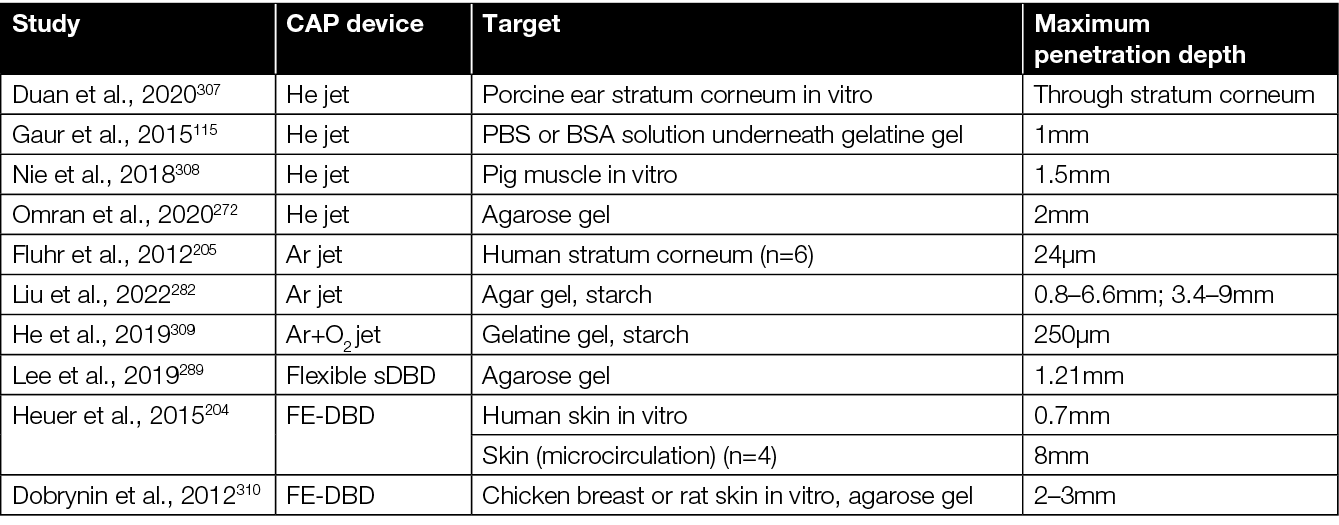
Reactive species produced by jet or DBD devices penetrate various tissues or substrates (Table 9), although the depth of penetration is still insufficient and exact mechanisms are unknown. Plasma settings and target composition do affect penetration of different reactive species; hydrophobic RONS is translocated more easily across the stratum corneum than hydrophilic RONS.307
Table 9: Penetration depth of RONS depends on CAP device and target surface.
Several plasma devices are relatively large or are not integrated into an operation room easily. Ambulant and out-patient treatment requires transport of CAP equipment, nurses and/or patients. Therefore, it will be more practical, comfortable, cost-efficient and effective (in terms of compliance and frequency of utilisation) when small and affordable portable devices are available. If indeed so, this creates potential for at-home applications. Portable CAP devices have been used for skincare,286,290 biomedical application,280,311 to treat residual tumour cells in surgical cavities,216 and as wearable fabrics.285
Although ozone is one of the reactive species involved in CAP-mediated bacterial killing,292 production of high levels of ozone by CAP can limit its use by HCPs. Ozone can cause irritation of skin, eyes and mucous membranes of the respiratory tract, as well as drowsiness, dizziness, headache and fatigue. Safety limits for ozone inhalation vary between 100μg/m3 (World Health Organization [WHO]312) and 120μg/m3 (European Union [EU]313) for 8 hours per day. Tests with different types of CAP devices have been performed on cells or skin in vitro14,74,80,204,212,314,315 or in vivo,212,316 and the results on cellular activity, inflammation or DNA damage were found to be within safe limits.
5.8 Plasma-activated solutions for indirect plasma treatment
The range of CAP applications in the biomedical area is limited by two characteristics – permeability and manipulation. CAP only affects superficial layers, while deeper layers of tissue (muscle, bone and organs) are difficult or not reached by CAP (Table 9). As for manipulation, the direct use of CAP is limited depending on the presence and shape of the device, potentially in combination with the characteristics of the area to treat. These limitations were also encountered in other industries, especially environmental technology and agriculture, where plasma-activated solutions (PAS) have already been used in water treatment and food preservation.317,318
Solutions such as distilled water, saline solution or cell culture media can be activated by treating them with plasma.261 After CAP treatment, PAS can be used for indirect plasma treatment. The benefits of this type of plasma treatment are highly controlled production parameters, higher plasma intensities or doses, limited safety issues, and availability of an off-the-shelf, easy-to-use and apply product for washing wounds or impregnating dressings. The shelf life of PAS solutions can range from hours to years depending on plasma dose and storage temperature,319–323 and can be improved by changing the composition of the medium.319
The various ROS and RNS that are produced during the plasma–liquid interaction of the CAP treatment of PAS diffuse into the liquids. As a result, PAS contains the same reactive species as plasma produced during direct treatment with DBD or PJ.296,324,325 The composition and efficacy of PAS for a specified condition can be improved by adjusting the production parameters. The chemistry of the reactive species in PAS and its application in the biomedical field was reviewed earlier by Zhou and colleagues.296
PAS has been found to be effective against bacteria,322,326–328 fungi and viruses.323,327,329–331 Initially, it was thought that acidification of the solution was the main antimicrobial factor of PAS. Studies, however, showed that both acidity and RONS influenced the efficacy of PAS for the inactivation of microorganisms.325,326,332–338 PAS can be used against bacterial biofilms339,340 and to disinfect medical devices. Furthermore, PAS was found effective for irrigating peritoneal cavities in a rat acute peritonitis model.341
5.9 Plasma-activated hydrogels for indirect plasma treatment
In addition to solutions, plasma can also be used to activate hydrogels. Hydrogels exhibit excellent water storage and absorption properties, as well as observed favourable biocompatibility.342–344 As such, they are used in a range of biomedical applications, including wound dressings,345,346 and may be loaded with antimicrobial agents, antibiotics or metal nanomaterials.347–349 Plasma-activated hydrogels (PAH) combine the properties of normal hydrogels with the capability to act as a carrier for reactive species.350–352 Furthermore, PAH have demonstrated the ability to preserve and release plasma-derived reactive species over extended periods of time,350,353 demonstrating long-term antimicrobial effects.350 PAH also presents advantages where plasma-activated liquids (PTLs) may be diluted and/or washed away when treating patients.354
Hydrogels can be activated via direct or indirect plasma treatment. Indirect activation of PAH involves either plasma treatment of the aqueous polymeric solution or use of plasma-activated water (PAW) instead of non-treated water prior to cross-linking, resulting in a hydrogel encapsulating plasma-generated RONS.351,354 Direct activation of PAH, however, involves plasma activation after cross-linking and hydrogel formation.351 The chemistry of the polymers used for hydrogels will affect the species and concentration of RONS generated.354 PAH have been formed from a wide range of polymers, including alginate,352 gelatine,355 methylcellulose,356 hydroxyethyl cellulose,351 carbomer 940,351 ammonium acryloyldimethy taurate/VP copolymers,351 polyethyleneoxyde based copolymers,357 and polyethyleneglycol–polylactide copolymers.353
Hydrogels also provide the ability to screen out short-lived and highly reactive species from direct plasma when placed between the plasma source and target. These include hydroxyl radicals (•OH) which have been linked to biological effects including phagocytosis, apoptosis and DNA damage.358 Longer lived species, such as H2O2, are still delivered through the hydrogel screen.358
PAH can also be used as drug delivery vehicles. Injectable hydrogels, treated with plasma prior to injection and cross-linking (in-situ polymerisation), have been proposed for drug delivery treatment in cancers.354 Hydrogels loaded with therapeutics such as antimicrobials, prior to plasma activation, have also been demonstrated, utilising a system whereby cationic drugs are loaded into sodium polyacrylate particles (PAA) contained within a secondary polymer matrix (e.g. PVA). Subsequent application of CAP releases the drug from the hydrogel “on demand”.359 To minimise systemic toxicity and improve tissue penetration of CAP, an injectable pluronic hydrogel was used as a delivery method. ROS and RNS in CAP were effectively preserved in the hydrogel and remained efficacious in inducing immunogenic cancer cell death after intratumoral injection.359
6. Safety aspects of plasma technology
6.1 Safety definition
Novel clinical and medical technologies and therapies need to be effective and safe. In 2013, two devices, one DBD operated in ambient air, and one atmospheric pressure Ar PJ, were approved for treating non-healing wounds and infected skin and appendices. Several other devices followed.360
When reviewing the topic of safety in plasma science, several things are important to note. First, clinical plasma devices are a class of therapeutic technologies, and not a single, uniform type of therapy. Instead, devices differ in geometries, discharge mechanisms, power and electric parameters, reactive species mixtures generated, and application. Therefore, safety assessment needs to be performed for each medical plasma device separately, not only from an academic point of view, but also – at least in Europe – under the requirements of Medical Device Regulation (MDR). It is important to note that there are a number of viewpoints on the safety of the application of a medical product. This includes regulatory aspects on the one hand, and caregiver and patient safety during medical product application on the other. In addition, molecular safety aspects should be considered. These may not affect overall health in general, but may be indicators that such effects may occur later, e.g. the “potentially carcinogenic” classification defined by the European Environment Agency (EEA) for suspected carcinogens. From a practical point of view, it is essential to acknowledge that ‘safe’ does not necessarily mean without side effects or risks. Safe also can also mean that potential side effects and risks are considered acceptable when weighed against the benefits of the specific therapy. Furthermore, such safety assessments are always relative and not absolute, as they are dependent on the boundary conditions of the specific applications defining the margins within a safe operation, e.g. maximum allowed plasma exposure times per area unit. It is therefore important to note that this chapter does not contain details on all aspects of all approved plasma devices (or their prototypes), since this chapter’s information relies on peer-reviewed international journal publications.
6.2 Physical safety of plasma devices
Medical gas plasma devices are systems based on physical modalities generated through electricity. Accordingly, several features of plasma need to be considered. Importantly, all items refer to the noted correct application of the device. If, for example, a PJ is held too close to its target, or a plasma DBD device is applied much longer than indicated, the plasma application can leave the indicated safety margins. The first to note is the temperature. Per definition, cold plasmas need to be cold, which is roughly defined as not being significantly above body temperature or 40˚C, a temperature at which protein denaturation can set in. Accordingly, it has been shown for several plasma devices that their temperatures are within this margin.21,53 The second noteworthy item is UV radiation. According to the International Commission on Non-Ionizing Radiation Protection (ICNIRP), the maximum effective (weighted) exposure of 30 J/m2 per day (8h; λ=180–400nm) should not be exceeded. This is the case for several plasma sources.21,361 The third item is the production of toxic gases, such as higher concentrations of ozone and nitrogen oxides. Analyses have shown gas levels below toxic thresholds.14,360 The fourth item is electrical safety, e.g. patient leakage current, which should be below 100µA, as is the case for all certified plasma devices in medicine.362–364 More on safety by design of plasma devices can also be found in Appendix II.
6.3 Safety of plasma application in humans
The following section reports on findings related to safety applications of CAP exposure to intact and wounded human skin, excluding tumour wounds and other dermatology disorders. For the latter, CAP treatment is not recommended to date, based on medical guidelines.
Most studies on patient safety and tolerability of CAP exposure were carried out for an Ar PJ approved as medical product.21 For its prototype, investigated prior to approval as medical product, treatments of the intact skin of fingertips of four human subjects at bactericidal exposure times were tolerated well. Specifically, the self-reported scores for paraesthesia, pain and heat on a scale from 0–10 for CAP exposure times 2–4 times longer (150–240 seconds) than bactericidal dosages (60 seconds) ranged from 0.5 to 2.0 for all three parameters investigated.165 For the Ar PJ, a complementary study in seven subjects found the treatment to be safe to skin physiology under clinical conditions in terms of a modest to absent change in trans-epidermal water loss and beta-carotenoid levels.205 In a third Ar PJ study in ten other volunteers, it was demonstrated by laser-scanning microscopy that the treatment of intact human skin does not change the properties of the upper skin layer.365 In addition, Ar PJ treatment of experimental laser wounds in five human subjects forearms promoted wound healing,98 while not inducing scar formation if investigated in a 1-year follow-up study,191 or a 5-year follow-up study in the same patients.366 None of them reported on any acute or long-term AEs. In the case of experimentally generated low pressure induced wounds in intact skin of six healthy volunteers, Ar PJ treatment was overall well tolerated, and induced only mild burning sensations in some subjects, which lasted only as long as the duration of the exposition (60 seconds).194 In four patients subjected to radial forearm free flap donor site surgery, creating acute wounds, Ar PJ exposure could be performed without occurrence of undesired AEs.367 With regard to Ar PJ treatment of chronic wounds, 37 patients, self-reporting on a numerical scale, reported less pain during CAP exposure compared to the application of standard wound antiseptics.170 In another clinical trial, 16 patients received either Ar PJ or antiseptic treatments, of which both treatments were tolerated well.175 Finally, in a prospective, randomised, placebo-controlled, patient-blinded clinical trial, Ar PJ treatments of chronic wounds of 29 patients were well tolerated.178 No SAEs related to the study intervention were described in Ar plasma exposure or the placebo group. The latter received a mock treatment with an Ar PJ being moved over the wound while its electric power was switched off. Other expected AEs, like for example scar formation, skin irritation and bleeding, were distributed evenly between both treatment groups. With time, all wounds healed, but Ar PJ-treated wounds healed significantly faster.178
Table 10 lists observed AEs and SAEs and, if available, their frequency in wound healing studies involving plasma, based on different aetiologies such as chronic ulcers, acute skin graft wounds, burn wounds and DFU. It has not been investigated whether the events listed are related causally to the plasma source used in the respective study. To date, there is no clinical study that investigated more than one plasma device. Due to the lack of a generally definable dosing concept, differences between plasma devices and their safety profiles may not be directly comparable. However, for each plasma device on the European market, at least one clinical study or case collection has reported its safety profile.
Table 10: Overview of AE/SAE reported in clinical studies involving plasma treatment.
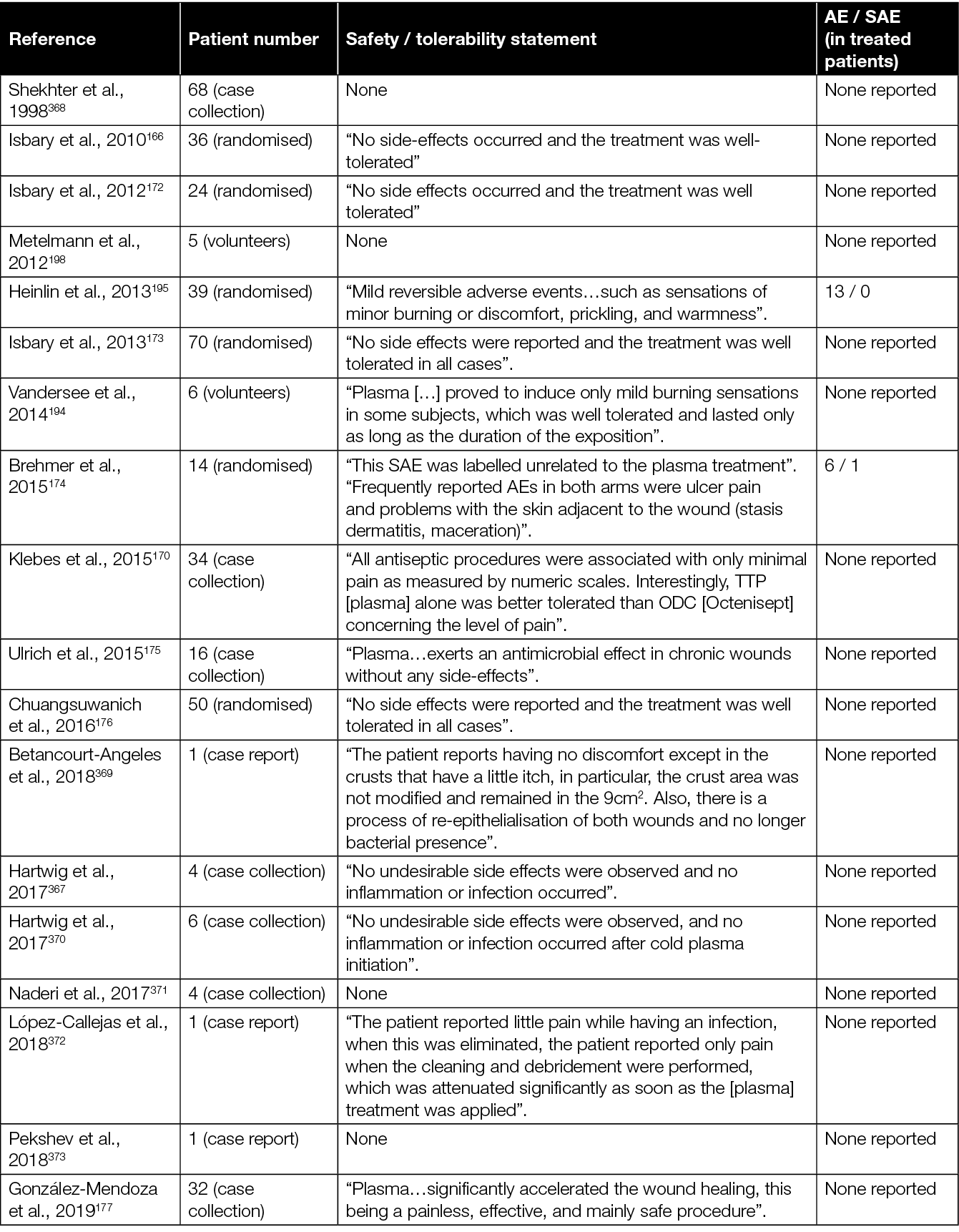

Specifically, regarding plasma sources approved for wound treatments in Europe for ambient air-operated or Ar gas-operated DBD, several studies were performed to assess the safety of treatment in human probands and patients suffering from chronic wounds. Acute wound treatment with an Ar-driven DBD was well tolerated in 34 patients.195 Exposure with the same regulatory approved plasma source, or its investigative precursor prototype, was also well tolerated in a total of 130 patients suffering from chronic wounds described in three reports.166,172,173 A single centre, two-armed, open, prospective RCT in 14 patients suffering from chronic wounds reported that treatments with another approved air-operated DBD were well tolerated.174 Six patients indicated some pain and mild hyperthermia. One SAE was reported, which was unrelated to the treatment.
Recently, the first regulatory approved plasma plaster patch air-operated DBD was investigated in a randomised, multicentre clinical trial in 47 chronic wound patients, where the control group received standard wound therapy according to the current guideline without CAP therapy. In an interim analysis, no SAEs were reported in both the CAP treatment and standard wound treatment group.187 Some patients perceive CAP treatment as tickle or mild burning during treatment, but not afterwards. Available studies seem to indicate an evidence-based favourable safety profile with regard to the exposure to, and the treatment of, human skin and acute and chronic wounds.
In September 2023, the German Federal Joint Committee (G-BA, Gemeinsamer Bundesausschuss), an institute independent of the German Ministry of Health authorised to make legally binding regulations and directives for German healthcare providers and payers, commissioned the manufacturers of CAP devices to supply clinical data in order to secure future treatment reimbursement. In response, three German manufacturers of cold plasma technologies have joined forces, and commissioned a scientific institute to perform a randomised, double-blinded, multicentre trial with a planned population of 700 patients. This trial will generate the first data on the direct comparability of the efficacy and safety of different plasma devices.375
6.4 Safety from the molecular and preclinical perspective
Preclinical and molecular studies are important to analyse the potential risks of new therapeutics, especially regarding their potential carcinogenic action. There are several common assays, of which two are the Organization for Economic Co-operation and Development (OECD) 471 Bacterial Reverse Mutation Test, also known as AMES test, and the OECD 490e In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, performed in mouse lymphoma cells. There are no publications available with results of either of these commonly used assays with any of the certified medical plasma devices. However, results with two other OECD assays with CAP devices do exist: the cytokinesis-block micronucleus cytome assay In Vitro Mammalian Cell Micronucleus Test (OECD 487) for testing chemicals, also referred to as cytokinesis-block micronucleus or MN test;376 and the second assay which is the hypoxanthine phosphoribosyl transferase (HPRT) test (OECD 476), validated for industry purposes. The studies performed with these two assays are summarised in Table 11. In these studies, where CAP was applied to the cells directly, without any time delays using additional carrier solutions, none showed evidence of plasma-induced genotoxicity in the in vitro laboratory cell culture models.
Table 11: Overview of OECD 476 and 487 assays performed with CAP devices.
In addition to these in vitro studies, three critical in vivo publications are available. The first is a quantitative genotoxicity evaluation by MN test in living chicken embryos. In a comparison of exposure times and conditions, i.e. liquid-covered versus dry tissue, no genotoxicity could be shown for a clinically compliant atmospheric pressure Ar PJ.382 The second study investigated repetitive Ar PJ treatments (seven applications in 2 weeks) in experimental wounds in mice, which were analysed after 12 months.144 When investigated with PET-CT, MRI and histopathological analysis, none of the CAP-treated animals showed any tumour growth, abnormal tissue proliferation or fibrosis. The third animal study investigated mucosal wounds treated with repeated Ar PJ exposure, i.e. once a month for 12 months at two different exposure times.161 None of the 43 mice showed any tissue abnormality or oncological formation. Additional performed mouse studies also observed no malignant or pre-malignant tissue formation in intact skin after long-term Ar PJ application for 3 months.383
7. Regulatory submission and approval: a critical step to reach patient bedsides
7.1 Regulators: addressing high wound healing unmet need
There are many critical milestones on the development path of an innovative and effective therapeutic solution. However, few of these milestones are as significant as the process of obtaining regulatory approval. After all, when a medicinal product or medical device does not finalise its journey from bench to bedside by gaining market access, all previous product development investments have been futile. That this simple fact also applies to potentially new wound healing solutions was confirmed again recently by the US Food and Drug Administration (FDA). In a perspective publication by Verma et al., the FDA shares that it understands that innovative product development is critical for addressing the significantly increasing wound healing pathology prevalence and incidence.384 Due to its high unmet medical need, and relatively limited research and funding, the FDA identifies non-healing chronic wounds as an area of priority, and therefore intends to help advance product development for non-healing chronic wounds for the ultimate betterment of patients.
Although the recognition and proffered help by the FDA are certainly to be appreciated, it will be interesting to learn what this help might mean for the regulatory obligations and requirements of new innovative wound healing solutions specifically. For example, Verma et al. confirm that “Regarding challenges in clinical trial execution, difficulties with patient enrolment, heterogeneous study designs with varying standard of care protocols and study populations, and difficulty achieving the most commonly utilized primary efficacy endpoint of complete wound healing result in high rates of trial failure, singly as well as collectively further impeding the development of innovative products”.385–389 It is therefore not surprising that, according to the authors, “FDA understands that there is external stakeholder interest to provide updated guidance to the 2006 ‘Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment’, especially since no new chemical entities have received FDA efficacy approval for the treatment of chronic wounds since decades, in part due to an inability to reach the FDA accepted end point of ‘complete wound closure’”.385–387,390 And although the FDA reminds stakeholders that “guidance documents are a set of recommendations. An alternate approach other than one proposed in guidance documents may be used”, the frequent inability to reach the full wound closure end point within a clinical study timeframe, and the repudiation of alternative healing end points for regulatory approval, might demonstrate the intricate and delicate nature of this last critical product development step in everyday practice.384,385
7.2 Determining mechanism of action and dose response
Although European regulators do not seem to share the FDA wound care focussed insight, attention and urgency, the base principles of regulatory evaluation of new wound healing therapies are surprisingly similar between the USA and Europe. Per definition, a regulatory evaluation classifies a new therapeutic solution into predefined categories, summarised crudely as a determination of what it is, what it does, and how it does it. The determination of the mechanism of action is one of the first basic elements of this process.
In 1857, Johann Heinrich Wilhelm Geissler, a German physicist and glassblower, produced a sealed glass tube filled with a “noble” gas and two electrodes at each end; this was the great-grandfather of the neon light tube and modern-day energy saving compact fluorescent lamp (CFL).391 Geissler did so on the request of Julius Plücker, professor of physics at the University of Bonn, who one year later published their observations with the ‘Geissler tube’. Inspired by their findings, Sir William Crookes presented his findings on “streams of radiant matter” after applying high voltages to the poles of “highly exhausted glass tubes” in 1897.392 Since then, “radiant matter”, dubbed plasma in 1928 by Irving Langmuir, has found its way to several medical applications (Chapters 4 and 5). Due to its complexity and multifaceted nature, the exact mechanism of action of CAP in wound healing is unknown, and therefore remains an ongoing subject for further study. As also described in detail in Appendix I of this EWMA document, plasma is an ionised gas composed of charged particles, electronically excited atoms and molecules, radicals and UV photons.99 The type of energy input, voltage or discharge power, gas component, gas pressure, and radiation type of electric field determine the exact composition and properties of produced plasma.393 By varying these plasma generation parameters, a low-temperature state (<40˚C) therapeutic plasma can be produced, with temperature values of ions and neutral particles much lower than those of electrons.8 During discharge, ROS, RNS, RONS, charged particles, and electromagnetic and UV radiation are formed, which can influence biological systems.394
As was also noted earlier in this document, it is still a significant subject of research to determine how the RONS are delivered into biological targets, and what the exact interaction with various components of a tissue is, especially when considering the lifetime, diffusion rate and major physical barriers to traverse.395 Despite this, the diffusion and delivery of plasma-generated RONS, or the stimulation of intracellular RONS generating mechanisms as a result of cold plasma treatment, have been suggested to regulate cell activity in both intensity- and time-dependent manners, at least indicating some kind of dose-response relationship.102,396 Appropriate low levels of ROS produced by cold plasma treatment at a suitable intensity and time have been reported to enhance directly the proliferation and migration of skin-related cells, extracellular matrix (ECM) protein synthesis, cytoskeletal architecture, cytokine and growth factor production, and changes of junctional proteins between the cells, and to increase wound healing enhancing angiogenesis and (micro) circulation (FGF, angiopoietin, VEGF, TGF) (Figures 5 & 6).57,58,150,168,202,397–401
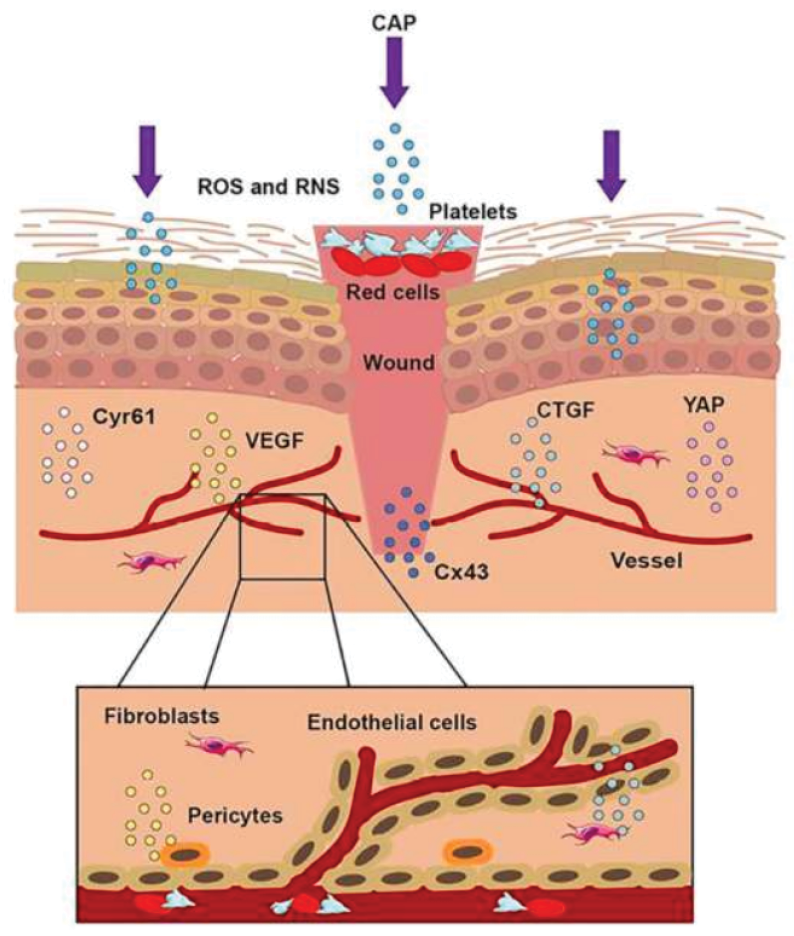
Figure 5: The treatment of CAP on a wound.
When the skin was injured, the first step was to form a blood scab to protect the wound. CAP could accomplish wound healing through short-lived and long-lived ROS and RNS. CAP could promote the formation of new blood vessels, strengthen the release of connective tissue growth factor (CTGF) and vascular endothelial growth factor (VEGF), activate the yes-associated protein (YAP) pathway, and upregulate the expression of Connexin 43 (Cx43) and Cysteine-rich angiogenic inducer 61 (Cyr61). Reprinted with kind permission 58
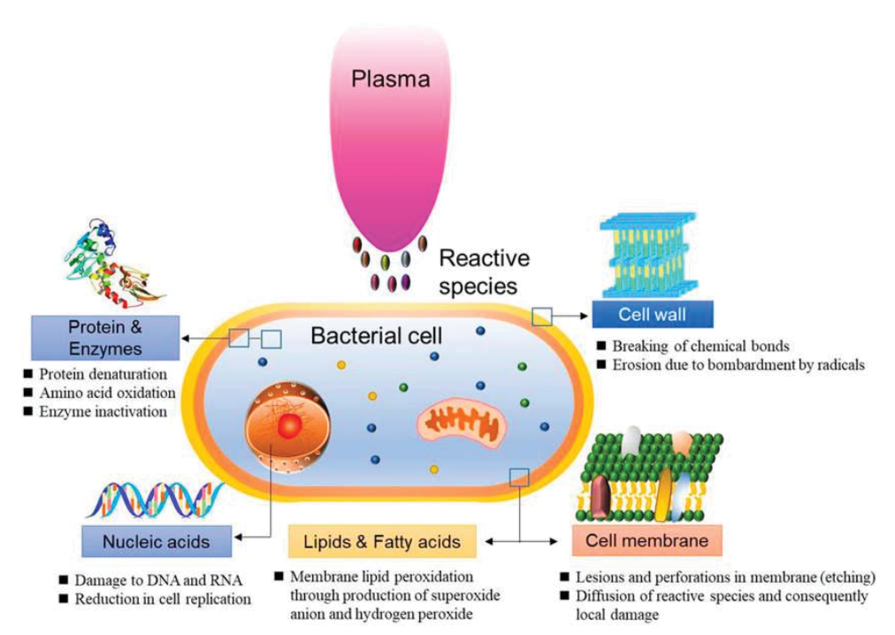
Figure 6: Schematic representation of bacterial reduction induced by CAP58
High levels of ROS, however, produced at a high intensity or over a long period of time, are seen to inhibit cell proliferation of, for example, endothelial cells, keratinocytes and fibroblasts.57,102,183,200,402,403 Furthermore, CAP-induced immunomodulation was observed, showing that the time to maximum ROS production in human polymorphonuclear cells (PMN)/granulocytes could be reduced in a dose dependant manner, while PMNs showed enhanced integrin and selectin expression, and an increase of activation markers on their cell surfaces as a result of more CAP treatment.404
7.3 What it does: clinical indications
However, when reviewing the fast-growing body of CAP peer-reviewed publications, it becomes clear that the most frequent and prominent clinical observation is the direct antimicrobial efficacy of CAP. In wounds, it is seen to reduce infection and bacterial load significantly, two factors which, according to many, are major contributors to impaired and incomplete wound healing. As can be read in more detail elsewhere in this document, CAP has shown its benefits in both in vitro and in vivo studies, where the reduction of various bacteria and fungi become apparent immediately after treatment.153,212,405,406 As early as 2010 and 2012, Isbary et al. reported that 2- and 5-minute clinical CAP treatments were able to decrease the microbial load in different types of chronic ulcers, including diabetic ulcers, irrespective of bacterial species.14,166,172 Due to its strong broad-spectrum antimicrobial ability to reduce microbial loads in wounds effectively, some position CAP as a potential alternative or adjuvant to conventional antibiotics for the treatment of bacterial infections, including those caused by antibiotic-resistant pathogens.407–409. Although the formation and presence of biofilms can impair the wound treatment efficacy of CAP significantly, and potential bacterial resistance has been observed, study results still show CAP to be a promising addition for the treatment of biofilm containing wounds.200,255,406,410–412
More recently, the efficacy of CAP for the treatment of DFU was investigated in several randomised trails. And although many of these studies were troubled by small population sizes, most did show beneficial CAP treatment effects in terms of bacterial load, wound surface reduction, and time to closure.178–180,182,186,190,413 It is therefore understandable that the 2022 German guideline, published on the therapeutic use of cold plasma, concludes that the main clinical benefit of CAP is the effective deactivation of microorganisms, including multi-resistant pathogens, followed subsequently by the stimulation of cell proliferation and micro-circulation, resulting in tissue regeneration. Its strong recommendation towards clinicians therefore is to consider the treatment of chronic and infected wounds with CAP.199
7.4 Next regulatory step: determining applicable legislation
Medical devices in the EU and European Free Trade Association (EFTA) countries are regulated by the MDR (EU 2017/745) and supervised by the Notified Bodies, augmented sometimes by scientific consultation of the European Medicine Agency.414,415 Medicinal products, however, are regulated by different legislation which is enforced by European Medicine Agency and the national Competent Authorities direct, i.e. Regulation (EC) 726/2004 and Directive 2001/83/EC, although the latter will be replaced by a new Pharmaceutical Regulation in the near future.416–420
Article 1 of Directive 2001/83/EC defines a medicinal product as “(a) any substance or combination of substances presented as having properties for treating or preventing disease in human beings; or (b) any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis”.417
Looking at Article 2 of the MDR, it defines a medical device as “any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease, [...] and which does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means”.414 From this, one could conclude that both definitions are mutually exclusive.
This means that in practice, whether in the USA or in Europe, regulators will always first attempt to determine which legislation applies, determining whether the submitted application should be regarded a medicinal product or medical device. Furthermore, this classification will also define which authority will be responsible for its evaluation, potential approval, and continued review after market entry. In their 2020 comprehensive review, von Woedtke et al. listed the EU available clinical plasma devices as medical devices, CE certification Class IIa according to the European Council Directive 93/42/EEC.421 This directive, also known as the Medical Device Directive, was, however, superseded in May 2021 by Regulation (EU) 2017/745, the MDR.414,422 This is relevant, since all medical devices approved in the EU under the former Medical Device Directive 93/42/EC, will need to obtain renewed market approval, as defined in Article 120 of MDR and its later amendment, Regulation (EU) 2023/607.414,418,423–426 Streamlining this process, submission for approval is now possible at a pan-European level, offering a more efficient route to the previous national level regulatory submission.427,428
7.5 Innovation at the edge of regulation
Regulatory frameworks are, due to their pre-defined and reactive nature, challenged continuously in everyday practice by the introduction of new therapies, especially when such therapies transcend pre-existing regulatory axioms and definitions due to their innovative approach.429,430 Paradoxically, that can mean that innovative therapeutic solutions, critical to address strong unmet medical needs, are halted or delayed significantly on their way to the bedside of patients. In order to anticipate this, the Borderline Manual by the Borderline and Classification Working Group (BCWG) can be consulted.431–434 The BCWG is chaired by the European Commission, and consists of representatives of Competent Authorities, the national medicine agencies of all member states, and a number of stakeholder associations as observers.
In short, the Borderline Manual is a record of previous cases where the classification of a submitted new product was unclear, and therefore was discussed individually within BCWG. In instances where all guidance is inconclusive, applicants, who are unclear on the correct classification of their product, should consult a national Competent Authority, and provide information on their product’s composition and constituents, a scientific explanation of its mode of action, and its intended indication for use. National Competent Authorities subsequently classify borderline products either as medicinal products or as medical devices on a case-by-case basis.428 With remaining uncertainty or dispute, cases can, and have been, submitted to the European Court of Justice for final deliberation and verdict, thus creating jurisprudence.435,436
This process, and all its challenges in real life, can be illustrated for a new imaginary, but highly innovative CAP wound treatment device. As outlined, the first question for such a device to answer would be whether the device should be regarded a medical device or a medicinal product. As described by von Woedtke et al. and CAP device manufacturer data, currently CE-marked CAP devices are approved as either medical devices class IIa or class IIb.421 The definitions of medical devices class IIa of the former Medical Device Directive, and the MDR now in force, do not differ significantly. According to MDR, “all non-invasive devices which come into contact with injured skin or mucous membrane are classified as [...] class IIa if they are principally intended to manage the micro-environment of injured skin or mucous membrane”.414 Although the earlier mentioned publications indeed do show that CAP seems to manage the micro-environment successfully, one could reflect on whether its clinical efficacy induces actively chronic wounds to close. If indeed our innovative CAP device does induce wound closure actively, the clinical efficacy of CAP could be more in line with the definition of a class IIb medical device, i.e. “[...] class IIb if they are intended to be used principally for injuries to skin which have breached the dermis or mucous membrane and can only heal by secondary intent”.414 The overarching question here, however, might not be whether the primary clinical wound healing efficacy of CAP is passive or active, but more fundamental, whether CAP “does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means”.417
Based on the available literature and guidelines, it is evident that the antimicrobial properties of CAP are regarded not as its sole, but certainly as its most prominent, clinical effect. And although all previous reports mention that the exact mechanism of action is unclear and subject to further study, the majority identify ROS as an active, and in some instances, dose-response dependant clinical component.102,396
7.6 The Borderline Manual
In cases of regulatory uncertainly, one of the first actions is to compare to previously approved products, decisions and, in some cases, jurisprudence. Following this standard procedure for the imaginary CAP device, one can find that, in the 2019 edition of the Borderline Manual, antimicrobial photodynamic therapy (APDT) disinfection systems were discussed. APDT is intended for the decolonisation of potentially-pathogenic bacteria, including MRSA, from the oral cavity or anterior nasal passages.431 The photosensitiser solutions used in APDT systems produce ROS, which are responsible for lethally disrupting the microbial cell wall. Since the primary action of the photosensitisers is not physical, their activation of anti-bacterial efficacy is not achieved via physical means, and they are sold typically separate from the energy supplying/ activating (laser) device, they were judged to not be medical devices. The lasers activating these ROS producing photosensitisers, however, were marked as a medical device class IIa.431 Although the involvement of ROS in this case makes it appear comparable to our imaginary CAP device, the fact that the ROS producing photosensitisers are a separate, independent component, makes comparability, and thus applicability questionable.
The 2022 editions of the Borderline Manual seem to give more guidance. This time, the Medical Device Coordination Group (MDCG) 2022-5 notes carefully that although “not an exhaustive criterion, the presence of a dose-response correlation is indicative of a pharmacological, metabolic or immunological mode of action”,432 while the December edition of the Borderline Manual of that year discusses another ROS based disinfectant.433 In the latter case, the outcome reads that “the antimicrobial action of ROS, which is considered as the principal intended action, should be considered pharmacological, immunological or metabolic mode of action. The decision of ECJ ruling 6 September 2012, case C-308/11, also supports that such antimicrobial actions on the human body should be considered pharmacological. Consequently, considering the principal mode of action, this product should not be qualified as a medical device”.434,436
Further complicating matters is the conclusion that, as pointed out by the MDCG, in the discussed cases the ROS (generating) components can be viewed as separate products which can be purchased separately. In the case of CAP, however, the ROS-containing plasma is only available, and can only be generated in conjunction with a plasma-generating device. Due to this, some might suggest that CAP devices might be combination devices as described in Special Rule 14 of MDR: “All devices incorporating, as an integral part, a substance which, if used separately, can be considered to be a medicinal product, as defined in point 2 of Article 1 of Directive 2001/83/EC, [...], and that has an action ancillary to that of the devices, are classified as class III”.414 Such a classification would indeed recognise the disinfecting ROS aspect of CAP plasma, but struggle to show how it could be used as a separate substance.
Another approach can be to consider the mode of action of CAP devices as not of a ;pharmacological, immunological or metabolic; but as of a physical nature. Examples of this can also be found in the Borderline Manual. The May 2022 edition states that gases intended to be used in anaesthesia and inhalation therapy are regarded medicinal products. Should these gases, however, be used with a physical mode of action, e.g. inflation during minimal access surgery, then these products can be regarded medical devices. Then again, later, confusingly, it can also be read that “the intention of the manufacturer regarding the action of the substance on the device or on the body is irrelevant for the decision on whether the substance would be considered a medicinal product, because intentionality is not mentioned in the MDR legal provisions under discussion”.432
Finally, in the latest December 2022 edition of the Borderline Manual, there is a more CAP comparable, but not fully compatible, case.434 Here, the MDCG discusses whether Ar coagulation units should be regarded as medicinal products or medical devices. These units are used in APC, a mono-polar electrosurgical technique, where the Ar plasma functions as the application electrode, making the intervention contactless. In their evaluation and outcome, the EU Competent Authority representatives within the BCWG conclude that Ar coagulation units are active therapeutic devices. More specifically, BCWG notes that such units influence directly the APC, where electrical energy is administered to body tissues by an Ar plasma stream, thus functioning as an application electrode. Based on a risk evaluation considering the common site of application and nature and density of the applied energy, BCWG classified Ar coagulation units as class IIb medical devices according to Rule 9 of MDR.434
7.7 The right regulation: checks, balances and choices
In order to become a new, useful tool at the bedside of patients instead of vapourware, new wound healing solutions must obtain their regulatory and market access approval expeditiously. After all, without the proper regulatory approval, a therapy can be life- and world-changing, but will never reach the patients it is supposed to help. With this thought in mind, one can ruminate on whether the current regulatory frameworks are flexible and adaptive enough to facilitate new and innovative therapeutic developments. As the imaginary new innovative cold plasma wound healing device example here has shown, the EU regulatory approach could hold an inherent rigidity which might not facilitate the introduction and availability of new effective wound healing therapies necessarily. Then again, some will argue rightly that regulatory frameworks and authorities first and foremost should safeguard a stable, albeit less flexible, base where new products just need to fit in and comply with the already defined and applicable standards and policies. Either way, the main objective must remain to provide patients with optimal care in treating their morbidity, and thus enabling their physicians to do so safely with the most effective tools possible. With this prime directive in mind, it seems self-explanatory and imperative to adjust a regulatory framework continuously, albeit vigilantly, to welcome never-before-encountered therapeutic approaches. Because, as the groundbreaking initiative by FDA has underlined again, ever increasing incidence, prevalence, and healthcare budget impact numbers do call urgently for new, inventive, multidisciplinary and effective solutions for chronic, non-healing wounds.384 It would therefore be exciting to see European regulatory authorities follow the lead of their American counterparts, and extend their continuous effort and commitment to ensure a “sound and flexible regulatory system”, as defined by the European Commission in their Pharmaceutical Strategy for Europe, to include the globally recognised unmet need of non-healing wounds.415
8. CAP in every day clinical wound practice
8.1 The potential of CAP as therapy for wound healing
Despite the fact that plasmas have been known for more than a century (paragraph 7.2), developments in plasma medicine only picked up speed in the 1990s with the improved availability of CAP technology for antibacterial treatment of medical devices, surfaces and materials (Chapter 2). The application of CAP to non-healing wounds and infections is one of earliest realised medical applications of CAP, with publications on clinical studies from 2010 onwards.166 These studies describe a broad range of wounds and protocols, with studies becoming more sophisticated over time (Chapters 3–5).
Studies of new technologies in wound healing are notoriously difficult. Conducting clinical trials at scale within a representative population, with suitable randomisation, blinding of participants and patients to the intervention, and inclusion of a control arm, is a difficult process even for the most established technologies. This is further complicated by selection of suitable endpoints. To aid with this process, ample standards and guidelines provide guidance for trials in wound healing.1,437–441
For CAP, this has meant that the approach into wound healing has been gradual, with many of the first studies resembling clinical case studies, later followed by more standardised, albeit frequently small scale, open label investigations. In 2023, Li et al. reported in the BMJ that they opened the undertaking of systematic review and meta-analysis of studies to date in CAP in wound healing.413 They note that, although studies of CAP in DFU report that CAP could improve wound healing speed compared to conventional therapy, to date, studies “are small and not sufficiently representative”. Nevertheless, the potential of CAP for treating hard-to-heal wounds has not gone unnoticed by the clinical community.
8.2 Working with CAP devices
Despite many clinical studies conducted and planned (Chapter 4–6), various issues remain to be addressed with regard to CAP since they might complicate and hamper its implementation in every day clinical practice. These open questions do not only concern academic studies regarding, for example, the exact mode of action, but also more practical queries which might be considered by wound healing HCPs.
In the previous chapters, an overview of the various types, modes of operation and specific characteristics and limitations of CAP devices was given (Chapters 2 and 5). A consideration for everyday therapeutic operation of medical devices is, however, also their practicability. Devices generating CAP can vary in size, geometry and technical setup, not seldom depending on the intended use.442 This might not be an issue for the stationary treatment of inpatients or ambulant patients able to visit a practice, but could present a bulky confutation for many regions where post-hospitalisation wound care and the treatment of non-healing wounds is performed mainly as in-home care. However, with device design progressing continuously, smaller more mobile CAP devices suitable for home care have become available, enabling care givers more flexibility in their wound healing treatment (Figure 11).
Another consideration can be that efficacy can be impacted considerably when a device is less flexible and/or suitable for the large variety of wounds patients present to the wound healing HCPs at their practice. For example, a flat CAP patch might not be well suited for wound cavities or very small wounds, while a hand-held PJ will prove impractical for wounds of larger sizes (Figure 7). Furthermore, case studies and RCTs tend to focus on results of CAP treatment of chronic wounds. Unfortunately, the in-practice ever-present variations and limitations with regard to local wound conditions are commonly under-reported. With this in mind, it is essential that HCPs weigh in the physiological and pathological variability that every patient will present inevitably, which here also means that CAP cannot be a ‘one size fits all’ wound therapy. Practically, CAP should be regarded as a valuable addition for the treatment of chronic wounds, which, like every medical intervention, will need tailoring, evaluation and potential adaptation by experienced wound care HCPs for optimal efficacy and result.
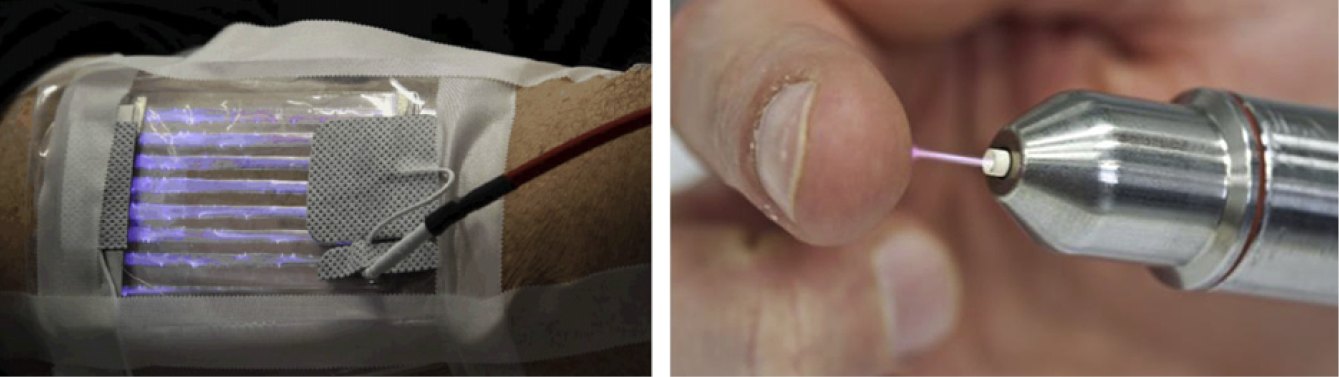
Figure 7: The efficacy of different device types.
Left) investigative flexible SDBD prototype (8.5x13cm2) applicable for treatment of larger curved surfaces, placed here on the proximal anterior forearm (INP Greifswald, Germany);13 Right) an Ar PJ.360
Another of such clinical considerations is the penetration depth of CAP, an ongoing task in plasma medicine research, especially since the distribution of bacteria in chronic non-healing wounds has been seen to vary significantly.443 When reviewing, a distinction must be made between real penetration of reactive species into deeper liquid or tissue layers, respectively, and in-depth biological effectiveness (Table 9). Diffusion of plasma-originated reactive species depends strongly on the reactivity of the species itself, as well as the characteristics of the (biological) environment, and the content of potential reaction partners. Available in vitro research does, however, suggest that the physical effects of plasma can be seen to depths of several hundred micrometres within tissues. Plasma-derived RONS are likely delivered into tissues for millimetres, since speciation reveals that RONS delivered by plasma into tissues and tissue fluid are predominately stable secondary RONS (e.g. H2O2, NO2−, and NO3−).18,26 Furthermore, plasma generation of RONS within a hydrated target is influenced by the target matrix, which can enhance or reduce the RONS concentrations and act as a reservoir of RONS. This suggests concentrations exceeding hundreds of micromoles, even at depths of several millimetres within tissue.395 This behaviour might be useful, especially since Melone et al. estimated that at least 78% of all chronic wounds are covered with biofilm or slough.444 Therefore, pre-procedural debridement as part of a CAP wound treatment plan is advised strongly since it will increase CAP efficacy by better targeting and killing of any remaining (biofilm) bacteria in the wound bed and wound margin.410,412,445
8.3 CAP and modalities of treatment
With a significant number of studies conducted in chronic wounds, the optimal treatment duration and frequency have proven difficult to establish. In practice, CAP device experts should instruct HCPs on the appropriate operation, since results can and will vary per device, or indeed per subsequent wound treatment (for some real-life examples, see Figures 8–11). Accordingly, no evidence-based standards on plasma application and therapy frequency exist to date.199 No doubt due to the broad range of different plasma technologies, devices and clinical study related variables, CAP treatment durations from 30 seconds per cm2 up to 7 minutes have been reported (Table 6). Furthermore, CAP treatment schedules varied from just once a week, multiple times per week, to even repetitive use up to five times a day over a period of several weeks. Although it is difficult to generalise due to the earlier mentioned variability, there seem to be indications that CAP wound treatment once or twice a week at the most might be sufficient,446 while for sole antimicrobial purposes, a more frequent treatment, once daily for 1 week, is suggested.166 This therapeutic approach is confirmed further by the observation of Moelleken and colleagues that results of a once weekly treatment were not inferior to those for CAP treatment three times a week. For such a regimen, investigators also found that treatment once a week was organisationally easier and more economical to implement in clinical routine.446
Figure 8: Wound healing example: 80-year-old male.
Male, 80 years old, with persistent, multidrug-resistant infected DFU for 19 months. Treated with microwave Ar CAP (Adtec Healthcare Limited® SteriPlas™), in combination with standard care (no antibiotics) for 16 weeks. Treatment frequency: once a week for 3 minutes. Clinical situation at: A) therapy start; B) after debridement and first treatment; and C) 16 weeks of treatment. Kettering General Hospital NHS Foundation Trust, United Kingdom.
Figure 9: Wound healing example: 80-year-old male.
Male, 80 years with persistent, multidrug-resistant infected DFU for 19 months. Treated with microwave Ar CAP (Adtec Healthcare Limited® SteriPlas™), in combination with standard care (no antibiotics) for 56 weeks. Treatment frequency: once a week for 3 minutes. Clinical situation at: A) therapy start; B) after 24 weeks; and C) 56 weeks of treatment. Kettering General Hospital NHS Foundation Trust, United Kingdom.
Figure 10: Wound healing example: 68-year-old female.
Female, 68 years with persistent DFU for more than a year. Treated with VDBD CAP (ColdPlasmaTech® CPT®patch), in combination with conventional treatment for 8 weeks. Treatment frequency: twice a week for 2 minutes. Clinical situation at: A) therapy start; and B) after 4 weeks of treatment. Martin Luther Hospital, Berlin, Germany.
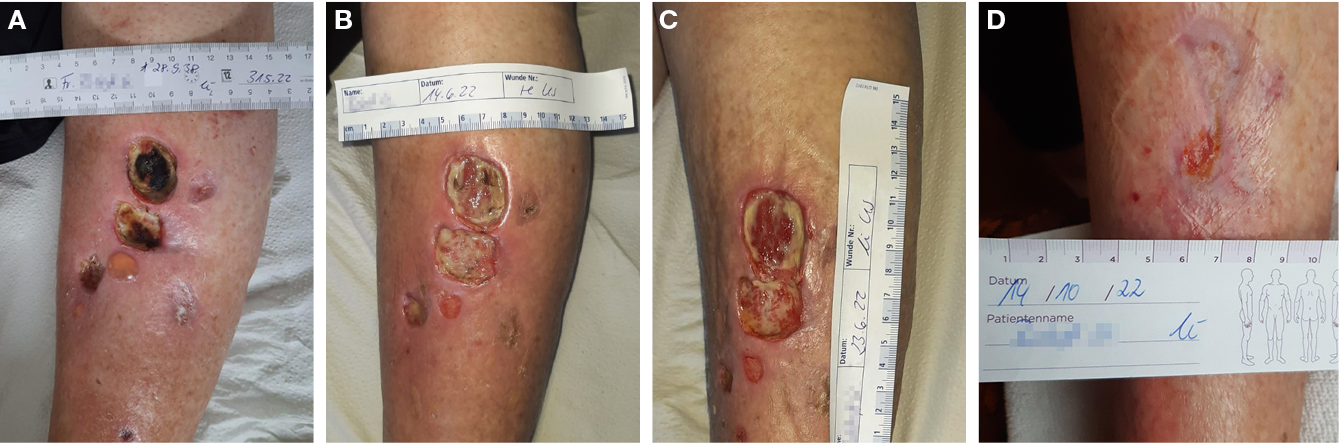
Figure 11: Wound healing example: 84-year-old female.
Female, 84 years with persistent infected VLU for more than a year. Treated with VDBD CAP (ColdPlasmaTech® CPT®patch), in combination with conventional treatment. Treatment regimen: twice a week for 2 minutes. Clinical situation at: A) therapy start; B) after debridement at 2 weeks; C) after debridement at 4 weeks; and D) after 20 weeks of treatment. MeckCura Plegedienst GmbH ambulant/ homecare, Rostock, Germany.
When considering optimal dosing in the everyday clinical practice for wound treatment, it is worth mentioning that clinical observations report that prolonged exposure of the skin/wound can potentially cause adverse effects such as dehydration, hypoxia and skin damage.137,143,149,447 Another basic therapeutic consideration might be how to minimise heat transfer to the patient, thus preventing potential thermal tissue damage and increased erythema.212 The issue of potential plasma induced heat damage to tissues will be addressed in a new, upcoming standard, a revision of the earlier German standard DIN SPEC 91315:2014-06 – General Requirements For Plasma Sources In Medicine (publication planned for 2024). Contrary to the earlier 2014 standard448 which focused on plasma temperatures exclusively, this revision will focus on the more relevant aspects of tissue temperature as a result of energy transfer from the plasma to the tissue, using ex vivo models in combination with optical methods for temperature quantification. Manufacturers of medical plasma devices have been aware and diligent of these treatment risks during the product development of their devices, subsequently integrating technical measures in the product design, preventing patients from excessive energy input, thus keeping temperature increases under the maximum temperature of 39˚C as specified by the earlier German DIN standard.448 For some of the medical plasma devices available on the market currently, this means that their (flat) sources produce sufficiently low tissue temperatures, mainly by specific electric signal characteristics, while alternative jet-like configurations apply physical spacers to prevent direct interaction of ‘hot’ parts of the plasma with the patient’s tissue. Nevertheless, it remains important to optimise the plasma dose, and find the delicate balance between both maximised CAP efficacy and minimised AEs in the wound and adjacent healthy tissues.301 Here, more clinical studies can and will help to gain further insight into the optimal treatment modalities for specific patient characteristics.
8.4 CAP reaching the patient with chronic wounds
Even though the clinical wherewithal of CAP for the treatment of chronic and hard-to-heal wounds is most promising, it can only unfold its full beneficial potential when patients have optimal access to said treatment. Where nowadays more and more younger patients (<65 years) present themselves with chronic and hard-to-heal wounds due to the rise of lifestyle disease, the typical chronic wound patient is (still) elderly and multi-morbid. This causes a sluggishness in visiting outpatient clinics and hospitals several times a week, the location where CAP treatments are mainly performed currently. Multiple weekly applications, such as those for antimicrobial wound therapies, must be considered difficult in this context.446 Therefore, sufficient evidence, mapping the efficacy of CAP, and investigating especially the minimum effective dose (e.g. once-weekly application to the wound) is urgently needed. Furthermore, the availability of affordable, small, portable devices, such as for example an air-fed CAP device already used to treat residual tumour cells in surgical cavities,216 would increase the therapeutic impact of CAP significantly. As it stands, CAP application by appropriately trained HCPs will most likely be of benefit to wound patients who are expected to gain the largest increase in QoL by CAP treatment. Having said this, however, even with CAP as an addition to the wound healing toolbox to control the bacterial burden of a wound, it should not invite HCPs to not diagnose and treat the underlying (causal) morbidity, as seen frequently in Europe today.449
9. Appendix I: The physics of CAP
9.1 Introduction
This chapter aims to help develop a basic understanding of the physics relevant to CAP. For this purpose, mathematical equations are avoided deliberately, and the focus is instead on phenomenological descriptions.
CAP is a special variant of physical plasma. In physics, plasma refers to a gaseous state of matter in which the integrity of atoms and molecules is at least partially violated. Being a natural phenomenon, the plasma state is well known to (astro)physicists as 99.9% of the radiating matter in universe. Materials scientists and engineers are well acquainted with physical plasma, having engineered it for a huge variety of (industrial) applications for decades. These applications encompass, for example, surface functionalisation of polymers,450 generation of radiation,451 production of semiconductors,452 thin film coatings,453 material synthesis,454 and decontamination purposes of surfaces, liquids and gas streams.35,455,456
However, despite vast scientific and research progress, physical plasma’s technological contributions to our modern society goes largely unnoticed by the general public and outside of most school curricula. In nature, plasma is not only more visible, but even the fundament of visibility as the main source of natural light in our solar system attributable to the enormous plasma ball in its centre.
In healthcare, the term plasma might be particularly confusing due to established definitions in relation to the circulatory system. In medicine, it usually gives name to the yellowish liquid component of blood that contains water, salts and proteins but no blood cells, white cells or platelets. Furthermore, in cell biology, the cytoplasm refers to all materials enclosed by the cell membrane, excluding the nucleus in the case of eukaryotic cells.
9.2 Physics basics
To understand the nature of physical plasma, it is helpful to briefly refresh three basic topics that most readers will be familiar with from physics or chemistry classes.
9.2.1 Atoms
An atom is the basic particle of chemical elements and consists of a nucleus surrounded by a cloud of electrons. The nucleus holds at least one or more positive charges (protons), while negative charges carried by electrons can be found in the cloud surrounding the nucleus (Figure 12a). When the amount of positive and negative charges is balanced, the net charge of that atom equals zero, and it is referred to as a neutral particle.
When a certain amount of energy, specific for each element, is applied to an atom, one of the electrons may overcome the forces that have bound it to the nucleus, and thus the outcome of this event leaves behind a positively charged ion and a free electron (Figure 12b). This process is referred to as ionisation. In an ensemble of many particles, the released electron, as well as the ion, can be subject to energy sources and interact with other particles.
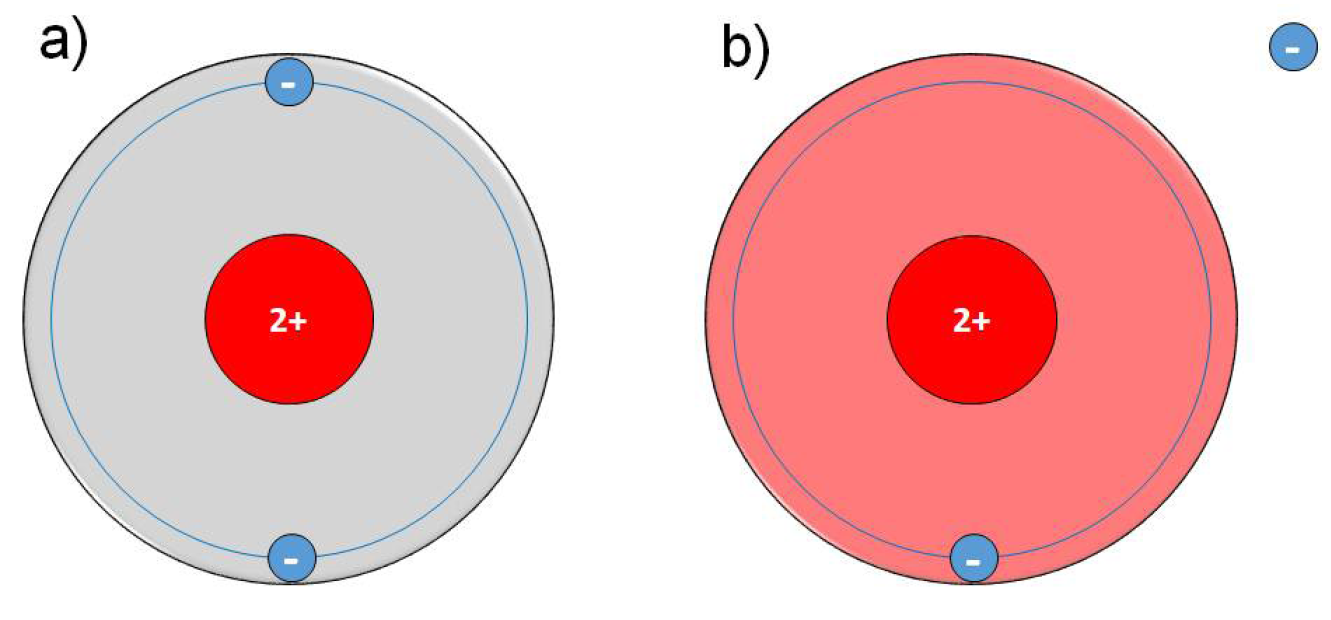
Figure 12: Shell model of the element He.
a) shows He in the neutral ground state configuration (grey); b) shows the first ionisation configuration forming a positively charged ion (red) and a negatively charged free electron (blue), with the latter being no longer bound to that atom nucleus.
At moderate energy supply, an electron might not be released, but rather excited to a higher energy state of that element as indicated by the dashed circles in Figure 13. When falling back spontaneously to ground state (relaxation), the energy difference between ground state (Figure 13a) and excited state (Figure 13b) is released in the form of electromagnetic radiation (photon) according to Figure 13c. At a certain wavelength range, the human eye can detect photons and identify this radiation as visible light. More energetic photons are referred to as UV radiation. As an alternative to emitting a photon, the excited particle can also store the absorbed energy for a certain period of time (metastable) and transfer it to other particles via collisions.
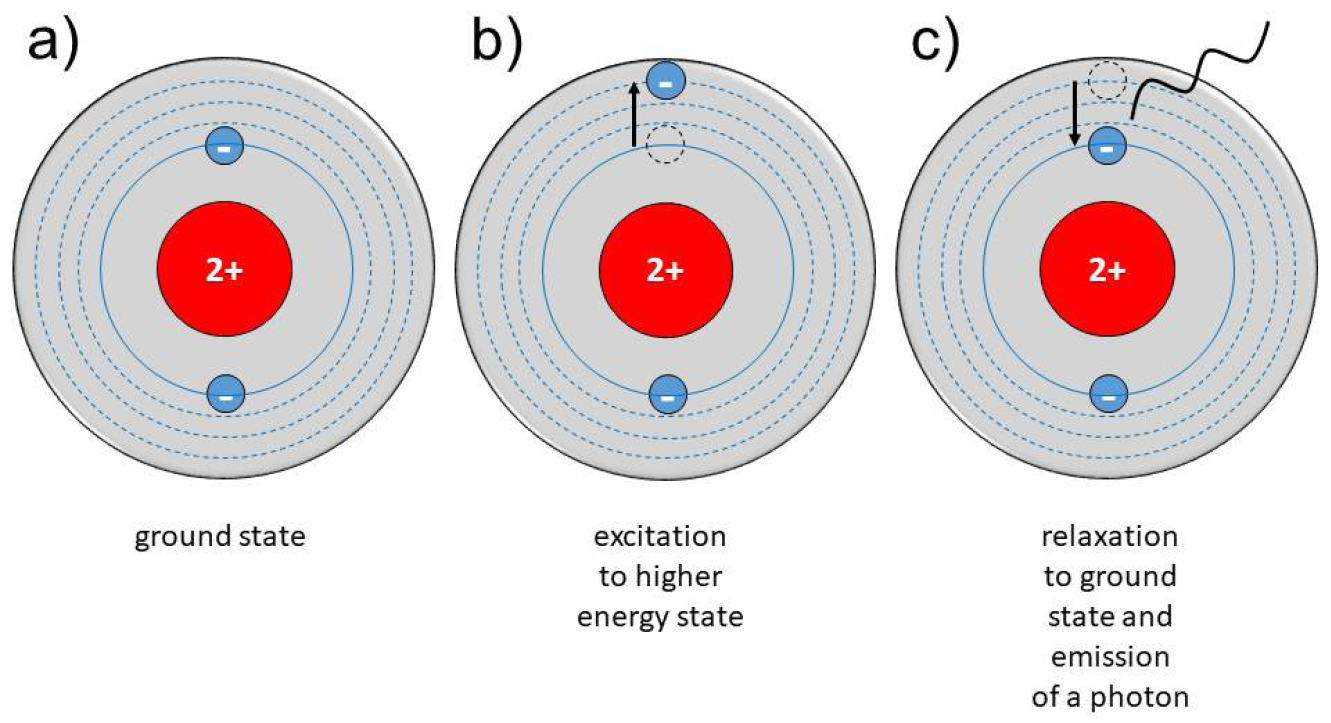
Figure 13: Shell model of the element He with moderate energy supply.
a) shows He in the ground state; whereby b) an electron can be excited to a higher energy level (dashed lines); and c) can relax back to the ground state. The energy above ground state is released in the form of electromagnetic radiation (photon).
9.2.2 Molecules
Atoms can attach to other atoms by chemical bonds, thereby forming molecules. Most relevant are covalent bonds, in which an electron of one atom together with an electron from another atom form an electron pair. Naturally, atoms in the molecular bond can still be subject to excitation processes, eventually leading to ionisation or photon emission, just like non-bounded atoms. In addition, molecules may give up their multi-atom configuration and decay into their atomic components (dissociation) as soon as sufficient energy is supplied to release the bond. The oxygen molecule O2 in Figure 14a is the common and stable configuration of two oxygen atoms at atmospheric conditions. As illustrated in Figure 14b, it can be dissociated to two oxygen atoms (O). However, the outer shell of each atom is now missing two electrons, which renders the atomic configuration of oxygen highly reactive, and offers the starting point for gas phase reactions involving other atoms and molecules.
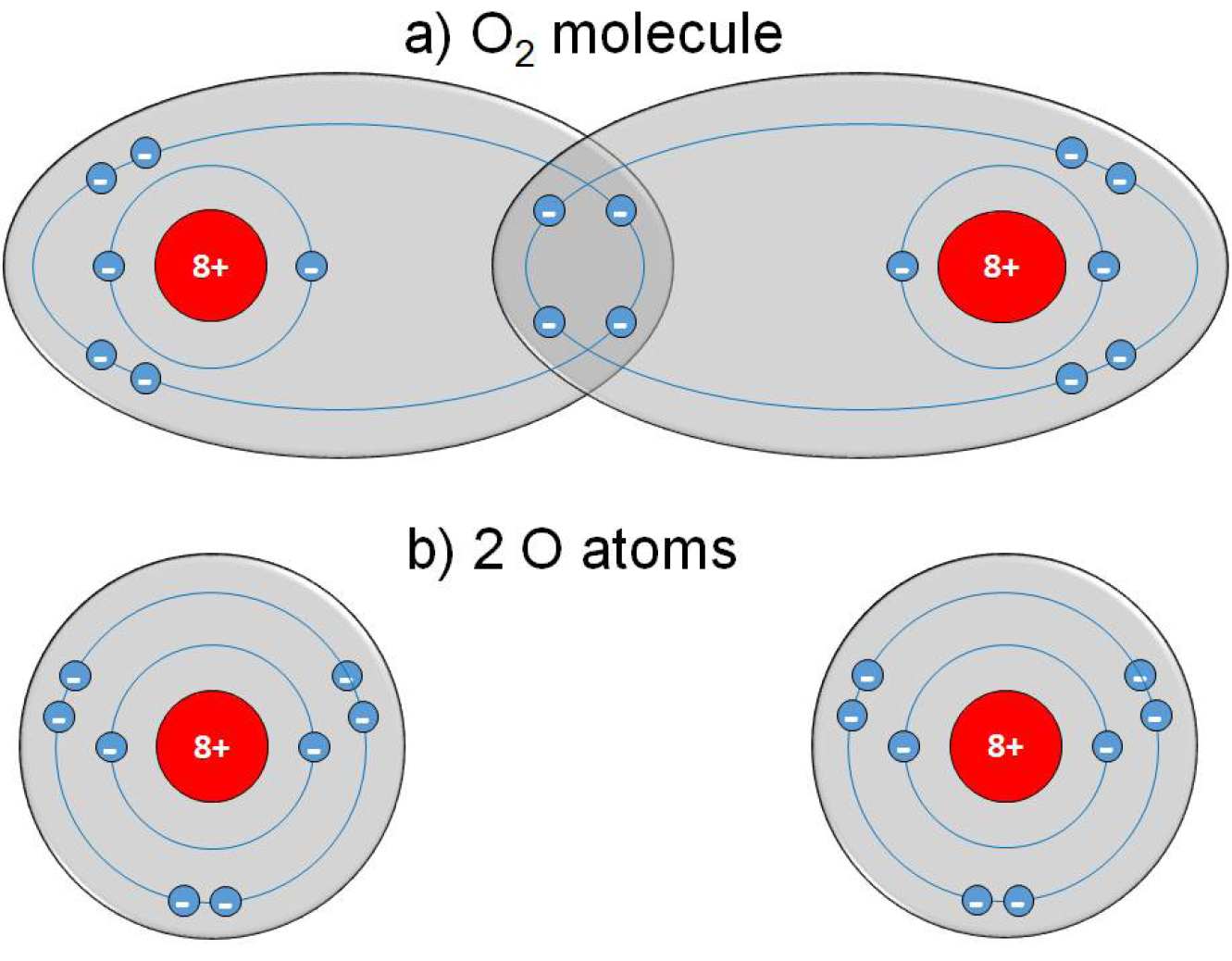
Figure 14: Electron configuration of an oxygen molecule.
(a) shows an oxygen molecule in which each atom shares two of their outer electrons in an electron pair; (b) shows two separate oxygen atoms not interacting with each other.
9.2.3 Thermodynamics and particle collisions
Atoms and molecules as building blocks of matter are in constant disordered motion, with a mean velocity of the particles being determined by, and proportional to, temperature and energy. One temperature that applies uniformly throughout an ensemble of gas particles exists only when the system is in a state of thermal equilibrium. Here, the particle velocities are subject to a defined statistical distribution – some particles have higher velocities than the average velocity, while others move at less speed. In air at atmospheric pressure and a temperature of 20˚C, about 1025 gas particles per cubic metre move at a mean velocity of more than 1600 kilometres per hour and collide with 7 billion other gas particles per second. At constant temperature, each particle of the ensemble of particles transfers energy and momentum continuously with other particles by collisions, but the overall amount of (heat) energy of the system is constant. As soon as energy is added to this ensemble of particles, the energy is distributed homogeneously between all particles by (elastic) collisions until the system adapts to thermal equilibrium at a higher temperature than before, which equals a higher mean velocity of the particles.
In our everyday life, we often experience systems in thermal equilibrium, e.g. the evenly warm water of a bathtub, the cold winter air, the hot stove top. But we also know systems that are not in thermal equilibrium, or at least not in thermal equilibrium for a certain amount of time. Deviations from thermal equilibrium often occur when a system is composed of subsystems. As long as the subsystems can exchange energy, the system strives towards thermal equilibrium by itself with time constants depending on temperature difference and efficiency of (heat) energy transfer. An example of such a mixed system on macroscopic scale is a glass of hot tap water filled with ice cubes. Due to efficient energy transfer, the heat of the hot water first melts the ice cubes and then heats the cold water until the liquid in the glass equilibrates at a uniform temperature. This process (thermalisation) does not occur instantaneously but requires a certain amount of time, e.g. minutes for the earlier ice cube example (Figure 15a).
However, if the energy exchange between subsystems is not efficient, it can take much longer to equilibrate. An example of a system where (heat) energy transfer is very inefficient is an insulated mug. Due to its double-walled construction, the heat energy exchange between the inside and outside of the mug is severely limited, so that a cool liquid inside equilibrates with the inside wall rather quickly, but only equilibrates with the temperature surrounding the mug within hours (Figure 15b).
When a system only exists for a certain amount of time, and time constants for thermalisation are significantly above this lifetime, a state can establish with subsystems continuously existing at different temperatures (Figure 15b). An important key for understanding the nature of CAP is that it is a gaseous system, composed of subsystems at the microscopic scale, existing at highly different temperatures.
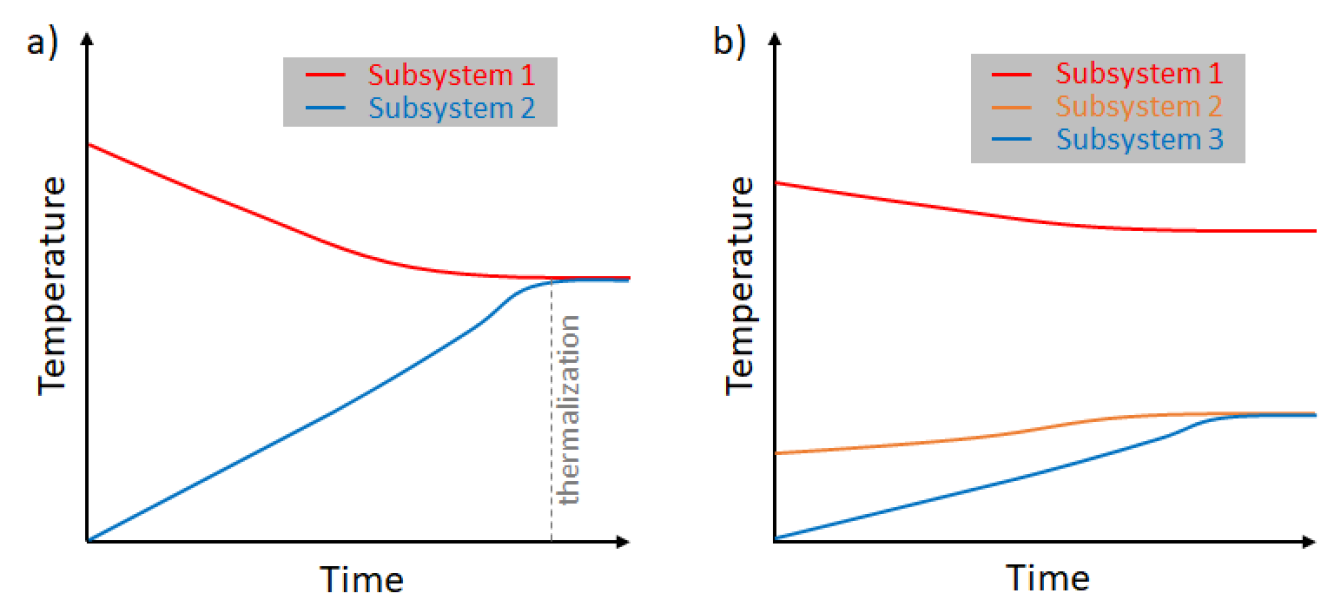
Figure 15: Graphs showing the process of thermalisation.
a) shows the thermodynamic system of two subsystems that equilibrate to the same temperature within a certain time;
b) shows a system consisting of three subsystems in which only two subsystems reach thermodynamic equilibrium.
9.2.4 Physical plasma as the fourth state of matter
Matter can exist in different states, with solid, liquid and gaseous being the classical states (Figure 16). On a microscopic scale, solids are characterised by fixed positions of each atom relative to other atoms because of attractive forces between them. The movement of each atom is restricted to oscillations around the fixed central position, with the intensity of oscillations being proportional to temperature. At a temperature specific for each element and material composition, the forces induced by the oscillations overcome the attractive forces of the atoms, thus provoking the phase shift to a liquid state. In liquid state, particles can almost move freely and collide with each other, attractive forces between particles being significantly lower compared to solids. With a further increase of temperature, a liquid becomes gaseous. Accompanied by this phase shift is a significant loss in density – the number of particles per volume unit. As a consequence, gas particles can move freely and experience less collisions compared to liquids.
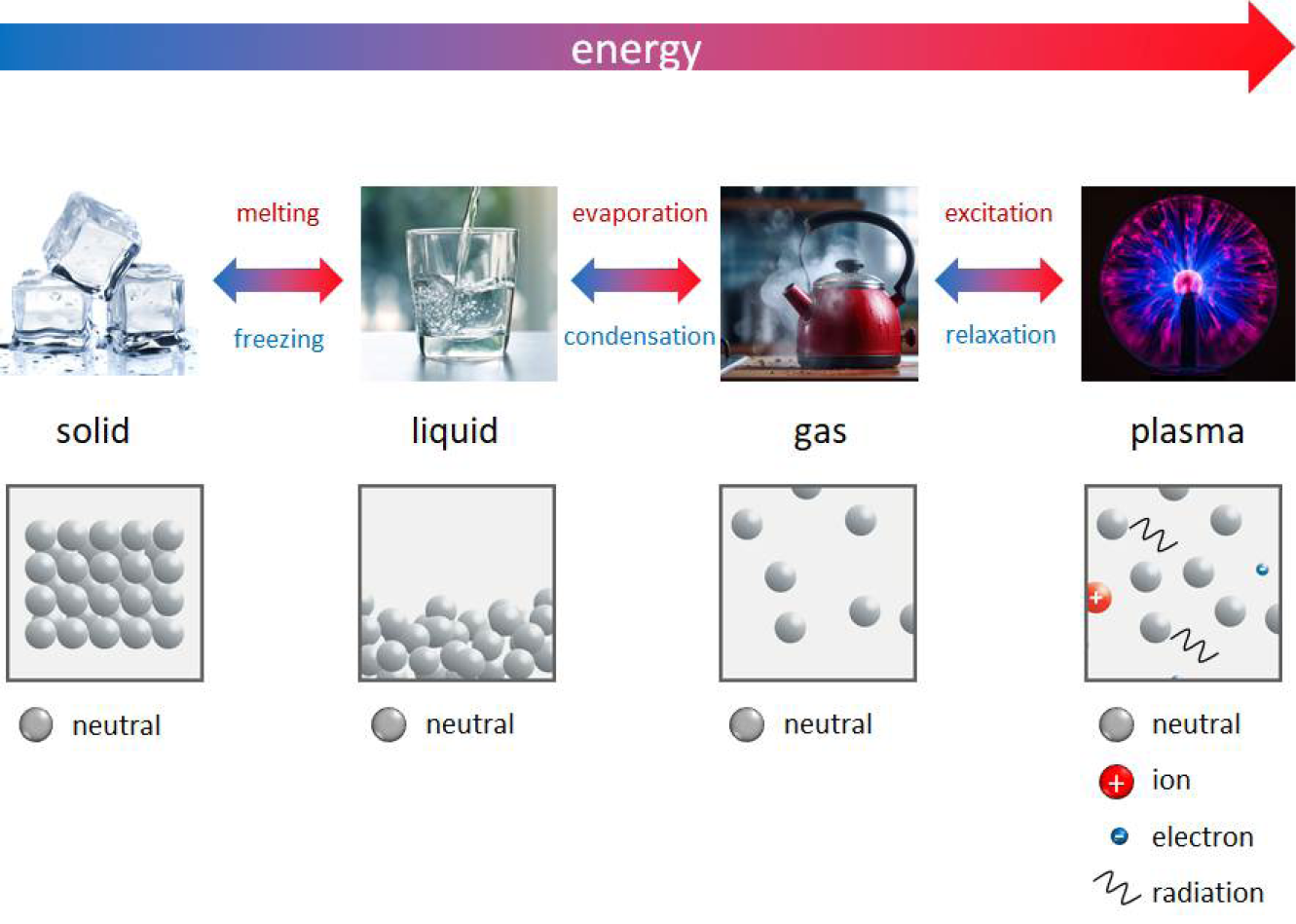
Figure 16: The three classical states of matter with their arrangement and density of neutral particles in comparison to the plasma state as a mix of neutral and charged particles as well as electromagnetic radiation (photons).
When energy is delivered to an ice cube in the form of heat, its molecular components lose their crystalline long-range order and the solid becomes liquid water. The molecular components of both, ice and water, are still identical, i.e. a molecule formed from two hydrogen atoms bound to one oxygen atom (H2O). When the temperature is increased further, liquid water evaporates to become gaseous water vapour. Hereby, 1 litre of liquid water can produce almost 1700 litres of water vapour, thus illustrating the massive decrease of particles per volume (density). Chemically, however, the gaseous components are still H2O. They feature a higher mobility and move at higher speeds, yet the number of electrons in the atomic shells is still identical to the number of protons in the nuclei, thus rendering the solid, liquid and gaseous H2O molecules electrically neutral.
As soon as the excitation of gaseous neutral particles exceeds a certain energy level, processes such as ionisation, dissociation and photon emission occur. The gas becomes plasma, i.e. a mix of free electrons, gaseous ions, gaseous neutral particles and photons. In terms of thermodynamic properties, all particle classes can be considered a separate subsystem. The temperatures of the electrons, ions and neutrals can be very close to each other (equilibrium) or differ significantly (non-equilibrium). In fact, the temperature depends on how efficient each subsystem is in gaining energy from an energy source after balancing the losses by distributing this energy-uptake throughout the (gaseous) system.
The degree of ionisation, which corresponds to the ratio of charged particles to neutral particles in a plasma state, can vary strongly in the range from only a few particles per million up to a fully ionised system. As a direct consequence of the presence of free charge carriers, plasmas carry electric currents and can be manipulated by electric and magnetic fields. They can also exist at a wide range of pressures and temperatures. After all, plasmas glow, and the spectral characteristics of the luminous appearance depend on the base gas, pressure and temperature.
9.3 CAP
CAP is a special variant of a physical plasma characterised by the following attributes:
- it operates close to atmospheric pressure;457
- the energy required to generate and maintain the plasma is supplied as electrical energy by so-called gas discharges;303
- particle collision is the dominant mechanism of energy transfer in the plasma;458
- the vast majority of the particles present are neutral particles, while ionised particles (electrons and ions) exist only at approx. one in a million;459
- the mean temperature of the electrons, corresponding to their energy, is orders of magnitudes higher than the mean temperature of ions and neutral particles;460
- heat transfer to the treated area is reduced to the technically possible minimum.314
CAP is a mixture of components existing simultaneously in a confined space as a result of supplying energy to a gas. Without energy supply, the plasma extinguishes, allowing CAP to be turned on and off virtually at the push of a button. As illustrated in Figure 17, CAP encompasses neutral gas particles that represent the main portion of particles. In addition, there are charged particles, such as electrons and ions, deriving from ionisation processes. Excitation and relaxation processes produce electromagnetic radiation (photons), mainly in the UV and visible spectral band. With molecular gases included, dissociation processes lead to the formation of reactive gas species. Since ambient air is always involved in medical applications of CAP, many of these reactive species belong to the group of RONS.461 Finally, the energy for CAP development derives from strong electromagnetic fields. Therefore, these fields can also be considered a component of CAP. Together with the electrical current flow and heat conduction induced by the plasma, electromagnetic fields can also have an impact on medical treatments by increasing tissue temperature.
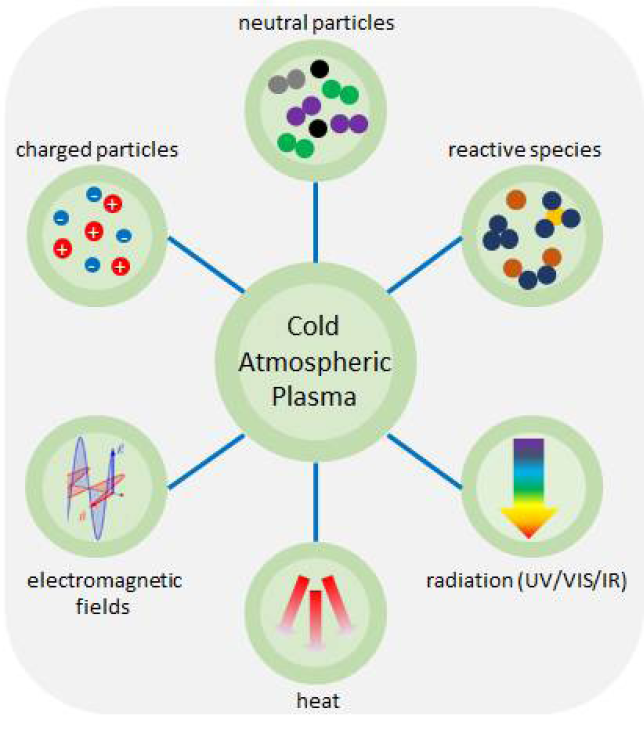
Figure 17: CAP as a mix of different components.
9.4 Gas discharges
Common for the generation of physical plasmas are electrical gas discharges. A variety of technical concepts exists to generate CAP at atmospheric pressure.462 However, the basic physical mechanisms in the gas phase are comparable in all concepts. A simple configuration to illustrate the concept of a gas discharge is an arrangement of two parallel plates (electrodes), made from electrically conducting materials, arranged at a distance of millimetres to centimetres as depicted in Figure 18.
Each electrode is connected to one pole of a voltage source, indicated typically by positive and negative polarity. The voltage induces an electric field in the gas volume that accelerates seed charge carriers by Coulomb forces. These seed charge carriers are sufficiently available through natural processes in habitable layers of the earth atmosphere. Specifically, positively charged ions are accelerated towards the negative electrode, while electrons gain speed on their way to the positive electrode. The stronger the electric field, the higher the velocity gain of the charge carriers per distance unit. Given balanced charges, neutral particles are not accelerated and cannot absorb energy directly from the electric fields. However, collisions with charge carriers that have previously absorbed energy in the electric field is a mechanism by which part of the field energy can also be transferred to neutral particles.
At the very beginning of each gas discharge, a naturally existing seed charge carrier (typically an electron) gains energy from the electric field (Figure 18a). In collisions with neutral particles, the seed electron loses part of this energy. However, immediately after the collision, the seed electron again receives energy from the electric field, so that the cycle of energy gain and loss continues. Electrons loose only a small amount of energy in collisions that heat up the much heavier neutral particles (elastic collision). However, given enough energy, they are very efficient in transferring energy to the electrons of the neutral particles (inelastic collisions). At an energy transfer specific for each element, the seed electron is finally able to release another electron from a neutral particle in a mechanism, referred to as electron impact ionisation. The released new electron, just like the seed electron, is also accelerated in the electric field, and on its turn can ionise further neutral particles on its way to the positive electrode. In a chain reaction called electron avalanche, this process repeats until finally an electrically conductive (ionised) gaseous volume forms between the two electrodes – the physical plasma (Figure 18b).
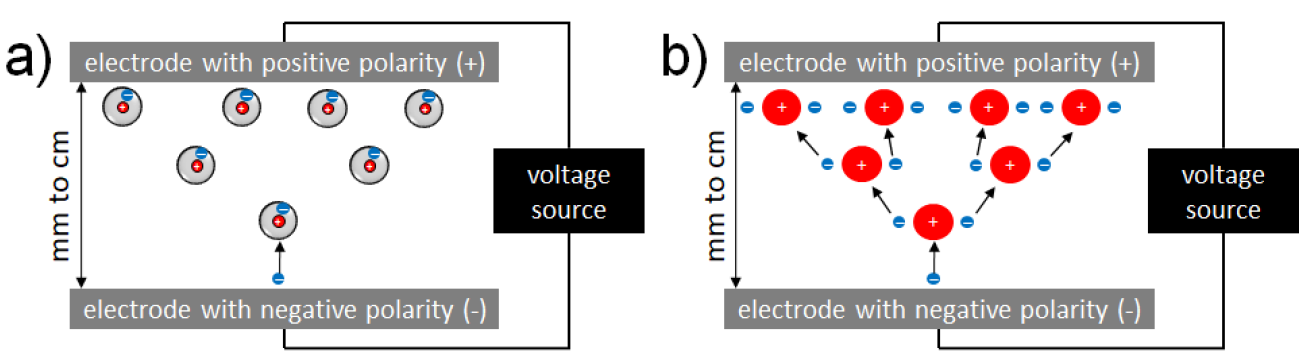
Figure 18: An example of electron impact ionisation of neutral particles.
Electrode arrangement with a) seed electron (bottom) inducing b) an electron avalanche in the gas volume between two oppositely charged electrodes.
For clarity, the illustration in Figure 18 is limited to one of the most important mechanisms in a gas discharge, i.e. electron impact ionisation of neutral particles. There are further processes, however, which are relevant for the formation of CAP. The impact of electrons with neutral particles can also result in excitation of the particles (metastable) or, eventually, the release of a photon. In the case of molecular gases, electron impact may also lead to rotational and vibrational excitation, as well as dissociation into atoms, which marks the starting point for chemical gas phase reactions leading to the formation of reactive species.463 All the aforementioned mechanisms induced by electron impact happen simultaneously in a plasma, each of them with different probability, essentially depending on the electron energy and the gas composition. Electron energy of the plasma can be controlled by parameters such as electrode configuration, signal characteristics of the power supply and gas composition. While these parameters are systematically researched during development, market-ready medical devices dispose a fixed electrode configuration that is supplied with constant electrical signal characteristics and operate in a defined gas composition comprising of either a combination of working gases with ambient air or pure ambient air.
As mentioned before, all charge carriers gain energy in electric fields. This also includes ions, whose mass is almost identical to the mass of neutral gas particles. Therefore, (elastic) collisions of ions with neutral particles can increase very efficiently the temperature of neutral particles, while simultaneously decreasing the ion temperature. At the very beginning of each gas discharge, the neutral particles are at ambient temperature and continuously develop toward thermal equilibrium with the ions. In weakly ionised plasmas such as CAP, the number of ions is orders of magnitudes lower compared to neutral particles. Consequently, the temperature where both subsystems reach thermal equilibrium, the gas temperature, is significantly below the initial ion temperature and above ambient temperature.
10. Appendix II: Available and emerging solutions for delivery of CAP therapies in wound healing
Plasma medicine has emerged as a promising field for various medical applications, particularly in wound healing. To pave the way for these applications, innovative plasma technologies have been developed, certified and declared conformant for medical use. Thereby, different technology pathways were applied to make the devices safe for medical use. While first plasma devices are certified under the MDR, ongoing innovations continue to expand the potential applications of CAP therapy.
10.1 Basic requirements for CAP sources in medical technology
Plasma sources are comprised of essential components before a device is operational and available for therapy in plasma medicine. A base unit includes various parts, but mainly provides power for a pre-defined duration of treatment and thus defines the manufacturers studied and approved ‘dose control’ for efficacy and safety reasons. In the case of systems requiring a working gas supply, a flow control system is similarly implemented into the base unit. A user interface either as part of the base unit or the hand-held device manages the device operation for on/off switching and error signalling. Although in theory many device operation parameters could be made variable as it is typical in laboratory settings, when facing utilisation in every day clinical practice, however, complexity is mostly reduced to an on/off functionality.
In different cases, some manufacturers offer a device family with a variety of individual ergonomic solutions for the treatment case. These exchangeable applicators are hosted in such a manner that, by design, high voltage connections present minimised risk for operator or patient. An additional safety element is a so-called spacer, allowing an intrinsic operation within pre-defined conditions. New trends propose sensors to track treatment time, enable device quality control, and monitor environmental impact like the humidity to post-qualify the performed therapy.421,464–466
For a medical use case, innovative technologies first have to prove their benefits on the intended therapy in vitro before operating under in vivo conditions. Thus, to pre-qualify devices for future trials requiring clinical ethics approval as well as to validate safety and efficacy, a group of scientists in Germany, together with plasma device manufacturers, initiated a so-called pre-standard, the DIN SPEC 91315.364,467 This German pre-standard proposes a set of in vitro studies designed specifically for plasma medical devices. Further refinement of the DIN SPEC 91315 in Germany is in progress, while internationally at other locations, i.e. Austria, Brazil, France, Korea, initiatives are also commencing to define electrical requirements for plasma devices.468 With both the electrical safety definitions and the local regulatory medical device requirements (e.g. MDR for the EU), an overall set of qualifiers is defined. Although medical and regulatory requirements vary globally, these criteria have also been used for guidance evaluating new device innovations outside Europe, due to its investigative frontrunner availability and position.469–474
Further requirements need consideration if the treatment area has a local sensitivity on respective plasma components. Plasma is commonly described as a cocktail of reactive agents, namely emission in the UV and vacuum UV spectral range, a large variety of RONS, transient electrical fields, local temperature and charged species. The treated areas have to be considered individually on these components so that a treatment near the nose should consider the local reactive species exposure with a direct pathway into the lung. Similarly, the vestibular organ is known to react to magnetic fields from nuclear magnetic resonance imaging via impacts on the local fluids and ions, thus highlighting a careful electrical safety consideration for plasma sources, as it would be covered within the electrical safety qualification.475
10.2 Available plasma medical sources for CAP therapy – device categories
Presently a variety of plasma medical sources are on the market, following different technological pathways to provide safe and efficient operation. When presenting an overview of these device categories and their differences here, it should also be noted that the international community so far has not defined a clear set of characteristics for a plasma device to be accountable as a CAP source for medical therapy. As a minimal foundation for this field, an accountable performance and safety validation in accordance to the DIN SPEC 91315 is recommended.364,467,476
Among the different types of plasma medical sources, a total of two major categories summarises the discharge principle within – the DBD and the plasma torch. Understanding the difference between both different technical approaches also clarifies the treatment philosophy.
A DBD uses a dielectric barrier to inhibit current flow from the power supply into the conductive plasma discharge onto the patient. This allows a direct treatment of the patient with the plasma under safe conditions. By properly selecting the dielectric barrier, an effective capacitance acts by inhibiting a current transmission. A dielectric material is non-conductive by nature and thus avoids a current flow from the power unit to the patient. At the same time, it is able to store charges on its surface, ideal to enhance consecutive plasma ignitions. In the case of alternating voltage, charges can be collected onto the dielectric and emitted again to supply a discharge with a tolerable current flow. Once a current limitation is in place, a transition from a filamentary non-thermal plasma into a thermal equilibrium plasma will not occur. While a thermal plasma can easily reach several thousand degree celsius, a non-thermal plasma can be operated at tissue tolerable temperatures down to room temperature. The DBD is the most common plasma medical source approach, branching into three sub categories – the VDBD, the SDBD and the PJ.19 Sometimes the PJ are put into a category of their own, for the dielectric is not always the only limiting building block, for an intrinsic current limitation can be established within the electrical circuit; this is especially relevant in the case of a device operation in close vicinity to the surface.
The plasma torch on the other side is categorised as a hot non-thermal plasma discharge. Yet, by considering an indirect treatment approach at an appropriate distance, the discharge arrangement can still provide a tissue tolerable temperature. In order to make this device-specific feature less dependent on experience of the user handling it, provided spacers of the devices provides an intrinsic ideal treatment distance. From the thermal discharge region, a recombining afterglow is emitted downstream, and the wound treatment performed underneath. No direct contact with the plasma region is required for the medical therapy.
An overview of four major discharge types is depicted in Figure 19. Overall technological solutions for CAP require a local electrical field enhancement to reach breakdown criteria. VDBD and SDBD are mostly operated in ambient air. Here, an electrical field of roughly 10kV/cm is required, meaning a high voltage of 10kV will ignite a discharge at a 1cm discharge gap. However, in the case of local electrical field enhancement by geometric shape adjustments, this simple ratio will not apply any more. For safety reasons, a minimal possible voltage is desired and reached by individual electrode geometries. In the case of DBD, the high voltage electrode is mostly placed behind a dielectric barrier to avoid direct contact with the electrode.
The placement of the grounded electrode defines the spreading of the discharge. DBD using ambient air and the treated target (e.g. a patient, the wound) as a counter electrode will ignite several plasma filaments, breaching the gap between dielectric and surface, and thus they are ignited into the enclosed volume (Figure 19a). The terminology VDBD therefore describes a discharge into open air between two electrodes.19 The cocktail of reactive components is initiated within the whole gap and close to the targeted wound size, enhancing the complexity of the cocktail acting onto it. The filaments will be ignited stochastically over the extension of the high voltage electrode and, assuming an accumulation over a recommended timeframe, result in a large area treatment.
If the discharge device includes a grounded grid electrode on top of the dielectric barrier, the discharge is limited on the surface and will not reach into the volume (Figure 19b). These type of discharges are called SDBD.19 Since a treatment surface such as the patient’s skin is not required here for proper operation, they are also not sensitive to the distance towards the wound surface. This can provide advantages in the case of uneven wound surface. Yet the so-called “cocktail composition” (paragraph 10.1) reaching the surface is reduced with the treatment distance. Since the dose definition is still a topic of present research in plasma medicine considering the composition of the cocktail components, neither an advantage nor disadvantage can be stated. Here the qualification via a pre-defined set of measurements allows potential users a comparative data set for intended therapy.477 Recent datasets showed clear decontamination efficacy.
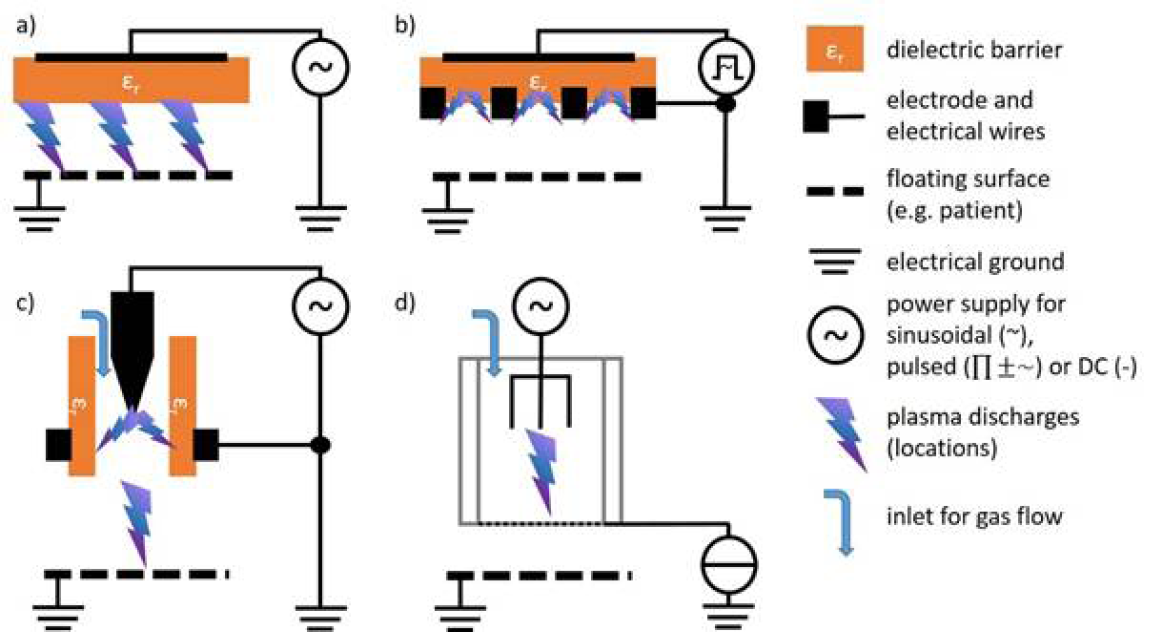
Figure 19: Electrical geometries for the four different discharge types.
a) VDBD; b) SDBD; c) PJ; and d) plasma torch
Besides VDBD and SDBD operating in ambient air, the PJ systems require an active gas flow, supplying rare gases for more convenient discharge ignition.19 The gas flushes in between the discharge system of a high voltage electrode, a dielectric barrier and an outer grounded electrode. The plasma is ignited inside the capillary, while the electrical field and the gas composition guide the discharge also into the ambient air. There, a mixture of working gas like Ar or He and ambient open air evolves and a variety of reactive species is generated. In special cases, even a temperature gradient can be initiated by the gas flow itself.474 Furthermore, the operation within a rare gas enhances the overall visibility of this discharge due to emission in the visible spectral range. The generated cocktail composition has a similar complexity to the VDBD with discharges onto the surface, yet, in the case of PJ, the distance is of similar importance. Recent findings show a strong dependence of the distance to the surface like the wound area on the discharge performance, indicating a mode shift for the device controlled by the distance.478 The so-called conductive and free mode differ in the sense that the free mode is limited to individual discharge filaments per voltage period travelling into ambient air, while in conductive mode the filament connects with the surface and a consecutive discharge initiate a secondary discharge onto the surface in an infrequent manner.
While the device types VDBD, SDBD and PJ operate either at sinusoidal, pulsed or mixed voltage at frequencies up to 26MHz, the plasma torch is a microwave powered system at 2.45GHz. Plasma ignition is initiated inside the device and, due to the high frequency and high distance to the surface, the discharge is not transmitted onto the wound directly. Since each device is associated with different compositions of cocktail components, a selection of the device to be used should be focused on the requirement for the desired therapy. These, however, are not solely limited on efficacy consideration, but also on ergonomic requirements or, in some healthcare sectors, on economic requirements like treatment time per patient instead of per area element. Other comparative perspectives might also be the impact of environmental conditions. PJ showed that the impact of ambient temperature for instance is less significant compared to the working gas humidity,120,479 yet requiring an external gas supply, while DBD are affected by certain range of humidity in the ambient air.480,481
10.3 Emerging plasma sources for CAP therapies and their fields of application
Out of these initial device types available to clinicians and patients in a limited amount of countries, ongoing research has led to the development of new CAP devices, sources and types. Within this chapter, new innovative discharge trends like the transferred discharge devices and upscaling solutions are presented. Furthermore, different new fields of applications that required and inspired new CAP devices are briefly addressed.
Beside further investigations into established discharge concepts of CAP for medical therapy like SDBD, VDBD and PJ, one new concept grows more attention – the transferred discharge method (TDM). This approach decouples the power unit circuit from the discharge relevant for the treatment by an in-between additional discharge due to the elementary conductivity of plasma itself. A primary discharge is ignited in a somewhat closed environment, while a secondary discharge is then generated towards the patient. The applied potential to ignite the primary discharge is transmitted through a dielectric barrier or a conductive wire to the treatment area. The TDM can be established as a PJ or a DBD, sometimes a CAP device even provides both varieties (Figure 21).469,482–486
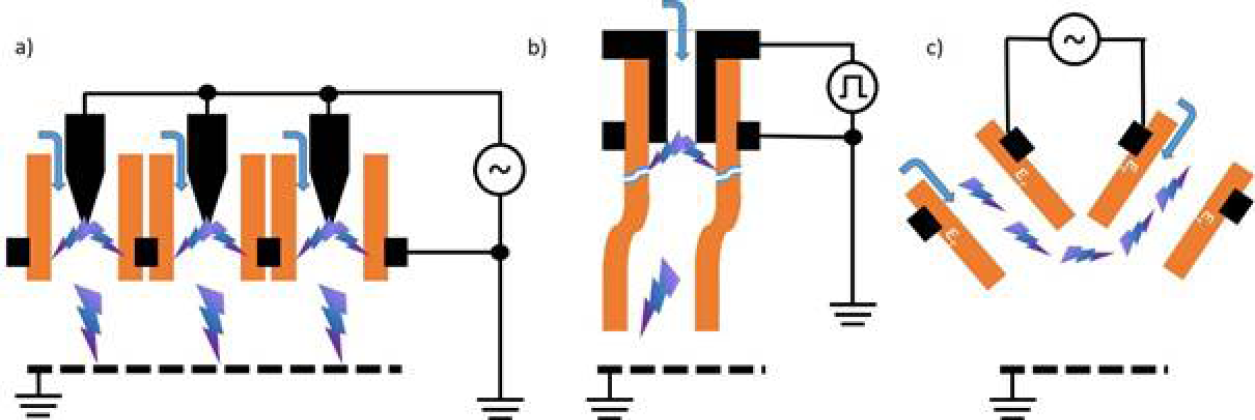
Figure 21: Innovative approaches to CAP solutions for medical therapy.
a) upscaling via a jet array; b) the plasma gun providing a remote ignition inside a capillary and a plasma propagation towards the tube exit; c) the V-jet igniting a discharge between two discharge systems.
Present PJ-based approaches of TDM focus on endoscopic treatment solutions or on treatment of infected, hard to reach treatment spots like in the tracheostomy tube (Figure 20a).271,469,483,487–491 The TDM intrinsically disconnects a possible continuous current transfer through the temporal limited primary discharge and hence the resulting secondary discharge is ignited but without a conductive connection to the power supply. Resulting investigations showed tolerable safety levels with proven efficacy based on a DIN SPEC 91315 characterisation in one system.473,474
Approaches based on VDBD combined with the TDM are not new but rather date back nearly a century.492 However, given modern regulations and the advances in scientific validation of plasma therapies, an adapted innovation of this technology took place leading to a variety of devices.469,484–486 Most devices operate by igniting the primary discharge inside a glass or quartz container. This container is previously vacuumed and filled to a low pressure level with a rare gas (Figure 20b). Here mostly neon is applied for its good breakdown criteria and possibly the visible emission colour. The secondary discharge outside the container is ignited inside ambient air and thus does not consume a constant gas flow. Also, it could in principle even generate discharge underneath a target if the tip is placed in full contact with a surface. Such systems are presently undergoing evaluations towards future therapy pathways in plasma medicine.484,485
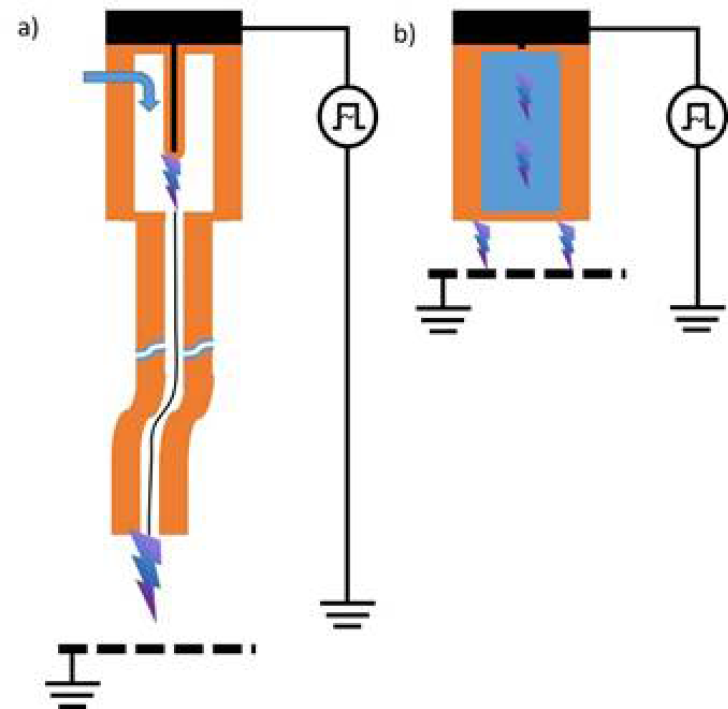
Figure 20: Scheme of transferred discharge systems found in the literature.
a) depicts a PJ based on the TDM; b) depicts a VDBD based on the TDM
Beside new discharge technologies, adaptations to previously presented technologies lead to new innovations in plasma medicine. One adaptation comes with the approach to upscale existing technologies. Based on the high complexity of the plasma technology, an upscaling does invoke several challenges that have to be solved before establishing a solution for a medical therapy. An upscaling allows the treatment of a larger area in one more generalised approach, while reducing treatment times and potential user burden. Such an upscaling is not just a matter of a multiplication factor, even full body dimensions could be considered.
In the case of DBD, the upscaling is commonly performed by exchanging the applicator and maintaining the same power supply unit.11,187,477,493–495 Most concepts of a DBD geometry open a pre-designed upscaling solution,480,481 given that the changing capacity would require adjustments in the power supply to sustain the electrical circuit. Continuous development of CAP therapeutic devices has led to new power sources which have the possibility to adjust their inner resonant circuit to the outer resonant circuit via an integrated matching network.
In the case of PJ, upscaling is similarly approached with an adjustment of the design. Due to breakdown conditions requiring a high electrical field described as voltage over distance, an increase of capillary diameter would require an upscaling of the voltage. Thus the preferred approach is the multiplication of the discharge systems to the desired dimensions, commonly called a PJ array (Figure 21a).274,494,496,497 While the treatment area can be increased, limitations on the ambient air admixture are induced, possibly altering the cocktail composition. One innovation aims to counteract this limitation by regulating the gas flow per channel and thus not invoking a drainage of ambient air.498 Another solution was found when adjusting the tube length in an upscaled fashion of the remote ignition technology.499 Considering new therapies, the variety of cocktail compositions possible with plasma devices is of special interest. Due to its complex nature, plasma can be generated in multiple different fashion, still hiding future potential.471,498,500,501 With the increase of treatment area, the balance of operation time defined by safety thresholds such as reactive species, temperature, ozone and leakage current have to be balanced with the improvement of efficacy.
As an adaptation of PJ concepts, two further methods are under research, focusing either on adjustment of the flow shaping or on the reorientation of the discharge by controlling the electrical field (Figure 21c).378,495,502–504 Shaping the gas flow results in a change of distribution of reactive species to a desired gradient on a surface based on fluid mechanics.503 An additional effect is the removal of the flow impulse that is applied onto the wound surface in that concept. Yet this approach could reduce or extinct components from the cocktail.504 The reorientation of the discharge generates a system in which the switch from the free jet to the conductive jet is controlled per design, while the enhanced discharge length along the ambient air generates an upscaled impact area on the surface.378 Such technical requirements might have a slight negative impact on handling and ergonomic suitability for the desired therapy.
The innovations in CAP sources until this time focus on new and advanced discharge devices while not explicitly being focussed on a therapy in itself. Meanwhile, in some investigations, a new therapy indication is under development, highlighting the device for use in case-specific device design.
Among the therapy concepts under investigation, one will become the focus of solutions in the future – combination therapies. When plasma sources are tuned to be tissue tolerable and soft, they meet all safety requirements. Yet under these settings, the attribute of a mechanical impact on the surface, as known from surface treatment like etching, is lost, thus reducing its efficiency potentially. However, to treat diseases reaching a certain depth or being shielded by layers of, for example, biofilm, a mechanical pre-treatment is required. Such approaches are already in the clinical trial phase in dentistry in order to treat peri-implantitis.471,505–507 Further combination therapies are frequently discussed at scientific events, showing the desire to broaden the field where it can be of help. Another known combination approach is together with pulsed electrical fields, known from the decontamination topics with plasma technology.508
As discussed in this Appendix, the potential of CAP innovations in plasma medicine is surely significant. Making these innovations reach the bedside of patients will, however, require dedicated multi-disciplinary effort and commitment from all parties involved directly or indirectly – researchers, HCPs, funding agencies and industry.
Author(s)
Jan Apelqvist, MD (Editor)
Department of Endocrinology University Hospital of Skåne in Malmö and Division for Clinical Sciences University of Lund, Sweden
Alexander Robson, PhD
Plasma Technology, University of Sheffield, United Kingdom
Andreas Helmke, PhD
University of Applied Sciences and Arts, Hildesheim/Holzminden/Göttingen, Germany
Antoine Rousseau, PhD
CNRS, Physics of Plasmas Laboratory, School of Polytechnics, Palaiseau, France
Bouke Boekema, PhD
Association of Dutch Burn Centres, Beverwijk, The Netherlands
Edwin den Braber, MD, PhD
(Co-editor)
Independent Consultant,
EWMA Innovation Alliance, Denmark
Endre Szili, PhD
Plasma Medicine Research Group, Future Industries Institute, University of South Australia, Australia
Ewa Stürmer, MD
Department of Vascular Medicine, University Heart Center, Division of Translational Research, University Medical Center Hamburg-Eppendorf (UKE), Germany
Lars Böckmann, PhD
Clinic and Policlinic for Dermatology and Venereology, University Medical Center Rostock, Germany
Nishta Gaur, PhD
Department of Chemistry, Lancaster University, United Kingdom
Robert Short, PhD
University of Sheffield and Lancaster University, United Kingdom
Sander Bekeschus, PhD
ZIK plasmatis, Leibniz Institute for Plasma Science and Technology (INP), Germany
Steffen Emmert, MD
Clinic and Policlinic for Dermatology and Venereology, University Medical Center Rostock, Germany
Thomas von Woedtke, PhD
Plasma Medicine, Leibniz Institute for Plasma Science and Technology (INP), Germany
Torsten Gerling, PhD
Plasma Source Concepts, Leibniz Institute for Plasma Science and Technology (INP), Germany
Corresponding author:
Jan Apelqvist, MD
Jan.Apelqvist@skane.se
Editorial support and coordination:
Peter Frank, PhD, EWMA Secretariat
References
- Gottrup F, Apelqvist J, Price P, European Wound Management Association Patient Outcome Group. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care. 2010 Jun;19(6):237–68.
- Gottrup F, Apelqvist J, Bjarnsholt T, Cooper R, Moore Z, Peters EJG, et al. EWMA document: Antimicrobials and non-healing wounds. Evidence, controversies and suggestions. J Wound Care. 2013;22(5 Suppl):S1-89.
- Piaggesi A, Bassetto F, den Braber E, Dalla Paola L, Marques A, Palla I, et al. New technologies for tissue replacement. Journal of Wound Management. 2023 Apr;24(1).
- Probst S, Apelqvist J, Bjarnsholt T, Lipsky BA, Ousey KJ, Peters EJG. Antimicrobials and Non-healing Wounds: An Update. Journal of Wound Management. 2022 Oct 7;23(3):S1–33.
- Piaggesi A, Låuchli S, Bassetto F, Biedermann T, Marques A, Najafi B, et al. Advanced therapies in wound management: cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J Wound Care. 2018 01;27(Sup6a):S1–137.
- Gottrup F, Dissemond J, Baines C, Frykberg R, Jensen PØ, Kot J, et al. Use of Oxygen Therapies in Wound Healing. J Wound Care. 2017 May 1;26(Sup5):S1–43.
- Apelqvist J, Willy C, Fagerdahl AM, Fraccalvieri M, Malmsjö M, Piaggesi A, et al. EWMA Document: Negative Pressure Wound Therapy. J Wound Care. 2017 Mar 1;26(Sup3):S1–154.
- Conrads H, Schmidt M. Plasma generation and plasma sources. Plasma Sources Sci Technol. 2000 Nov;9(4):441.
- Fridman A, Chirokov A, Gutsol A. Non-thermal atmospheric pressure discharges. Journal of Physics D: Applied Physics. 2005;38(2):R1–24.
- Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P. Atmospheric pressure plasmas: A review. Spectrochimica Acta Part B: Atomic Spectroscopy. 2006;61(1):2–30.
- von Woedtke T, Schmidt A, Bekeschus S, Wende K, Weltmann KD. Plasma Medicine: A Field of Applied Redox Biology. In Vivo. 2019;33(4):1011–26.
- Wende K, Brandenburg R. Cold Physical Plasma: A Short Introduction. In: Metelmann HR, von Woedtke T, Weltmann KD, Emmert S, editors. Textbook of Good Clinical Practice in Cold Plasma Therapy. Cham: Springer International Publishing; 2022. p. 37–62.
- von Woedtke Th, Reuter S, Masur K, Weltmann KD. Plasmas for medicine. Physics Reports. 2013 Sep 30;530(4):291–320.
- Isbary G, Shimizu T, Li YF, Stolz W, Thomas HM, Morfill GE, et al. Cold atmospheric plasma devices for medical issues. Expert Rev Med Devices. 2013 May;10(3):367–77.
- Kim YJ, Jin S, Han GH, Kwon GC, Choi JJ, Choi EH, et al. Plasma Apparatuses for Biomedical Applications. IEEE Transactions on Plasma Science. 2015;43(4):944–50.
- Setsuhara Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch Biochem Biophys. 2016/04/26 ed. 2016 Sep 1;605:3–10.
- Weltmann KD, von Woedtke T. Plasma medicine—current state of research and medical application. Plasma Physics and Controlled Fusion. 2017;59(1).
- Von Woedtke T, Laroussi M, Gherardi M. Foundations of plasmas for medical applications. Plasma Sources Sci Technol. 2022 May 1;31(5):054002.
- Brandenburg R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Science and Technology. 2017;26(5):53001–53001.
- Winter J, Brandenburg R, Weltmann KD. Atmospheric pressure plasma jets: an overview of devices and new directions. Plasma Sources Science and Technology. 2015;24(6).
- Reuter S, von Woedtke T, Weltmann KD. The kINPen—a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. Journal of Physics D: Applied Physics. 2018 May 16;51(23):233001.
- Lin L, Keidar M. A map of control for cold atmospheric plasma jets: From physical mechanisms to optimisations. Applied Physics Reviews. 2021;8(1).
- Kim JY, Ballato J, Kim S. Intense and Energetic Atmospheric Pressure Plasma Jet Arrays. Plasma Processes & Polymers. 2012 Mar;9(3):253–60.
- Lu X, Naidis GV, Laroussi M, Reuter S, Graves DB, Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Physics Reports. 2016;630:1–84.
- Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics. 2012;45(26).
- Privat-Maldonado A, Schmidt A, Lin A, Weltmann KD, Wende K, Bogaerts A, et al. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid Med Cell Longev. 2019/11/07 ed. 2019;2019:9062098.
- Desmet T, Morent R, De Geyter N, Leys C, Schacht E, Dubruel P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules. 2009 Sep 14;10(9):2351–78.
- Vasilev K, Griesser SS, Griesser HJ. Antibacterial Surfaces and Coatings Produced by Plasma Techniques. Plasma Processes and Polymers. 2011;8(11):1010–23.
- Bazaka K, Jacob MV, Chrzanowski W, Ostrikov K. Anti-bacterial surfaces: natural agents, mechanisms of action, and plasma surface modification. RSC Advances. 2015;5(60):48739–59.
- Recek N. Biocompatibility of Plasma-Treated Polymeric Implants. Materials (Basel). 2019/01/16 ed. 2019 Jan 12;12(2).
- Tan F, Fang Y, Zhu L, Al-Rubeai M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Critical Reviews in Biotechnology. 2021 Apr 3;41(3):425–40.
- Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia L. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. International Journal of Pharmaceutics. 2001 Sep 11;226(1):1–21.
- Laroussi M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Processes and Polymers. 2005;2(5):391–400.
- Moreau M, Orange N, Feuilloley MG. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008/09/09 ed. 2008 Nov;26(6):610–7.
- Scholtz V, Pazlarova J, Souskova H, Khun J, Julak J. Nonthermal plasma--A tool for decontamination and disinfection. Biotechnol Adv. 2015/01/18 ed. 2015 Nov 1;33(6 Pt 2):1108–19.
- Barjasteh A, Dehghani Z, Lamichhane P, Kaushik N, Choi EH, Kaushik NK. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Applied Sciences. 2021;11(8).
- von Woedtke T, Kramer A, Weltmann K. Plasma Sterilization: What are the Conditions to Meet this Claim? Plasma Processes and Polymers. 2008;5(6):534–9.
- Denis B, Steves S, Semmler E, Bibinov N, Novak W, Awakowicz P. Plasma Sterilization of Pharmaceutical Products: From Basics to Production. Plasma Processes and Polymers. 2012;9(6):619–29.
- Marsit NM, Sidney LE, Branch MJ, Wilson SL, Hopkinson A. Terminal sterilization: Conventional methods versus emerging cold atmospheric pressure plasma technology for non-viable biological tissues. Plasma Processes and Polymers. 2016;14(7).
- Kramer A, Bekeschus S, Matthes R, Bender C, Stope MB, Napp M, et al. Cold Physical Plasmas in the Field of Hygiene—Relevance, Significance, and Future Applications. Plasma Processes and Polymers. 2015;12(12):1410–22.
- Bourke P, Ziuzina D, Boehm D, Cullen PJ, Keener K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018/01/14 ed. 2018 Jun;36(6):615–26.
- Puač N, Gherardi M, Shiratani M. Plasma agriculture: A rapidly emerging field. Plasma Processes and Polymers. 2017;15(2).
- Ranieri P, Sponsel N, Kizer J, Rojas-Pierce M, Hernández R, Gatiboni L, et al. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Processes and Polymers. 2020;18(1).
- Ucar Y, Ceylan Z, Durmus M, Tomar O, Cetinkaya T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends in Food Science & Technology. 2021;114:355–71.
- Sanito RC, You SJ, Wang YF. Degradation of contaminants in plasma technology: An overview. J Hazard Mater. 2021/12/10 ed. 2022 Feb 15;424(Pt A):127390.
- Raiser J, Zenker M. Argon plasma coagulation for open surgical and endoscopic applications: state of the art. Journal of Physics D: Applied Physics. 2006;39(16):3520–3.
- Taheri A, Mansoori P, Sandoval LF, Feldman SR, Pearce D, Williford PM. Electrosurgery: part I. Basics and principles. J Am Acad Dermatol. 2014/03/19 ed. 2014 Apr;70(4):591 e1-591 e14.
- Stoffels E, Kieft IE, Sladek REJ. Superficial treatment of mammalian cells using plasma needle. Journal of Physics D: Applied Physics. 2003 Nov 19;36(23):2908.
- Stoffels E. “Tissue Processing” with Atmospheric Plasmas. Contributions to Plasma Physics. 2007;47(1–2):40–8.
- Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied Plasma Medicine. Plasma Processes and Polymers. 2008;5(6):503–33.
- Lloyd G, Friedman G, Jafri S, Schultz G, Fridman A, Harding K. Gas Plasma: Medical Uses and Developments in Wound Care. Plasma Processes and Polymers. 2010;7(3–4):194–211.
- Kramer A, Hübner NO, Weltmann KD, Lademann J, Ekkernkamp A, Hinz P, et al. Polypragmasia in the therapy of infected wounds – conclusions drawn from the perspectives of low temperature plasma technology for plasma wound therapy. GMS Krankenhaushygiene Interdisziplinär. 2008;3(1):8.
- Arndt S, Schmidt A, Karrer S, Von Woedtke T. Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clinical Plasma Medicine. 2018 Mar;9:24–33.
- Schmidt A, von Woedtke T, Vollmar B, Hasse S, Bekeschus S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics. 2019/03/15 ed. 2019;9(4):1066–84.
- Schmidt A, Liebelt G, Niessner F, von Woedtke T, Bekeschus S. Gas plasma-spurred wound healing is accompanied by regulation of focal adhesion, matrix remodeling, and tissue oxygenation. Redox Biol. 2020/12/04 ed. 2021 Jan;38:101809.
- Bekeschus S, von Woedtke T, Emmert S, Schmidt A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021 Oct;46:102116.
- Wu Y, Yu S, Zhang X, Wang X, Zhang J. The Regulatory Mechanism of Cold Plasma in Relation to Cell Activity and Its Application in Biomedical and Animal Husbandry Practices. Int J Mol Sci. 2023 Apr 12;24(8):7160.
- Bolgeo T, Maconi A, Gardalini M, Gatti D, Di Matteo R, Lapidari M, et al. The Role of Cold Atmospheric Plasma in Wound Healing Processes in Critically Ill Patients. Journal of personalized medicine. 2023 Apr 26;13(5).
- Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta S, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer. 2011/10/08 ed. 2011 Oct 25;105(9):1295–301.
- Dubuc A, Monsarrat P, Virard F, Merbahi N, Sarrette JP, Laurencin-Dalicieux S, et al. Use of cold-atmospheric plasma in oncology: a concise systematic review. Ther Adv Med Oncol. 2018/07/27 ed. 2018;10:1758835918786475.
- Yan D, Malyavko A, Wang Q, Ostrikov KK, Sherman JH, Keidar M. Multi-Modal Biological Destruction by Cold Atmospheric Plasma: Capability and Mechanism. Biomedicines. 2021/09/29 ed. 2021 Sep 18;9(9).
- Ishikawa K, Takeda K, Yoshimura S, Kondo T, Tanaka H, Toyokuni S, et al. Generation and measurement of low-temperature plasma for cancer therapy: a historical review. Free Radical Research. 2023 Mar 4;57(3):239–70.
- Khalili M, Daniels L, Lin A, Krebs FC, Snook AE, Bekeschus S, et al. Non-Thermal Plasma-Induced Immunogenic Cell Death in Cancer: A Topical Review. J Phys D Appl Phys. 2019/09/06 ed. 2019 Oct 22;52(42).
- Semmler ML, Bekeschus S, Schafer M, Bernhardt T, Fischer T, Witzke K, et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers (Basel). 2020/01/26 ed. 2020 Jan 22;12(2).
- Bekeschus S, Freund E, Spadola C, Privat-Maldonado A, Hackbarth C, Bogaerts A, et al. Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers (Basel). 2019/08/28 ed. 2019 Aug 23;11(9).
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford: Oxford University Press; 2007.
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008/06/05 ed. 2008 Aug;10(8):1343–74.
- Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta. 2008 Nov;1780(11):1348–61.
- Schmidt A, Bekeschus S. Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants. 2018;7(10):146.
- Nosenko T, Shimizu T, Morfill GE. Designing plasmas for chronic wound disinfection. New Journal of Physics. 2009;11(11):115013.
- Ermolaeva SA, Varfolomeev AF, Chernukha MYu, Yurov DS, Vasiliev MM, Kaminskaya AA, et al. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. Journal of Medical Microbiology. 2011 Jan 1;60(1):75–83.
- Kang SK, Seo YS, Lee HW, Aman ur R, Kim GC, Lee JK. Slit shaped microwave induced atmospheric pressure plasma based on a parallel plate transmission line resonator. Journal of Physics D: Applied Physics. 2011;44(43):435201.
- Kim PY, Kim YS, Koo IG, Jung JC, Kim GJ, Choi MY, et al. Bacterial Inactivation of Wound Infection in a Human Skin Model by Liquid-Phase Discharge Plasma. PLoS ONE. 2011;6(8):e24104.
- Laurita R, Miserocchi A, Ghetti M, Gherardi M, Stancampiano A, Purpura V, et al. Cold Atmospheric Plasma Treatment of Infected Skin Tissue: Evaluation of Sterility, Viability, and Integrity. IEEE Trans Radiat Plasma Med Sci. 2017 May;1(3):275–9.
- Ghimire B, Patenall BL, Szili EJ, Gaur N, Lamichhane P, Thet NT, et al. The influence of a second ground electrode on hydrogen peroxide production from an atmospheric pressure argon plasma jet and correlation to antibacterial efficacy and mammalian cell cytotoxicity. Journal of Physics D: Applied Physics. 2021 Dec 28;55(12):125207.
- Ghimire B, Szili EJ, Patenall BL, Lamichhane P, Gaur N, Robson AJ, et al. Enhancement of hydrogen peroxide production from an atmospheric pressure argon plasma jet and implications to the antibacterial activity of plasma activated water. Plasma Sources Science and Technology. 2021 Mar 1;30(3):035009.
- Szili EJ, Ghimire B, Patenall BL, Rohaim M, Mistry D, Fellows A, et al. On-demand cold plasma activation of acetyl donors for bacteria and virus decontamination. Applied Physics Letters. 2021;119(5):054104.
- Mohd Nasir N, Lee BK, Yap SS, Thong KL, Yap SL. Cold plasma inactivation of chronic wound bacteria. Archives of Biochemistry and Biophysics. 2016;605:76–85.
- Burts ML, Alexeff I, Meek ET, McCullers JA. Use of atmospheric non-thermal plasma as a disinfectant for objects contaminated with methicillin-resistant Staphylococcus aureus. American Journal of Infection Control. 2009;37(9):729–33.
- Maisch T, Shimizu T, Li YF, Heinlin J, Karrer S, Morfill G, et al. Decolonisation of MRSA, S. aureus and E. coli by Cold-Atmospheric Plasma Using a Porcine Skin Model In Vitro. Otto M, editor. PLoS ONE. 2012 Apr 27;7(4):e34610.
- Sladek REJ, Filoche SK, Sissons CH, Stoffels E. Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Letters in Applied Microbiology. 2007;45(3):318–23.
- Xiong Z, Du T, Lu X, Cao Y, Pan Y. How deep can plasma penetrate into a biofilm? Applied Physics Letters. 2011;98(22):221503–3.
- Alkawareek MY, Algwari QT, Laverty G, Gorman SP, Graham WG, O’Connell D, et al. Eradication of Pseudomonas aeruginosa Biofilms by Atmospheric Pressure Non-Thermal Plasma. PLoS ONE. 2012;7(8):e44289.
- Pei X, Lu X, Liu J, Liu D, Yang Y, Ostrikov K, et al. Inactivation of a 25.5 µm Enterococcus faecalis biofilm by a room-temperature, battery-operated, handheld air plasma jet. Journal of Physics D: Applied Physics. 2012;45(16):165205.
- Pan J, Sun K, Liang Y, Sun P, Yang X, Wang J, et al. Cold Plasma Therapy of a Tooth Root Canal Infected with Enterococcus faecalis Biofilms In Vitro. Journal of Endodontics. 2013;39(1):105–10.
- Niemira BA, Boyd G, Sites J. Cold Plasma Rapid Decontamination of Food Contact Surfaces Contaminated with Salmonella Biofilms. Journal of Food Science. 2014;79:M917–22.
- Patenall BL, Hathaway H, Sedgwick AC, Thet NT, Williams GT, Young AE, et al. Limiting Pseudomonas aeruginosa Biofilm Formation Using Cold Atmospheric Pressure Plasma. 2018 Sep 26;8(3):269–77.
- Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS. Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas aeruginosa. Journal of Bacteriology. 2006 Nov 1;188(21):7344–53.
- Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. Journal of Antimicrobial Chemotherapy. 2012;67(7):1589–96.
- Veal EA, Day AM, Morgan BA. Hydrogen Peroxide Sensing and Signaling. Molecular Cell. 2007;26(1):1–14.
- Dröge W. Free Radicals in the Physiological Control of Cell Function. Physiological Reviews. 2002 Jan 1;82(1):47–95.
- Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017 Feb;14(1):89–96.
- Schäffer MR, Tantry U, Gross SS, Wasserkrug HL, Barbul A. Nitric Oxide Regulates Wound Healing. Journal of Surgical Research. 1996 Jun 1;63(1):237–40.
- Luo J dong, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacologica Sinica. 2005 Mar 1;26(3):259–64.
- Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep. 2006/01/18 ed. 2005;57 Suppl:108–19.
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB j. 1999 Nov;13(14):2002–14.
- Endre JS, Frances JH, Sung-Ha H, Franziska H, Nicolas HV, Robert DS. The hormesis effect of plasma-elevated intracellular ROS on HaCaT cells. Journal of Physics D: Applied Physics. 2015;48(49):495401.
- Arjunan KP, Clyne AM. A Nitric Oxide Producing Pin-to-Hole Spark Discharge Plasma Enhances Endothelial Cell Proliferation and Migration. 2013 Feb 11;1(3–4):279–93.
- Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011 Jan 21;6(1):e16270.
- Liebmann J, Scherer J, Bibinov N, Rajasekaran P, Kovacs R, Gesche R, et al. Biological effects of nitric oxide generated by an atmospheric pressure gas-plasma on human skin cells. Nitric Oxide. 2011;24(1):8–16.
- Hasse S, Hahn O, Kindler S, Woedtke T von, Metelmann HR, Masur K. Atmospheric Pressure Plasma Jet Application on Human Oral Mucosa Modulates Tissue Regeneration. 2015 Feb 11;4(1–4):117–29.
- Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann Biomed Eng. 2010 Mar;38(3):748–57.
- Ngo MHT, Liao JD, Shao PL, Weng CC, Chang CY. Increased Fibroblast Cell Proliferation and Migration Using Atmospheric N2/Ar Micro-Plasma for the Stimulated Release of Fibroblast Growth Factor-7. Plasma Processes and Polymers. 2014;11(1):80–8.
- Haertel B, Straßenburg S, Oehmigen K, Wende K, von Woedtke T, Lindequist U. Differential Influence of Components Resulting from Atmospheric-Pressure Plasma on Integrin Expression of Human HaCaT Keratinocytes. BioMed Research International. 2013;2013:9.
- Schmidt A, von Woedtke T, Weltmann KD, Masur K. Identification of the Molecular Basis of Non-thermal Plasma-Induced Changes in Human Keratinocytes. 2014 Oct 1;3(1–2):15–25.
- Landsberg K, Scharf Ch, Darm K, Wende K, Daeschlein G, Kindel E, et al. Use of Proteomics to Investigate Plasma-Cell Interactions. 2010 Sep 10;1(1):55–63.
- Rodriguez PG, Felix FN, Woodley DT, Shim EK. The Role of Oxygen in Wound Healing: A Review of the Literature. Dermatologic Surgery. 2008;34(9):1159–69.
- Sen CK. Wound Healing Essentials: Let There Be Oxygen. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(1):1–18.
- Arjunan KP, Friedman G, Fridman A, Clyne AM. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J R Soc Interface. 2012 Jan 7;9(66):147–57.
- Oh JS, Strudwick X, Short RD, Ogawa K, Hatta A, Furuta H, et al. How plasma induced oxidation, oxygenation, and de-oxygenation influences viability of skin cells. Applied Physics Letters. 2016;109(20):203701.
- Collet G, Robert E, Lenoir A, Vandamme M, Darny T, Dozias S, et al. Plasma jet-induced tissue oxygenation: potentialities for new therapeutic strategies. Plasma Sources Science and Technology. 2014;23(1):012005.
- Endre JS, Jun-Seok O, Hideo F, Rishabh B, Nishtha G, Cuong KN, et al. Modelling the helium plasma jet delivery of reactive species into a 3D cancer tumour. Plasma Sources Science and Technology. 2018;27(1):014001.
- Jun-Seok O, Endre JS, Nishtha G, Sung-Ha H, Hiroshi F, Hirofumi K, et al. How to assess the plasma delivery of RONS into tissue fluid and tissue. Journal of Physics D: Applied Physics. 2016;49(30):304005.
- Oh JS, Szili EJ, Ito S, Hong SH, Gaur N, Furuta H, et al. Slow Molecular Transport of Plasma-Generated Reactive Oxygen and Nitrogen Species and O2 through Agarose as a Surrogate for Tissue. Plasma Medicine. 2015;5:125–43.
- Gaur N, Szili EJ, Oh JS, Hong SH, Michelmore A, Graves DB, et al. Combined effect of protein and oxygen on reactive oxygen and nitrogen species in the plasma treatment of tissue. Applied Physics Letters. 2015 Sep 7;107(10):103703.
- Jun-Seok O, James LW, James WB. Plasma bullet current measurements in a free-stream helium capillary jet. Plasma Sources Science and Technology. 2012;21(3):034020.
- Bakalyar SR, Bradley MPT, Honganen R. The role of dissolved gases in high-performance liquid chromatography. Journal of Chromatography A. 1978 Oct 1;158:277–93.
- Jablonowski H, Hänsch MACh, Dünnbier M, Wende K, Hammer MU, Weltmann KD, et al. Plasma jet’s shielding gas impact on bacterial inactivation. Biointerphases. 2015;10(2):029506.
- Reuter S, Tresp H, Wende K, Hammer MU, Winter J, Masur K, et al. From RONS to ROS: Tailoring Plasma Jet Treatment of Skin Cells. Plasma Science, IEEE Transactions on. 2012;40(11):2986–93.
- Winter J, Wende K, Masur K, Iseni S, Dnnbier M, Hammer MU, et al. Feed gas humidity: a vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. Journal of Physics D: Applied Physics. 2013;46(29):295401–295401.
- Oh JS, Szili EJ, Hatta A, Ito M, Shirafuji T. Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet. Plasma. 2019;2(2):127–37.
- Kotaro O, Jun-Seok O, Nishtha G, Sung-Ha H, Hirofumi K, Akira M, et al. Modulating the concentrations of reactive oxygen and nitrogen species and oxygen in water with helium and argon gas and plasma jets. Japanese Journal of Applied Physics. 2019;58(SA):SAAB01.
- Dubey SK, Parab S, Alexander A, Agrawal M, Achalla VPK, Pal UN, et al. Cold atmospheric plasma therapy in wound healing. Process Biochemistry. 2022 Jan 1;112:112–23.
- Mohseni P, Ghorbani A, Fariborzi N. Exploring the potential of cold plasma therapy in treating bacterial infections in veterinary medicine: opportunities and challenges. Front Vet Sci. 20230901st ed. 2023;10:1240596.
- Bekeschus S, Kramer A, Schmidt A. Gas Plasma-Augmented Wound Healing in Animal Models and Veterinary Medicine. Molecules. 2021;26(18).
- Nastuta AV, Topala I, Grigoras C, Pohoata V, Popa G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. Journal of Physics D: Applied Physics. 2011 Feb 21;44(10):105204.
- Yu Y, Tan M, Chen H, Wu Z, Xu L, Li J, et al. Non-thermal plasma suppresses bacterial colonization on skin wound and promotes wound healing in mice. J Huazhong Univ Sci Technolog Med Sci. 20110614th ed. 2011 Jun;31(3):390–4.
- Arndt S, Unger P, Wacker E, Shimizu T, Heinlin J, Li YF, et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One. 20131112th ed. 2013;8(11):e79325.
- García-Alcantara E, López-Callejas R, Morales-Ramírez PR, Peña-Eguiluz R, Fajardo-Muñoz R, Mercado-Cabrera A, et al. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch Med Res. 20130316th ed. 2013 Apr;44(3):169–77.
- Wu AS, Kalghatgi S, Dobrynin D, Sensenig R, Cerchar E, Podolsky E, et al. Porcine intact and wounded skin responses to atmospheric nonthermal plasma. J Surg Res. 20120310th ed. 2013 Jan;179(1):e1–12.
- Jacofsky MC, Lubahn C, McDonnell C, Seepersad Y, Fridman G, Fridman AA, et al. Spatially Resolved Optical Emission Spectroscopy of a Helium Plasma Jet and its Effects on Wound Healing Rate in a Diabetic Murine Model. Plasma Medicine. 2015 Feb 11;4(1–4):177–91.
- Ngo Thi MH, Shao PL, Liao JD, Lin CCK, Yip HK. Enhancement of Angiogenesis and epithelialisation Processes in Mice with Burn Wounds through ROS/RNS Signals Generated by Non-Thermal N2/Ar Micro-Plasma. Plasma Processes and Polymers. 2014 Nov 1;11(11):1076–88.
- Nasruddin, Nakajima Y, Mukai K, Rahayu HSE, Nur M, Ishijima T, et al. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialisation and wound contraction. Clinical Plasma Medicine. 2014 Jul 1;2(1):28–35.
- Nasruddin, Nakajima Y, Mukai K, Komatsu E, Rahayu HSE, Nur M, et al. A Simple Technique to Improve Contractile Effect of Cold Plasma Jet on Acute Mouse Wound by Dropping Water. Plasma Processes and Polymers. 2015 Oct 1;12(10):1128–38.
- Ngo H, Huynh L, Jiunn Der L, Nguyen T. Stimulation of wound healing process through ROS/RNS signals indirectly generated by N2/Ar micro-plasma – in vitro and in vivo studies. Science and Technology Development Journal. 2015;18(2):29–37.
- Kim HY, Kang SK, Park SM, Jung HY, Choi BH, Sim JY, et al. Characterization and Effects of Ar/Air Microwave Plasma on Wound Healing. Plasma Processes and Polymers. 2015 Dec 1;12(12):1423–34.
- Xu GM, Shi XM, Cai JF, Chen SL, Li P, Yao CW, et al. Dual effects of atmospheric pressure plasma jet on skin wound healing of mice. Wound Repair Regen. 2015;23(6):878–84.
- Shao PL, Liao JD, Wong TW, Wang YC, Leu S, Yip HK. Enhancement of Wound Healing by Non-Thermal N2/Ar Micro-Plasma Exposure in Mice with Fractional-CO2-Laser-Induced Wounds. PLOS ONE. 2016;11(6):e0156699.
- Hung YW, Lee LT, Peng YC, Chang CT, Wong YK, Tung KC. Effect of a nonthermal-atmospheric pressure plasma jet on wound healing: An animal study. J Chin Med Assoc. 20160329th ed. 2016 Jun;79(6):320–8.
- Kim DW, Park TJ, Jang SJ, You SJ, Oh WY. Plasma treatment effect on angiogenesis in wound healing process evaluated in vivo using angiographic optical coherence tomography. Applied Physics Letters. 2016;109(23):233701.
- Schmidt A, Bekeschus S, Wende K, Vollmar B, von Woedtke T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp Dermatol. 2017 Feb;26(2):156–62.
- Nasruddin, Putri IK, Kamal S, Esti Rahayu HS, Lutfiyati H, Pribadi P, et al. Evaluation the effectiveness of combinative treatment of cold plasma jet, Indonesian honey, and micro-well dressing to accelerate wound healing. Clinical Plasma Medicine. 2017 Jun 1;5–6:14–25.
- Kos S, Blagus T, Cemazar M, Filipic G, Sersa G, Cvelbar U. Safety aspects of atmospheric pressure helium plasma jet operation on skin: In vivo study on mouse skin. PLoS One. 2017;12(4):e0174966.
- Schmidt A, Woedtke T von, Stenzel J, Lindner T, Polei S, Vollmar B, et al. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int J Mol Sci. 2017 Apr 19;18(4):868.
- Shahbazi Rad Z, Abbasi Davani F, Etaati G. Determination of proper treatment time for in vivo blood coagulation and wound healing application by non-thermal helium plasma jet. Australas Phys Eng Sci Med. 20181001st ed. 2018 Dec;41(4):905–17.
- Arndt S, Unger P, Berneburg M, Bosserhoff AK, Karrer S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J Dermatol Sci. 20171126th ed. 2018 Feb;89(2):181–90.
- Choi BBR, Choi JH, Ji J, Song KW, Lee HJ, Kim GC. Increment of growth factors in mouse skin treated with non-thermal plasma. Int J Med Sci. 20180730th ed. 2018;15(11):1203–9.
- Duchesne C, Frescaline N, Lataillade JJ, Rousseau A. Comparative Study between Direct and Indirect Treatment with Cold Atmospheric Plasma on In Vitro and In Vivo Models of Wound Healing. Plasma Medicine. 2018 Nov 7;8(4):379–401.
- Wahyuningtyas ES, Iswara A, Sari Y, Kamal S, Santosa B, Ishijima T, et al. Comparative study on Manuka and Indonesian honeys to support the application of plasma jet during proliferative phase on wound healing. Clinical Plasma Medicine. 2018 Dec 1;12:1–9.
- Duchesne C, Banzet S, Lataillade JJ, Rousseau A, Frescaline N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J Pathol. 2019 Nov;249(3):368–80.
- Darmawati S, Rohmani A, Nurani LH, Prastiyanto ME, Dewi SS, Salsabila N, et al. When plasma jet is effective for chronic wound bacteria inactivation, is it also effective for wound healing? Clinical Plasma Medicine. 2019 Jun 1;14:100085.
- Shahbazi Rad Z, Abbasi Davani F. Measurements of the electrical parameters and wound area for investigation on the effect of different non-thermal atmospheric pressure plasma sources on wound healing time. Measurement. 2020 Apr 1;155:107545.
- He R, Li Q, Shen W, Wang T, Lu H, Lu J, et al. The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. Int Wound J. 2020 Jun;17(3):851–63.
- Xu D, Wang S, Li B, Qi M, Feng R, Li Q, et al. Effects of Plasma-Activated Water on Skin Wound Healing in Mice. Microorganisms. 20200721st ed. 2020 Jul 21;8(7).
- Amini M, Jahandideh A, Dehghanpisheh P, Momeni M, Asghari A. Comparative Study of Histological Change After Local Treatments with Zinc Oxide, Infrared Rays, Ultraviolet Rays, and Cold Plasma in Rat Model of Diabetic Foot. Indian Journal of Surgery. 2020 Dec 1;82(6):1094–9.
- Martines E, Brun P, Cavazzana R, Cordaro L, Zuin M, Martinello T, et al. Wound healing improvement in large animals using an indirect helium plasma treatment. Clinical Plasma Medicine. 2020 Mar 1;17–18:100095.
- Wang S, Xu D, Qi M, Li B, Peng S, Li Q, et al. Plasma-Activated Water Promotes Wound Healing by Regulating Inflammatory Responses. Biophysica. 2021;1(3):297–310.
- Dang CP, Weawseetong S, Charoensappakit A, Sae-Khow K, Thong-Aram D, Leelahavanichkul A. Non-Thermal Atmospheric Pressure Argon-Sourced Plasma Flux Promotes Wound Healing of Burn Wounds and Burn Wounds with Infection in Mice through the Anti-Inflammatory Macrophages. Applied Sciences. 2021 Jun 9;11(12):5343.
- Schmidt A, Nießner F, Woedtke T von, Bekeschus S. Hyperspectral Imaging of Wounds Reveals Augmented Tissue Oxygenation Following Cold Physical Plasma Treatment in Vivo. IEEE Transactions on Radiation and Plasma Medical Sciences. 2021;5(3):412–9.
- Darmawati S, Nasruddin N, Putri GSA, Iswara A, Kurniasiwi P, Wahyuningtyas ES, et al. Accelerated Healing of Chronic Wounds under a Combinatorial Therapeutic Regimen Based on Cold Atmospheric Plasma Jet Using Contact and Noncontact Styles. Plasma Medicine. 2021 Jul 30;11(2):1–18.
- Evert K, Kocher T, Schindler A, Muller M, Muller K, Pink C, et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci Rep. 20211019th ed. 2021 Oct 19;11(1):20672.
- Amini M, Momeni M, Jahandideh A, Ghoranneviss M, Soudmand S, Yousefi P, et al. Tendon repair by plasma jet treatment. J Diabetes Metab Disord. 2021 Jun;20(1):621–6.
- Melotti L, Martinello T, Perazzi A, Martines E, Zuin M, Modenese D, et al. Could cold plasma act synergistically with allogeneic mesenchymal stem cells to improve wound skin regeneration in a large size animal model? Res Vet Sci. 20210128th ed. 2021 May;136:97–110.
- Borchardt T, Ernst J, Helmke A, Tanyeli M, Schilling AF, Felmerer G, et al. Effect of direct cold atmospheric plasma (diCAP) on microcirculation of intact skin in a controlled mechanical environment. Microcirculation (New York, NY : 1994). 2017;24(8).
- Daeschlein G, Napp M, Lutze S, Arnold A, von Podewils S, Guembel D, et al. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. J Dtsch Dermatol Ges. 2015 Feb;13(2):143–50.
- Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010 Jul;163(1):78–82.
- Jensen JO, Schulz L, Schleusser S, Matzkeit N, Stang FH, Mailaender P, et al. The repetitive application of cold atmospheric plasma (CAP) improves microcirculation parameters in chronic wounds. Microvasc Res. 2021 Nov;138:104220.
- Kisch T, Helmke A, Schleusser S, Song J, Liodaki E, Stang FH, et al. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): Results of a controlled, prospective cohort study. Microvasc Res. 2016 Mar;104:55–62.
- Kisch T, Schleusser S, Helmke A, Mauss KL, Wenzel ET, Hasemann B, et al. The repetitive use of non-thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvascular Research. 2016 Jul;106:8–13.
- Klebes M, Ulrich C, Kluschke F, Patzelt A, Vandersee S, Richter H, et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J Biophotonics. 2014/03/25 ed. 2015 May;8(5):382–91.
- Bernhardt T, Semmler ML, Schafer M, Bekeschus S, Emmert S, Boeckmann L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid Med Cell Longev. 2019/10/01 ed. 2019;2019:3873928.
- Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012 Aug;167(2):404–10.
- Isbary G, Stolz W, Shimizu T, Monetti R, Bunk W, Schmidt HU, et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clinical Plasma Medicine. 2013;1(2):25–30.
- Brehmer F, Haenssle HA, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, et al. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm(®) VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J Eur Acad Dermatol Venereol. 2015 Jan;29(1):148–55.
- Ulrich C, Kluschke F, Patzelt A, Vandersee S, Czaika VA, Richter H, et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J Wound Care. 2015 May;24(5):196, 198–200, 202–3.
- Chuangsuwanich A, Assadamongkol T, Boonyawan D. The Healing Effect of Low-Temperature Atmospheric-Pressure Plasma in Pressure Ulcer: A Randomized Controlled Trial. Int J Low Extrem Wounds. 2016/09/02 ed. 2016 Dec;15(4):313–9.
- González-Mendoza B, López-Callejas R, Rodríguez-Méndez BG, Eguiluz RP, Mercado-Cabrera A, Valencia-Alvarado R, et al. Healing of wounds in lower extremities employing a non-thermal plasma. Clinical Plasma Medicine. 2019;16:100094.
- Stratmann B, Costea TC, Nolte C, Hiller J, Schmidt J, Reindel J, et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Network Open. 2020 Jul 16;3(7):e2010411.
- Mirpour S, Fathollah S, Mansouri P, Larijani B, Ghoranneviss M, Mohajeri Tehrani M, et al. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci Rep. 2020 Jun 26;10(1):10440.
- Amini MR, Sheikh Hosseini M, Fatollah S, Mirpour S, Ghoranneviss M, Larijani B, et al. Beneficial effects of cold atmospheric plasma on inflammatory phase of diabetic foot ulcers; a randomized clinical trial. J Diabetes Metab Disord. 2020 Dec;19(2):895–905.
- Moelleken M, Jockenhöfer F, Wiegand C, Buer J, Benson S, Dissemond J. Pilotstudie zum Einfluss von kaltem atmosphärischem Plasma auf bakterielle Kontamination und Heilungstendenz chronischer Wunden. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2020;18(10):1094–102.
- Samsavar S, Mahmoudi H, Shakouri R, Khani MR, Molavi B, Moosavi J, et al. The evaluation of efficacy of atmospheric pressure plasma in diabetic ulcers healing: A randomized clinical trial. Dermatol Ther. 2021 Nov;34(6):e15169.
- Schleusser S, Schulz L, Song J, Deichmann H, Griesmann AC, Stang FH, et al. A single application of cold atmospheric plasma (CAP) improves blood flow parameters in chronic wounds. Microcirculation. 2022 Apr;29(3):e12754.
- Strohal R, Dietrich S, Mittlböck M, Hämmerle G. Chronic wounds treated with cold atmospheric plasmajet versus best practice wound dressings: a multicenter, randomized, non-inferiority trial. Sci Rep. 2022 Mar 7;12(1):3645.
- Weigand C, Haycocks S, Chadwick P, Russell D, Cutting K. The efficacy of non-thermal gas plasma in the treatment of diabetic foot ulcers stalled by subclinical, biofilm-related wound infection. 2022 Dec 8;Volume 13:14–21.
- Lagrand RS, Sabelis LW, de Groot V, Peters EJ. Cold plasma treatment is safe for diabetic foot ulcers and decreases Staphylococcus aureus bacterial load. J Wound Care. 2023 Apr 2;32(4):247–51.
- Abu Rached N, Kley S, Storck M, Meyer T, Stücker M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J Clin Med. 2023 Aug 4;12(15):5121.
- Bakker O, Smits P, Van Weersch C, Quaaden M, Bruls E, Van Loon A, et al. Improved Wound Healing by Direct Cold Atmospheric Plasma Once or Twice a Week: A Randomized Controlled Trial on Chronic Venous Leg Ulcers. Advances in Wound Care. 2024 Jun 3;wound.2023.0196.
- Ligresti C, Malan F, Motolese A, Del Zotti M. Use of Cold Plasma in the Treatment of Infected Wounds. J Surg Res Prac. 2024 Apr 30;5(1):1–10.
- Hiller J, Stratmann B, Timm J, Costea TC, Tschoepe D. Enhanced growth factor expression in chronic diabetic wounds treated by cold atmospheric plasma. Diabet Med. 2022 Jun;39(6):e14787.
- Metelmann HR, Vu TT, Do HT, Le TNB, Hoang THA, Phi TTT, et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clinical Plasma Medicine. 2013;1(1):30–5.
- Nishijima A. A New Energy Device for Skin Activation to Acute Wound Using Cold Atmospheric Pressure Plasma: A Randomized Controlled Clinical Trial. BJSTR. 2019;21(1).
- Nishijima A, Fujimoto T, Hirata T, Nishijima J. Effects of Cold Atmospheric Pressure Plasma on Accelerating Acute Wound Healing: A Comparative Study among 4 Different Treatment Groups. MPS. 2019;09(01):18–31.
- Vandersee S, Richter H, Lademann J, Beyer M, Kramer A, Knorr F, et al. Laser scanning microscopy as a means to assess the augmentation of tissue repair by exposition of wounds to tissue tolerable plasma. Laser Phys Lett. 2014 Nov;11(11):115701.
- Heinlin J, Zimmermann JL, Zeman F, Bunk W, Isbary G, Landthaler M, et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013/08/14 ed. 2013 Nov;21(6):800–7.
- van Welzen A, Hoch M, Wahl P, Weber F, Rode S, Tietze JK, et al. The Response and Tolerability of a Novel Cold Atmospheric Plasma Wound Dressing for the Healing of Split Skin Graft Donor Sites: A Controlled Pilot Study. Skin Pharmacol Physiol. 2021;34(6):328–36.
- Matzkeit N, Schulz L, Schleusser S, Jensen JO, Stang FH, Mailaender P, et al. Cold atmospheric plasma improves cutaneous microcirculation in standardized acute wounds: Results of a controlled, prospective cohort study. Microvasc Res. 2021 Nov;138:104211.
- Metelmann HR, von Woedtke T, Bussiahn R, Weltmann KD, Rieck M, Khalili R, et al. Experimental Recovery of CO2-Laser Skin Lesions by Plasma Stimulation. The American Journal of Cosmetic Surgery. 2012;29(1):52–6.
- Metelmann H, Emmert S, Gümbel D, Harréus U, Kebschull M, Kersten H, et al. S2k-Leitlinie Rationaler therapeutischer Einsatz von kaltem physikalischem Plasma (Registernummer 007–107). Deutsche Gesellschaft für Mund-, Kiefer- und Gesichtschirurgie e.V. (DGMKG); 2022
- Gan L, Jiang J, Duan JW, Wu XJZ, Zhang S, Duan XR, et al. Cold atmospheric plasma applications in dermatology: A systematic review. J Biophotonics. 2021 Mar;14(3):e202000415.
- Lee Y, Ricky S, Lim TH, Kim H, Lee EJ, Song Y, et al. An Atmospheric Plasma Jet Induces Expression of Wound Healing Genes in Progressive Burn Wounds in a Comb Burn Rat Model: A Pilot Study. J Burn Care Res. 2023 May 2;44(3):685–92.
- Marches A, Clement E, Albérola G, Rols MP, Cousty S, Simon M, et al. Cold Atmospheric Plasma Jet Treatment Improves Human Keratinocyte Migration and Wound Closure Capacity without Causing Cellular Oxidative Stress. Int J Mol Sci. 2022 Sep 13;23(18):10650.
- Balzer J, Demir E, Kogelheide F, Fuchs PC, Stapelmann K, Opländer C. Cold atmospheric plasma (CAP) differently affects migration and differentiation of keratinocytes via hydrogen peroxide and nitric oxide-related products. Clinical Plasma Medicine. 2019 Mar;13:1–8.
- Heuer K, Hoffmanns MA, Demir E, Baldus S, Volkmar CM, Röhle M, et al. The topical use of non-thermal dielectric barrier discharge (DBD): Nitric oxide related effects on human skin. Nitric Oxide. 2015 Jan;44:52–60.
- Fluhr JW, Sassning S, Lademann O, Darvin ME, Schanzer S, Kramer A, et al. In vivo skin treatment with tissue-tolerable plasma influences skin physiology and antioxidant profile in human stratum corneum. Experimental dermatology. 2011/12/07 ed. 2012 Feb;21(2):130–4.
- Ganzarolli De Oliveira M. S-Nitrosothiols as Platforms for Topical Nitric Oxide Delivery. Basic Clin Pharma Tox. 2016 Oct;119(S3):49–56.
- Vercelino R, Cunha TM, Ferreira ES, Cunha FQ, Ferreira SH, De Oliveira MG. Skin vasodilation and analgesic effect of a topical nitric oxide-releasing hydrogel. J Mater Sci: Mater Med. 2013 Sep;24(9):2157–69.
- Suschek CV, Opländer C. The application of cold atmospheric plasma in medicine: The potential role of nitric oxide in plasma-induced effects. Clinical Plasma Medicine. 2016 Jul;4(1):1–8.
- Wu Y, Nieuwenhoff MD, Huygen FJPM, Van Der Helm FCT, Niehof S, Schouten AC. Characterizing human skin blood flow regulation in response to different local skin temperature perturbations. Microvascular Research. 2017 May;111:96–102.
- Del Pozzi AT, Miller JT, Hodges GJ. The effect of heating rate on the cutaneous vasomotion responses of forearm and leg skin in humans. Microvascular Research. 2016 May;105:77–84.
- Hodges GJ, Mallette MM, Tew GA, Saxton JM, Moss J, Ruddock AD, et al. Effect of age on cutaneous vasomotor responses during local skin heating. Microvascular Research. 2017 Jul;112:47–52.
- Boekema B, Stoop M, Vlig M, van Liempt J, Sobota A, Ulrich M, et al. Antibacterial and safety tests of a flexible cold atmospheric plasma device for the stimulation of wound healing. Appl Microbiol Biotechnol. 2021 Mar;105(5):2057–70.
- Daeschlein G, Scholz S, Ahmed R, Majumdar A, Von Woedtke T, Haase H, et al. Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture: Cold plasma well-tolerated by skin. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2012 Jul;10(7):509–15.
- G, Scholz S, Ahmed R, von Woedtke T, Haase H, Niggemeier M, et al. Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. The Journal of hospital infection. 2012/06/12 ed. 2012 Jul;81(3):177–83.
- Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regeneration. 2014 Mar;22(2):174–86.
- Chen G, Chen Z, Wang Z, Obenchain R, Wen D, Li H, et al. Portable air-fed cold atmospheric plasma device for postsurgical cancer treatment. Sci Adv. 2021 Sep 3;7(36):eabg5686.
- Bekeschus S, Brüggemeier J, Hackbarth C, Von Woedtke T, Partecke LI, Van Der Linde J. Platelets are key in cold physical plasma-facilitated blood coagulation in mice. Clinical Plasma Medicine. 2017 Dec;7–8:58–65.
- Bekeschus S, Poschkamp B, Van Der Linde J. Medical gas plasma promotes blood coagulation via platelet activation. Biomaterials. 2021 Nov;278:120433.
- Canullo L, Genova T, Naenni N, Nakajima Y, Masuda K, Mussano F. Plasma of argon enhances the adhesion of murine osteoblasts on different graft materials. Annals of Anatomy – Anatomischer Anzeiger. 2018 Jul;218:265–70.
- Canullo L, Genova T, Tallarico M, Gautier G, Mussano F, Botticelli D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces: An In Vitro Comparative Study. J Dent Res. 2016 May;95(5):566–73.
- Canullo L, Genova T, Wang HL, Carossa S, Mussano F. Plasma of Argon Increases Cell Attachment and Bacterial Decontamination on Different Implant Surfaces. Int J Oral Maxillofac Implants. 2017 Nov;32(6):1315–23.
- Hui WL, Perrotti V, Iaculli F, Piattelli A, Quaranta A. The Emerging Role of Cold Atmospheric Plasma in Implantology: A Review of the Literature. Nanomaterials. 2020 Jul 31;10(8):1505.
- Den Braber ET, De Ruijter JE, Smits HTJ, Ginsel LA, Von Recum AF, Jansen JA. Effect of parallel surface microgrooves and surface energy on cell growth. J Biomed Mater Res. 1995 Apr;29(4):511–8.
- Ji MK, Oh G, Kim JW, Park S, Yun KD, Bae JC, et al. Effects on Antibacterial Activity and Osteoblast Viability of Non-Thermal Atmospheric Pressure Plasma and Heat Treatments of TiO 2 Nanotubes. j nanosci nanotechnol. 2017 Apr 1;17(4):2312–5.
- Pan H, Wang G, Pan J, Ye G, Sun K, Zhang J, et al. Cold plasma-induced surface modification of heat-polymerized acrylic resin and prevention of early adherence of Candida albicans . Dental Materials Journal. 2015;34(4):529–36.
- Schendzielorz P, Schmitz T, Moseke C, Gbureck U, FrFlich K, Rak K, et al. Plasma-Assisted Hydrophilization of Cochlear Implant Electrode Array Surfaces Enables Adhesion of Neurotrophin-Secreting Cells. ORL. 2014;76(5):257–65.
- Wang M, Zhou Y, Shi D, Chang R, Zhang J, Keidar M, et al. Cold atmospheric plasma (CAP)-modified and bioactive protein-loaded core–shell nanofibers for bone tissue engineering applications. Biomater Sci. 2019;7(6):2430–9.
- Bogle MA, Arndt KA, Dover JS. Evaluation of Plasma Skin Regeneration Technology in Low-Energy Full-Facial Rejuvenation. Arch Dermatol. 2007 Feb 1 [cited 2024 Jun 27];143(2).
- Elsaie ML, Kammer JN. Evaluation of plasma skin regeneration technology for cutaneous remodeling. J of Cosmetic Dermatology. 2008 Dec;7(4):309–11.
- Gentile RD, McCoy JD. Pulsed and Fractionated Techniques for Helium Plasma Energy Skin Resurfacing. Facial Plastic Surgery Clinics of North America. 2020 Feb;28(1):75–85.
- Fitzpatrick R, Bernstein E, Iyer S, Brown D, Andrews P, Penny K. A histopathologic evaluation of the plasma skin regeneration system (PSR) versus a standard carbon dioxide resurfacing laser in an animal model. Lasers Surg Med. 2008 Feb;40(2):93–9.
- Kilmer S, Semchyshyn N, Shah G, Fitzpatrick R. A pilot study on the use of a plasma skin regeneration device (Portrait® PSR3) in full facial rejuvenation procedures. Lasers Med Sci. 2007 Jun;22(2):101–9.
- Gonzalez MJ, Sturgill WH, Ross EV, Uebelhoer NS. Treatment of acne scars using the plasma skin regeneration (PSR) system. Lasers Surg Med. 2008 Feb;40(2):124–7.
- Bentkover SH. Plasma Skin Resurfacing: Personal Experience and Long-Term Results. Facial Plastic Surgery Clinics of North America. 2012 May;20(2):145–62.
- Zeng J, Dou J, Gao L, Xiang Y, Huang J, Ding S, et al. Topical ozone therapy restores microbiome diversity in atopic dermatitis. International Immunopharmacology. 2020 Mar;80:106191.
- Glover JL, Bendick PJ, Link WJ, Plunkett RJ. The plasma scalpel: A new thermal knife. Lasers Surg Med. 1982 Jan;2(1):101–6.
- Holcomb JD. Plasma Energy Skin Rejuvenation. Facial Plastic Surgery Clinics of North America. 2020 Feb;28(1):67–74.
- Choi JH, Lee HW, Lee JK, Hong J woo, Kim G cheon. Low-temperature atmospheric plasma increases the expression of anti-aging genes of skin cells without causing cellular damages. Arch Dermatol Res. 2013 Mar;305(2):133–40.
- Friedman PC, Miller V, Fridman G, Lin A, Fridman A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: A case series. Journal of the American Academy of Dermatology. 2017 Feb;76(2):349–50.
- Khan A, Malik S, Walia J, Fridman G, Fridman A, Friedman P. Tolerability of Six Months Indirect Cold (Physical) Plasma Treatment of the Scalp for Hair Loss. JDD. 2020 Dec 1;19(12):1177–80.
- Isbary G, Shimizu T, Zimmermann JL, Heinlin J, Al-Zaabi S, Rechfeld M, et al. Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clinical Plasma Medicine. 2014 Dec;2(2):50–5.
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: A case series. Pediatric Dermatology. 2020 Jul;37(4):706–9.
- Becker S, Zimmermann JL, Baumeister P, Brunner TF, Shimizu T, Li YF, et al. Effects of cold atmospheric plasma (CAP) on bacteria and mucosa of the upper aerodigestive tract. Auris Nasus Larynx. 2019 Apr;46(2):294–301.
- Borges AC, Lima GDMG, Nishime TMC, Gontijo AVL, Kostov KG, Koga-Ito CY. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. Nguyen MH, editor. PLoS ONE. 2018 Jun 27;13(6):e0199832.
- Eggers B, Wagenheim AM, Jung S, Kleinheinz J, Nokhbehsaim M, Kramer FJ, et al. Effect of Cold Atmospheric Plasma (CAP) on Osteogenic Differentiation Potential of Human Osteoblasts. Int J Mol Sci. 2022 Feb 24;23(5):2503.
- Ding C, Chen C, Ouyang W, Liu Q, Lin L, Wu Z. Cold air plasma: A potential strategy for inducing apoptosis of rheumatoid arthritis fibroblast‐like synoviocytes. High Voltage. 2022 Feb;7(1):106–16.
- Ercan UK, İbiş F, Dikyol C, Horzum N, Karaman O, Yıldırım Ç, et al. Prevention of bacterial colonization on non-thermal atmospheric plasma treated surgical sutures for control and prevention of surgical site infections. Kaushik NK, editor. PLoS ONE. 2018 Sep 5;13(9):e0202703.
- Malik S, Gill M, Fridman G, Fridman A, Friedman PC. Cold atmospheric plasma reduces demodex count on the face comparably to topical ivermectin, as measured by reflectance confocal microscopy. Experimental Dermatology. 2022 Sep;31(9):1352–4.
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: A case report. SAGE Open Medical Case Reports. 2020 Jan;8:2050313X2092270.
- Zhong SY, Dong YY, Liu DX, Xu DH, Xiao SX, Chen HL, et al. Surface air plasma-induced cell death and cytokine release of human keratinocytes in the context of psoriasis. British Journal of Dermatology. 2016 Mar 1;174(3):542–52.
- Reitberger HH, Czugala M, Chow C, Mohr A, Burkovski A, Gruenert AK, et al. Argon Cold Plasma-A Novel Tool to Treat Therapy-resistant Corneal Infections. Am J Ophthalmol. 2018/03/28 ed. 2018 Jun;190:150–63.
- Marx AH, Oltmanns H, Meißner J, Verspohl J, Fuchsluger T, Busse C. Argon cold atmospheric plasma eradicates pathogens in vitro that are commonly associated with canine bacterial keratitis. Front Vet Sci. 2024 Jan 9;10:1320145.
- Seebauer C, Metelmann HR, Witzke K, Pouvesle JM. Palliative Treatment of Head and Neck Cancer. In: Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application. 2018. p. 185–95.
- Canady J, Gordon S, Zhuang T, Wigh S, Rowe W, Shashurin A, et al. Cold Atmospheric Plasma (CAP) Combined with Chemo-Radiation and Cytoreductive Surgery: The First Clinical Experience for Stage IV Metastatic Colon Cancer. In: Metelmann HR, Von Woedtke T, Weltmann KD, editors. Comprehensive Clinical Plasma Medicine. Cham: Springer International Publishing; 2018. p. 163–83.
- Limanowski R, Yan D, Li L, Keidar M. Preclinical Cold Atmospheric Plasma Cancer Treatment. Cancers (Basel). 2022 Jul 16;14(14):3461.
- Metelmann HR, Nedrelow DS, Seebauer C, Schuster M, Von Woedtke T, Weltmann KD, et al. Head and neck cancer treatment and physical plasma. Clinical Plasma Medicine. 2015 Jun;3(1):17–23.
- Metelmann HR, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB, et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clinical Plasma Medicine. 2018 Mar;9:6–13.
- Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017 Feb 28;8(9):15977–95.
- Gebel J, Exner M, French G, Chartier Y, Christiansen B, Gemein S, et al. The role of surface disinfection in infection prevention. GMS hygiene and infection control. 2013 Apr 29;8:Doc10.
- Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016 Dec;5(1):10.
- O’Connor N, Cahill O, Daniels S, Galvin S, Humphreys H. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? Journal of Hospital Infection. 2014 Oct;88(2):59–65.
- Sainz-García A, Toledano P, Muro-Fraguas I, Álvarez-Erviti L, Múgica-Vidal R, López M, et al. Mask disinfection using atmospheric pressure cold plasma. International Journal of Infectious Diseases. 2022 Oct;123:145–56.
- Zheng C, Kou Y, Liu Z, Li C, Huang Y, Yan K. Rapid Disinfection Performance of a Touchable Pulsed SDBD Nonthermal Plasma. IEEE Trans Plasma Sci. 2016 Nov;44(11):2667–72.
- Gilmore BF, Flynn PB, O’Brien S, Hickok N, Freeman T, Bourke P. Cold Plasmas for Biofilm Control: Opportunities and Challenges. Trends in Biotechnology. 2018 Jun;36(6):627–38.
- Zhang L, Guo Y, Chang X, Yao Z, Wei X, Feng Z, et al. In-duct grating-like dielectric barrier discharge system for air disinfection. Journal of Hazardous Materials. 2022 Aug;435:129075.
- Gao H, Wang G, Chen B, Zhang Y, Liu D, Lu X, et al. Atmospheric-pressure non-equilibrium plasmas for effective abatement of pathogenic biological aerosols. Plasma Sources Sci Technol. 2021 May 1;30(5):053001.
- Loizou C, Kniazeva V, Apostolou T, Kornev A, Kostevitch S, Roslyakov E, et al. Effect of Cold Atmospheric Plasma on SARS-CoV-2 Inactivation: A Pilot Study in the Hospital Environment. COVID. 2022 Sep 30;2(10):1396–404.
- Wang SN, Li JJ, Liu YX, Lin Z, Qiao JJ, Chen LH, et al. Pulsed xenon ultraviolet and non-thermal atmospheric plasma treatments are effective for the disinfection of air in hospital blood sampling rooms. Photodiagnosis and Photodynamic Therapy. 2019 Sep;27:137–40.
- Chen Z, Chen G, Obenchain R, Zhang R, Bai F, Fang T, et al. Cold atmospheric plasma delivery for biomedical applications. Materials Today. 2022 Apr;54:153–88.
- Mirpour S, Piroozmand S, Soleimani N, Jalali Faharani N, Ghomi H, Fotovat Eskandari H, et al. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci Rep. 2016 Jul 7;6(1):29048.
- Decauchy H, Pavy A, Camus M, Fouassier L, Dufour T. Cold plasma endoscopy applied to biliary ducts: feasibility risk assessment on human-like and porcine models for the treatment of cholangiocarcinoma. J Phys D: Appl Phys. 2022 Nov 10;55(45):455401.
- Omran AV, Busco G, Ridou L, Dozias S, Grillon C, Pouvesle JM, et al. Cold atmospheric single plasma jet for RONS delivery on large biological surfaces. Plasma Sources Sci Technol. 2020 Oct 1;29(10):105002.
- Robert E, Darny T, Dozias S, Iseni S, Pouvesle JM. New insights on the propagation of pulsed atmospheric plasma streams: From single jet to multi jet arrays. Physics of Plasmas. 2015 Dec 1;22(12):122007.
- Maho T, Binois R, Brulé-Morabito F, Demasure M, Douat C, Dozias S, et al. Anti-Bacterial Action of Plasma Multi-Jets in the Context of Chronic Wound Healing. Applied Sciences. 2021 Oct 15;11(20):9598.
- Cao Z, Walsh JL, Kong MG. Atmospheric plasma jet array in parallel electric and gas flow fields for three-dimensional surface treatment. Applied Physics Letters. 2009 Jan 12;94(2):021501.
- Ma S, Lee MH, Kang SU, Lee YS, Kim CH, Kim K. Development of an atmospheric nonthermal multineedle dielectric barrier discharge jet for large area treatment of skin diseases. Current Applied Physics. 2021 Apr;24:24–31.
- Nguyen DB, Trinh QH, Lee WG, Mok YS. Analysis of an Ar plasma jet in a dielectric barrier discharge conjugated with a microsecond pulse. Plasma Sci Technol. 2019 Sep;21(9):095401.
- Viegas P, Slikboer E, Bonaventura Z, Guaitella O, Sobota A, Bourdon A. Physics of plasma jets and interaction with surfaces: review on modelling and experiments. Plasma Sources Sci Technol. 2022 May 1;31(5):053001.
- Lu X, Jiang Z, Xiong Q, Tang Z, Hu X, Pan Y. An 11cm long atmospheric pressure cold plasma plume for applications of plasma medicine. Applied Physics Letters. 2008 Feb 25;92(8):081502.
- Xu N, Cui X, Fang Z, Shi Y, Zhou R. A Two-Mode Portable Atmospheric Pressure Air Plasma Jet Device for Biomedical Applications. IEEE Trans Plasma Sci. 2018 Apr;46(4):947–53.
- Boekema BKHL, Vlig M, Guijt D, Hijnen K, Hofmann S, Smits P, et al. A new flexible DBD device for treating infected wounds: in vitro and ex vivo evaluation and comparison with a RF argon plasma jet. J Phys D: Appl Phys. 2016 Feb 3;49(4):044001.
- Liu G, Shi F, Wang Q, Zhang Z, Guo J, Zhuang J. Penetration effect of the kINPen plasma jet investigated with a 3D agar-entrapped bacteria model. Microchemical Journal. 2022 Dec;183:107973.
- Liu B, Qi F, Zhou D, Nie L, Xian Y, Lu X. A novel flexible plasma array for large-area uniform treatment of an irregular surface. Plasma Sci Technol. 2022 Mar 1;24(3):035403.
- Gershman S, Harreguy MB, Yatom S, Raitses Y, Efthimion P, Haspel G. A low power flexible dielectric barrier discharge disinfects surfaces and improves the action of hydrogen peroxide. Sci Rep. 2021 Feb 25;11(1):4626.
- Jung H, Seo JA, Choi S. Wearable Atmospheric Pressure Plasma Fabrics Produced by Knitting Flexible Wire Electrodes for the Decontamination of Chemical Warfare Agents. Sci Rep. 2017 Jan 18;7(1):40746.
- Lee KH, Kim S, Jo H, Son BK, Shin MS, Cho G. Plasma skincare device based on floating electrode dielectric barrier discharge. Plasma Sci Technol. 2019 Dec;21(12):125403.
- Sun Y, Zhang B, Wang C, Zhang G. Polyimide-Based Flexible Plasma Sheet and Surface ionisation Waves Propagation. Adv Elect Materials. 2021 Nov;7(11):2100369.
- Zhao Y, Liu Y, Liu Z, Zhang X, Zhang L, Jin S, et al. A 3D-printed fence-surface plasma source for skin treatment and its potential for personalized medical application. J Phys D: Appl Phys. 2024 Mar 22;57(12):125207.
- Lee YR, Lee S, Kim DG. Enhancement of emulsion penetration in agarose gel model using flexible plasma treatment. Biomed Phys Eng Express. 2019 Jul 1;5(4):045027.
- Liu Y, Wang S, Zhou R, Fang Z, Ostrikov K (Ken). Development of a battery-operated floating-electrode dielectric barrier discharge plasma device and its characteristics. Plasma Sci Technol. 2021 Jun 1;23(6):064008.
- Arserim EH, Salvi D, Fridman G, Schaffner DW, Karwe MV. Microbial Inactivation by Non-equilibrium Short-Pulsed Atmospheric Pressure Dielectric Barrier Discharge (Cold Plasma): Numerical and Experimental Studies. Food Eng Rev. 2021 Mar;13(1):136–47.
- Pavlovich MJ, Chen Z, Sakiyama Y, Clark DS, Graves DB. Effect of Discharge Parameters and Surface Characteristics on Ambient‐Gas Plasma Disinfection. Plasma Processes & Polymers. 2013 Jan;10(1):69–76.
- Stryczewska HD, Boiko O. Applications of Plasma Produced with Electrical Discharges in Gases for Agriculture and Biomedicine. Applied Sciences. 2022 Apr 27;12(9):4405.
- Shang K, Wang M, Peng B, Li J, Lu N, Jiang N, et al. Characterization of a novel volume-surface DBD reactor: discharge characteristics, ozone production and benzene degradation. J Phys D: Appl Phys. 2020 Feb 6;53(6):065201.
- Yuan D, Zhang G, Ling Z, Wu A, He Y, Wang Z. Characteristics of temperature distribution in atmospheric pulsed surface dielectric barrier discharge for ozone production. Vacuum. 2020 Jun;176:109351.
- Zhou R, Zhou R, Wang P, Xian Y, Mai-Prochnow A, Lu X, et al. Plasma-activated water: generation, origin of reactive species and biological applications. J Phys D: Appl Phys. 2020 Jul 22;53(30):303001.
- Wu S, Huang G, Cheng W, Chen W, Hai B, Shao T, et al. The influences of the electrode dimension and the dielectric material on the breakdown characteristics of coplanar dielectric barrier discharge in ambient air. Plasma Processes & Polymers. 2017 Dec;14(12):1700112.
- Guo Y, Liu P, Zhang L, Peng S, Wang X, Luo H, et al. Disinfection of Escherichia coli in ice by surface dielectric barrier discharge plasma. Applied Physics Letters. 2021 Aug 30;119(9):090601.
- Roy S, Choudhury B, Johnson J, Schindler-Tyka A. Application of dielectric barrier discharge for improving food shelf life and reducing spoilage. Sci Rep. 2021 Sep 28;11(1):19200.
- Nassour K, Brahami M, Nemmich S, Hammadi N, Zouzou N, Tilmatine A. New Hybrid Surface–Volume Dielectric Barrier Discharge Reactor for Ozone Generation. IEEE Trans on Ind Applicat. 2017 May;53(3):2477–84.
- Morfill GE, Zimmermann JL. Plasma Health Care – Old Problems, New Solutions. Contrib Plasma Phys. 2012 Aug;52(7):655–63.
- Keidar M, Yan D, Beilis II, Trink B, Sherman JH. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends in Biotechnology. 2018 Jun;36(6):586–93.
- Weltmann KD, Kindel E, Von Woedtke T, Hähnel M, Stieber M, Brandenburg R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure and Applied Chemistry. 2010 Apr 20;82(6):1223–37.
- Chang YT, Chen G. Oral bacterial inactivation using a novel low-temperature atmospheric-pressure plasma device. Journal of Dental Sciences. 2016 Mar;11(1):65–71.
- Weltmann KD, Fricke K, Stieber M, Brandenburg R, Von Woedtke T, Schnabel U. New Nonthermal Atmospheric-Pressure Plasma Sources for Decontamination of Human Extremities. IEEE Trans Plasma Sci. 2012 Nov;40(11):2963–9.
- Lei H, Ji H, Liu X, Lu B, Xie L, Lim EG, et al. Self-Assembled Porous-Reinforcement Microstructure-Based Flexible Triboelectric Patch for Remote Healthcare. Nano-Micro Lett. 2023 Dec;15(1):109.
- Duan J, Ma M, Yusupov M, Cordeiro RM, Lu X, Bogaerts A. The penetration of reactive oxygen and nitrogen species across the stratum corneum. Plasma Processes & Polymers. 2020 Oct;17(10):2000005.
- Nie L, Yang Y, Duan J, Sun F, Lu X, He G. Effect of tissue thickness and liquid composition on the penetration of long-lifetime reactive oxygen and nitrogen species (RONS) generated by a plasma jet. J Phys D: Appl Phys. 2018 Aug 30;51(34):345204.
- He T, Liu D, Liu Z, Wang S, Liu Z, Rong M, et al. Transportation of ROS in model tissues treated by an Ar + O 2 plasma jet. J Phys D: Appl Phys. 2019 Jan 23;52(4):045204.
- Dobrynin D, Fridman G, Friedman G, Fridman AA. Deep Penetration into Tissues of Reactive Oxygen Species Generated in Floating-Electrode Dielectric Barrier Discharge (FE-DBD): An In Vitro Agarose Gel Model Mimicking an Open Wound. Plasma Med. 2012;2(1–3):71–83.
- Pei X, Liu J, Xian Y, Lu X. A battery-operated atmospheric-pressure plasma wand for biomedical applications. J Phys D: Appl Phys. 2014 Apr 9;47(14):145204.
- World Health Organization (WHO). What are the WHO Air quality guidelines? 2021. Available from: https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines
- European Commission. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Official Journal of the European Union. 2008; Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX%3A32008L0050
- Awakowicz P, Bibinov N, Born M, Busse B, Gesche R, Helmke A, et al. Biological Stimulation of the Human Skin Applying HealthPromoting Light and Plasma Sources. Contrib Plasma Phys. 2009 Nov;49(9):641–7.
- Dijksteel GS, Ulrich MMW, Vlig M, Sobota A, Middelkoop E, Boekema BKHL. Safety and bactericidal efficacy of cold atmospheric plasma generated by a flexible surface Dielectric Barrier Discharge device against Pseudomonas aeruginosa in vitro and in vivo. Ann Clin Microbiol Antimicrob. 2020 Dec;19(1):37.
- Rajasekaran P, Opländer C, Hoffmeister D, Bibinov N, Suschek CV, Wandke D, et al. Characterization of Dielectric Barrier Discharge (DBD) on Mouse and Histological Evaluation of the Plasma-Treated Tissue. Plasma Processes & Polymers. 2011 Mar 22;8(3):246–55.
- López M, Calvo T, Prieto M, Múgica-Vidal R, Muro-Fraguas I, Alba-Elías F, et al. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front Microbiol. 2019 Apr 2;10:622.
- Jiang B, Zheng J, Qiu S, Wu M, Zhang Q, Yan Z, et al. Review on electrical discharge plasma technology for wastewater remediation. Chemical Engineering Journal. 2014 Jan;236:348–68.
- Yan D, Nourmohammadi N, Bian K, Murad F, Sherman JH, Keidar M. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci Rep. 2016 May 13;6(1):26016.
- Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Utsumi F, Kajiyama H, et al. Cell survival and proliferation signaling pathways are downregulated by plasma-activated medium in glioblastoma brain tumor cells. Plasma Med. 2012;2(4):207–20.
- Ercan UK, Wang H, Ji H, Fridman G, Brooks AD, Joshi SG. Nonequilibrium Plasma-Activated Antimicrobial Solutions are Broad-Spectrum and Retain their Efficacies for Extended Period of Time. Plasma Processes & Polymers. 2013 Jun;10(6):544–55.
- Shen J, Tian Y, Li Y, Ma R, Zhang Q, Zhang J, et al. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci Rep. 2016 Jun 27;6(1):28505.
- Guo L, Xu R, Gou L, Liu Z, Zhao Y, Liu D, et al. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Elkins CA, editor. Appl Environ Microbiol. 2018 Sep;84(17):e00726-18.
- Suwal S, Coronel-Aguilera CP, Auer J, Applegate B, Garner AL, Huang JY. Mechanism characterization of bacterial inactivation of atmospheric air plasma gas and activated water using bioluminescence technology. Innovative Food Science & Emerging Technologies. 2019 May;53:18–25.
- Girard F, Badets V, Blanc S, Gazeli K, Marlin L, Authier L, et al. Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. RSC Adv. 2016;6(82):78457–67.
- Ganesh Subramanian PS, Harsha R, Manju DK, Hemanth M, Lakshminarayana R, Anand MS, et al. Characterization of Plasma Activated Water for Medical Applications. Adv Mater Lett. 2019 Dec 1;10(12):919–23.
- Lee HR, Lee YS, You YS, Huh JY, Kim K, Hong YC, et al. Antimicrobial effects of microwave plasma-activated water with skin protective effect for novel disinfectants in pandemic era. Sci Rep. 2022 Apr 8;12(1):5968.
- Rathore V, Patel D, Butani S, Nema SK. Investigation of Physicochemical Properties of Plasma Activated Water and its Bactericidal Efficacy. Plasma Chem Plasma Process. 2021 May;41(3):871–902.
- Takamatsu T, Uehara K, Sasaki Y, Hidekazu M, Matsumura Y, Iwasawa A, et al. Microbial Inactivation in the Liquid Phase Induced by Multigas Plasma Jet. Yousfi M, editor. PLoS ONE. 2015 Jul 14;10(7):e0132381.
- Su X, Tian Y, Zhou H, Li Y, Zhang Z, Jiang B, et al. Inactivation Efficacy of Nonthermal Plasma-Activated Solutions against Newcastle Disease Virus. Schaffner DW, editor. Appl Environ Microbiol. 2018 May;84(9):e02836-17.
- Pan J, Li YL, Liu CM, Tian Y, Yu S, Wang KL, et al. Investigation of Cold Atmospheric Plasma-Activated Water for the Dental Unit Waterline System Contamination and Safety Evaluation in Vitro. Plasma Chem Plasma Process. 2017 Jul;37(4):1091–103.
- Oehmigen K, Hähnel M, Brandenburg R, Wilke Ch, Weltmann K-D., Von Woedtke Th. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Processes & Polymers. 2010 Mar 22;7(3–4):250–7.
- Satoh K, MacGregor SJ, Anderson JG, Woolsey GA, Fouracre RA. Pulsed-Plasma Disinfection of Water Containing Escherichia coli. jjap. 2007 Mar 1;46(3R):1137.
- Korshunov SS, Imlay JA. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Molecular Microbiology. 2002 Jan;43(1):95–106.
- Lukes P, Dolezalova E, Sisrova I, Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H 2 O 2 and HNO 2. Plasma Sources Sci Technol. 2014 Feb 4;23(1):015019.
- Van Gils CAJ, Hofmann S, Boekema BKHL, Brandenburg R, Bruggeman PJ. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J Phys D: Appl Phys. 2013 May 1;46(17):175203.
- Shainsky N, Dobrynin D, Ercan U, Joshi S, Ji H, Brooks A, et al. Non-equilibrium plasma treatment of liquids, formation of plasma acid. In: Proceedings of the ISPC-20 20th International Symposium on Plasma Chemistry, Philadelphia, PA, USA. 2011. p. 24–9.
- Kojtari A, Ercan U, Smith JB, Fridman G, Sensenig R, Tyagi, et al. Chemistry for Antimicrobial Properties of Water Treated With Non-Equilibrium Plasma. J Nanomedine Biotherapeutic Discov. 2013 Jan 1;4.
- Chen TP, Su TL, Liang J. Plasma-Activated Solutions for Bacteria and Biofilm Inactivation. CBC. 2016 Dec 23;13(1):59–65.
- Tan J, Karwe MV. Inactivation and removal of Enterobacter aerogenes biofilm in a model piping system using plasma-activated water (PAW). Innovative Food Science & Emerging Technologies. 2021 May;69:102664.
- Oztan MO, Ercan UK, Aksoy Gokmen A, Simsek F, Ozdemir GD, Koyluoglu G. Irrigation of peritoneal cavity with cold atmospheric plasma treated solution effectively reduces microbial load in rat acute peritonitis model. Sci Rep. 2022 Mar 7;12(1):3646.
- Dos Santos JFR, Couceiro R, Concheiro A, Torres-Labandeira JJ, Alvarez-Lorenzo C. Poly(hydroxyethyl methacrylate-co-methacrylated-β-cyclodextrin) hydrogels: Synthesis, cytocompatibility, mechanical properties and drug loading/release properties. Acta Biomaterialia. 2008 May;4(3):745–55.
- Zou W, Chen Y, Zhang X, Li J, Sun L, Gui Z, et al. Cytocompatible chitosan based multi-network hydrogels with antimicrobial, cell anti-adhesive and mechanical properties. Carbohydrate Polymers. 2018 Dec;202:246–57.
- Ma X, Zhang L, Fan D, Xue W, Zhu C, Li X, et al. Physicochemical properties and biological behavior of injectable crosslinked hydrogels composed of pullulan and recombinant human-like collagen. J Mater Sci. 2017 Apr;52(7):3771–85.
- Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound Healing Dressings and Drug Delivery Systems: A Review. Journal of Pharmaceutical Sciences. 2008 Aug;97(8):2892–923.
- Lay-Flurrie K. The properties of hydrogel dressings and their impact on wound healing. Prof Nurse. 2004 Jan;19(5):269–73.
- Gong C, Wu Q, Wang Y, Zhang D, Luo F, Zhao X, et al. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013 Sep;34(27):6377–87.
- Martínez-Higuera A, Rodríguez-Beas C, Villalobos-Noriega JMA, Arizmendi-Grijalva A, Ochoa-Sánchez C, Larios-Rodríguez E, et al. Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci Rep. 2021 May 28;11(1):11312.
- Fu M, Gan Y, Jiang F, Lv X, Tan N, Zhao X, et al. Interpenetrating Polymer Network Hydrogels Formed Using Antibiotics as a Dynamic Crosslinker for Treatment of Infected Wounds. Adv Healthcare Materials. 2022 Aug;11(15):2200902.
- Liu Z, Zheng Y, Dang J, Zhang J, Dong F, Wang K, et al. A Novel Antifungal Plasma-Activated Hydrogel. ACS Appl Mater Interfaces. 2019 Jul 3;11(26):22941–9.
- Chen J, Wang Z, Sun J, Zhou R, Guo L, Zhang H, et al. Plasma-Activated Hydrogels for Microbial Disinfection. Advanced Science. 2023 May;10(14):2207407.
- Labay C, Hamouda I, Tampieri F, Ginebra MP, Canal C. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Sci Rep. 2019 Nov 6;9(1):16160.
- Zhang H, Xu S, Zhang J, Wang Z, Liu D, Guo L, et al. Plasma-activated thermosensitive biogel as an exogenous ROS carrier for post-surgical treatment of cancer. Biomaterials. 2021 Sep;276:121057.
- Živanić M, Espona-Noguera A, Lin A, Canal C. Current State of Cold Atmospheric Plasma and Cancer-Immunity Cycle: Therapeutic Relevance and Overcoming Clinical Limitations Using Hydrogels. Advanced Science. 2023 Jan 20;n/a(n/a):2205803.
- Labay C, Roldán M, Tampieri F, Stancampiano A, Bocanegra PE, Ginebra MP, et al. Enhanced Generation of Reactive Species by Cold Plasma in Gelatin Solutions for Selective Cancer Cell Death. ACS Appl Mater Interfaces. 2020 Oct 21;12(42):47256–69.
- Solé-Martí X, Vilella T, Labay C, Tampieri F, Ginebra MP, Canal C. Thermosensitive hydrogels to deliver reactive species generated by cold atmospheric plasma: a case study with methylcellulose. Biomater Sci. 2022;10(14):3845–55.
- Hamouda I, Labay C, Ginebra MP, Nicol E, Canal C. Investigating the atmospheric pressure plasma jet modification of a photo-crosslinkable hydrogel. Polymer. 2020 Mar;192:122308.
- Gaur N, Kurita H, Oh JS, Miyachika S, Ito M, Mizuno A, et al. On cold atmospheric-pressure plasma jet induced DNA damage in cells. J Phys D: Appl Phys. 2021 Jan 21;54(3):035203.
- Gaur N, Patenall BL, Ghimire B, Thet NT, Gardiner JE, Le Doare KE, et al. Cold Atmospheric Plasma-Activated Composite Hydrogel for an Enhanced and On-Demand Delivery of Antimicrobials. ACS Appl Mater Interfaces. 2023 Apr 26;15(16):19989–96.
- Bekeschus S, Schmidt A, Weltmann KD, von Woedtke T. The plasma jet kINPen – A powerful tool for wound healing. Clinical Plasma Medicine. 2016;4(1):19–28.
- Boxhammer V, Li YF, Koritzer J, Shimizu T, Maisch T, Thomas HM, et al. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutation research. 20130214th ed. 2013 Apr 30;753(1):23–8.
- Isbary G, Köritzer J, Mitra A, Li YF, Shimizu T, Schroeder J, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clinical Plasma Medicine. 2013;1(1):36–44.
- Tiede R, Hirschberg J, Viöl W, Emmert S. A μs-Pulsed Dielectric Barrier Discharge Source: Physical Characterization and Biological Effects on Human Skin Fibroblasts. 2016;13(8):775–87.
- Mann MS, Tiede R, Gavenis K, Daeschlein G, Bussiahn R, Weltmann KD, et al. Introduction to DIN-specification 91315 based on the characterization of the plasma jet kINPen® MED. Clinical Plasma Medicine. 2016 Dec 1;4(2):35–45.
- Lademann J, Richter H, Schanzer S, Patzelt A, Thiede G, Kramer A, et al. Comparison of the antiseptic efficacy of tissue-tolerable plasma and an octenidine hydrochloride-based wound antiseptic on human skin. Skin Pharmacol Physiol. 2012;25(2):100–6.
- Rutkowski R, Daeschlein G, von Woedtke T, Smeets R, Gosau M, Metelmann HR. Long-term Risk Assessment for Medical Application of Cold Atmospheric Pressure Plasma. Diagnostics. 20200411th ed. 2020 Apr 11;10(4).
- Hartwig S, Doll C, Voss JO, Hertel M, Preissner S, Raguse JD. Treatment of Wound Healing Disorders of Radial Forearm Free Flap Donor Sites Using Cold Atmospheric Plasma: A Proof of Concept. J Oral Maxillofac Surg. 2016/09/18 ed. 2017 Feb;75(2):429–35.
- Shekhter AB, Kabisov RK, Pekshev AV, Kozlov NP, Perov YL. Experimental and clinical validation of plasmadynamic therapy of wounds with nitric oxide. B Exp Biol Med+. 1998 Aug;126(8):829–34.
- Betancourt-Angeles M, Pena-Eguiluz R, Lopez-Callejas R, Dominguez-Cadena NA, Mercado-Cabrera A, Munoz-Infante J, et al. Treatment in the healing of burns with a cold plasma source. International Journal of Burns and Trauma. 2018/01/20 ed. 2017 received;7(7):142–6.
- Hartwig S, Preissner S, Voss JO, Hertel M, Doll C, Waluga R, et al. The feasibility of cold atmospheric plasma in the treatment of complicated wounds in cranio-maxillo-facial surgery. J Craniomaxillofac Surg. 2017/08/28 ed. 2017 Oct;45(10):1724–30.
- Naderi N, Zaefizadeh M. Expression of growth factors in re-epithelialisation of diabetic foot ulcers after treatment with non-thermal plasma radiation. Biomed Res-India. 2017;28(8):3402–7.
- López-Callejas R, Peña-Eguiluz R, Valencia-Alvarado R, Mercado-Cabrera A, Rodríguez-Méndez BG, Serment-Guerrero JH, et al. Alternative method for healing the diabetic foot by means of a plasma needle. Clinical Plasma Medicine. 2018;9:19–23.
- Pekshev AV, Shekhter AB, Vagapov AB, Sharapov NA, Vanin AF. Study of plasma-chemical NO-containing gas flow for treatment of wounds and inflammatory processes. Nitric Oxide. 2017/06/13 ed. 2018 Feb 28;73:74–80.
- Gao J, Wang L, Xia C, Yang X, Cao Z, Zheng L, et al. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int Wound J. 2019/06/18 ed. 2019 Oct;16(5):1103–11.
- Gemeinsamer Bundesausschuss. Press release. 2023. Erprobungsstudie Kaltplasmabehandlung bei chronischen Wunden: Herstellerkonsortium finanziert wissenschaftliche Studienbegleitung. Available from: https://www.g-ba.de/service/fachnews/87/
- Fenech M. Cytokinesis-block micronucleus assay evolves into a ‘cytome’ assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res-Fund Mol M. 2006/07/11 ed. 2006 Aug 30;600(1–2):58–66.
- Maisch T, Bosserhoff AK, Unger P, Heider J, Shimizu T, Zimmermann JL, et al. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environmental and Molecular Mutagenesis. 2017/04/04 ed. 2017 Apr;58(3):172–7.
- Miebach L, Freund E, Horn S, Niessner F, Sagwal SK, von Woedtke T, et al. Tumor cytotoxicity and immunogenicity of a novel V-jet neon plasma source compared to the kINPen. Scientific Reports. 20210108th ed. 2021 Jan 8;11(1):136.
- Bekeschus S, Schutz CS, Niessner F, Wende K, Weltmann KD, Gelbrich N, et al. Elevated H2AX Phosphorylation Observed with kINPen Plasma Treatment Is Not Caused by ROS-Mediated DNA Damage but Is the Consequence of Apoptosis. Oxidative Medicine and Cellular Longevity. 2019/10/24 ed. 2019;2019:8535163.
- Wende K, Bekeschus S, Schmidt A, Jatsch L, Hasse S, Weltmann KD, et al. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutation Research – Genetic Toxicology and Environmental Mutagenesis. 2016/03/21 ed. 2016 Mar;798–799:48–54.
- Bekeschus S, Schmidt A, Kramer A, Metelmann HR, Adler F, von Woedtke T, et al. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environmental and Molecular Mutagenesis. 2018/02/09 ed. 2018 May;59(4):268–77.
- Kluge S, Bekeschus S, Bender C, Benkhai H, Sckell A, Below H, et al. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS One. 2016;11(9):e0160667.
- Pasqual-Melo G, Nascimento T, Sanches LJ, Blegniski FP, Bianchi JK, Sagwal SK, et al. Plasma Treatment Limits Cutaneous Squamous Cell Carcinoma Development In Vitro and In Vivo. Cancers. 20200721st ed. 2020 Jul 21;12(7):1993.
- Verma KD, Lewis F, Mejia M, Chalasani M, Marcus KA. Food and Drug Administration perspective: Advancing product development for non-healing chronic wounds. Wound Repair Regen. 2022 May;30(3):299–302.
- Eaglstein WH, Kirsner RS, Robson MC. Food and Drug Administration (FDA) drug approval end points for chronic cutaneous ulcer studies. Wound Repair Regen. 2012;20(6):793–6.
- Darwin E, Tomic-Canic M. Healing Chronic Wounds: Current Challenges and Potential Solutions. Curr Dermatol Rep. 2018 Dec;7(4):296–302.
- Maderal AD, Vivas AC, Eaglstein WH, Kirsner RS. The FDA and designing clinical trials for chronic cutaneous ulcers. Semin Cell Dev Biol. 2012 Dec;23(9):993–9.
- Carter MJ, Fife CE, Walker D, Thomson B. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv Skin Wound Care. 2009 Jul;22(7):316–24.
- Vivas AC, Maderal AD, Than MP, Kirsner RS. Designing clinical trials to bring wound products to market. Int Wound J. 2013 Feb;10(1):114–5.
- FDA. Chronic Cutaneous Ulcer and Burn Wounds – Developing Products for Treatment. US Food and Drug Administration. 2006; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/chronic-cutaneous-ulcer-and-burn-wounds-developing-products-treatment
- Busco G, Robert E, Chettouh-Hammas N, Pouvesle JM, Grillon C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic Biol Med. 2020 Dec;161:290–304.
- Crookes W. On Radiant Matter; In: Popular Science Monthly. Sheffield, UK; 1879. p. 157–67.
- Haertel B, von Woedtke T, Weltmann KD, Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther (Seoul). 2014 Nov;22(6):477–90.
- Garner AL, Mehlhorn TA. A Review of Cold Atmospheric Pressure Plasmas for Trauma and Acute Care. Frontiers in Physics. 2021;9.
- Szili EJ, Hong SH, Oh JS, Gaur N, Short RD. Tracking the Penetration of Plasma Reactive Species in Tissue Models. Trends Biotechnol. 2018 Jun;36(6):594–602.
- Martusevich AK, Surovegina AV, Bocharin IV, Nazarov VV, Minenko IA, Artamonov MY. Cold Argon Athmospheric Plasma for Biomedicine: Biological Effects, Applications and Possibilities. Antioxidants (Basel). 2022 Jun 27;11(7):1262.
- Braný D, Dvorská D, Halašová E, Škovierová H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int J Mol Sci. 2020 Apr 22;21(8):2932.
- Hirata T, Kishimoto T, Tsutsui C, Kanai T, Mori A. Healing burns using atmospheric pressure plasma irradiation. Jpn J Appl Phys. 2013 Dec 12;53(1):010302.
- Fathollah S, Mirpour S, Mansouri P, Dehpour AR, Ghoranneviss M, Rahimi N, et al. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci Rep. 2016 Feb 23;6:19144.
- Velnar T, Gradisnik L. Tissue Augmentation in Wound Healing: the Role of Endothelial and Epithelial Cells. Med Arch. 2018 Dec;72(6):444–8.
- Frescaline N, Duchesne C, Favier M, Onifarasoaniaina R, Guilbert T, Uzan G, et al. Physical plasma therapy accelerates wound re-epithelialisation and enhances extracellular matrix formation in cutaneous skin grafts. J Pathol. 2020 Dec;252(4):451–64.
- Wende K, Straßenburg S, Haertel B, Harms M, Holtz S, Barton A, et al. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in HaCaT keratinocytes and influences cell physiology. Cell Biol Int. 2014 Apr;38(4):412–25.
- Balzer J, Heuer K, Demir E, Hoffmanns MA, Baldus S, Fuchs PC, et al. Non-Thermal Dielectric Barrier Discharge (DBD) Effects on Proliferation and Differentiation of Human Fibroblasts Are Primary Mediated by Hydrogen Peroxide. PLoS One. 2015;10(12):e0144968.
- Kupke LS, Arndt S, Lenzer S, Metz S, Unger P, Zimmermann JL, et al. Cold Atmospheric Plasma Promotes the Immunoreactivity of Granulocytes In Vitro. Biomolecules. 2021 Jun 17;11(6):902.
- Lührmann A, Matthes R, Kramer A. Impact of cold atmospheric pressure argon plasma on antibiotic sensitivity of methicillin-resistant Staphylococcus aureus strains in vitro. GMS Hyg Infect Control. 2016;11:Doc17.
- Abbasi E, Mehrabadi JF, Nourani M, Namini YN, Mohammadi S, Esmaeili D, et al. Evaluation of cold atmospheric-pressure plasma against burn wound infections and gene silencing. Iran J Microbiol. 2021 Aug;13(4):544–52.
- Bourke P, Ziuzina D, Han L, Cullen PJ, Gilmore BF. Microbiological interactions with cold plasma. J Appl Microbiol. 2017 Aug;123(2):308–24.
- Fallon M, Kennedy S, Daniels S, Humphreys H. Technologies to decontaminate bacterial biofilm on hospital surfaces: a potential new role for cold plasma? J Med Microbiol. 2022 Oct;71(10).
- Maybin JA, Thompson TP, Flynn PB, Skvortsov T, Hickok NJ, Freeman TA, et al. Cold atmospheric pressure plasma-antibiotic synergy in Pseudomonas aeruginosa biofilms is mediated via oxidative stress response. Biofilm. 2023 Dec;5:100122.
- Plattfaut I, Besser M, Severing AL, Stürmer EK, Opländer C. Plasma medicine and wound management: Evaluation of the antibacterial efficacy of a medically certified cold atmospheric argon plasma jet. Int J Antimicrob Agents. 2021 May;57(5):106319.
- Shabani H, Dezhpour A, Jafari S, Moghaddam MJM, Nilkar M. Antimicrobial activity of cold atmospheric-pressure argon plasma combined with chicory (Cichorium intybus L.) extract against P. aeruginosa and E. coli biofilms. Sci Rep. 2023 Jun 9;13(1):9441.
- Baz A, Bakri A, Butcher M, Short B, Ghimire B, Gaur N, et al. Staphylococcus aureus strains exhibit heterogenous tolerance to direct cold atmospheric plasma therapy. Biofilm. 2023 Dec;5:100123.
- Li Z, Zhou Q, Yang J, Qiu X, Fu S, Chen Q. Effect of cold atmospheric plasma therapy on wound healing in patients with diabetic foot ulcers: protocol for a systematic review and meta-analysis. BMJ Open. 2023 Apr 27;13(4):e066628.
- European Commission. Regulation (EU) 2017/745 of the European Parliment and the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Official Journal of the European Union. 2017 Apr 5; Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745&qid=1634137114533
- European Commission. COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS: Pharmaceutical Strategy for Europe. Brussels: European Commission; 2020 Nov [cited 2023 Aug 1]. Report No.: {SWD(2020) 286 final}. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52020DC0761
- European Commission. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 Laying Down Community Procedures for the Authorisation and Supervision of Medicinal Products for Human and Veterinary Use and Establishing a European Medicines Agency. Official Journal of the European Union. 2004 Mar 31; Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ. do?uri=OJ:L:2004:136:0001:0033:en:PDF
- European Commission. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. Official Journal of the European Union. 2001 Nov 6; Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:311:0067:0128:en:PDF
- European Commission. Regulation (EU) 2023/607 of the European Parliament and the Council of 15 March 2023 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices. Official Journal of the European Union. 2023 Mar 15; Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32023R0607&qid=1688833292117
- European Commission. European Commission – European Commission. 2023 Q&A: Revision of the Pharmaceutical legislation. Available from: https://ec.europa.eu/commission/presscorner/detail/en/qanda_23_1844
- European Commission. Revision of the EU general pharmaceuticals legislation. Revision of the EU general pharmaceuticals legislation. 2023 Apr 26; Available from: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12963-Revision-of-the-EU-general-pharmaceuticals-legislation_en
- von Woedtke T, Emmert S, Metelmann HR, Rupf S, Weltmann KD. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Physics of Plasmas. 2020 Jul;27(7):070601.
- European Commission. New EU rules on medical devices to enhance patient safety and modernise public health. Press Release IP/17/847. 2017 Apr 5; Available from: https://ec.europa.eu/commission/presscorner/detail/en/IP_17_847
- European Commission. Transition Timelines from the Directives to the Medical Revices Regulation. 2020. Available from: https://ec.europa.eu/health/sites/health/files/md_newregulations/docs/timeline_mdr_en.pdf
- European Commission. Proposal for a Regulation of the European Parliament and of the Council amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices. 2023/0005 (COD) Jan 6, 2023. Available from: https://health.ec.europa.eu/system/files/2023-01/mdr_proposal.pdf
- Medical Device Coordination Group (MDCG). MDCG 2020–3; Guidance on significant changes regarding the transitional provision under Article 120 of the MDR with regard to devices covered by certificates according to MDD or AIMDD. 2020.
- Medical Device Coordination Group (MDCG). UPDATE – MDCG 2020-3 Rev.1 – Guidance on significant changes regarding the transitional provision under Article 120 of the MDR – May 2023. European Commission; 2023. Available from: https://health.ec.europa.eu/latest-updates/update-mdcg-2020-3-rev1-guidance-significant-changes-regarding-transitional-provision-under-article-2023-05-12_en
- European Commission. New EU rules to ensure safety of medical devices. 2017. Available from: https://ec.europa.eu/docsroom/documents/22522/attachments/1/translations/en/renditions/pdf
- European Medicines Agency. Human regulatory – Medical devices. European Medicines Agency. 2018 Nov 26; Available from: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices
- Kramer A, Conway BR, Meissner K, Scholz F, Rauch BH, Moroder A, et al. Cold atmospheric pressure plasma for treatment of chronic wounds: drug or medical device? J Wound Care. 2017 Aug 2;26(8):470–5.
- Woedtke T von, Weltmann KD, Metelmann HR, Bekeschus S, Emmert S, Lademann J, et al. Letter. In response to: ‘Cold atmospheric pressure plasma for treatment of chronic wounds: drug or medical device?’ J Wound Care. 2018 Dec 2;27(12):892–3.
- Directorate-General for Health and Food Safety. Manual on Borderline and Classification in the Community Regulatory Framework for Medical Devices – Version 1.22 (05-2019). European Commission; 2019. Available from: https://health.ec.europa.eu/latest-updates/manual-borderline-and-classification-community-regulatory-framework-medical-devices-september-2022-2022-09-07_en
- Directorate-General for Health and Food Safety. MDCG 2022-5 – Guidance on borderline between medical devices and medicinal products under Regulation (EU) 2017/745 on medical devices. European Commission; 2022. Available from: https://health.ec.europa.eu/latest-updates/mdcg-2022-5-guidance-borderline-between-medical-devices-and-medicinal-products-under-regulation-eu-2022-04-26_en
- Directorate-General for Health and Food Safety. Manual on Borderline and Classification in the Community Regulatory Framework for Medical Devices (Version 1 – September 2022). European Commission; 2022. Available from: https://health.ec.europa.eu/latest-updates/manual-borderline-and-classification-community-regulatory-framework-medical-devices-september-2022-2022-09-07_en
- Directorate-General for Health and Food Safety. Manual on borderline and classification for medical devices under Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (Version 2 – December 2022). European Commission; 2022. Available from: https://health.ec.europa.eu/latest-updates/manual-borderline-and-classification-under-regulations-eu-2017745-and-2017746-version2-december-2022-2022-12-15_en
- European Court of Justice. Case C-140/07: Judgment of the Court (First Chamber) of 15 January 2009 (reference for a preliminary ruling from the Bundesverwaltungsgericht — Germany) — Hecht-Pharma GmbH v Staatliches Gewerbeaufsichtsamt Lüneburg (Directive 2001/83/EC — Articles 1(2) and 2(2) — Concept of medicinal product by function — Product in respect of which it has not been established that it is a medicinal product by function — Account taken of the content in active substances). 2007. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A62007CA0140
- European Court of Justice. Case C308/11: Judgment of the Court (Fifth Chamber), 6 September 2012. Chemische Fabrik Kreussler & Co. GmbH v Sunstar Deutschland GmbH, formerly John O. Butler GmbH. Reference for a preliminary ruling from the Oberlandesgericht Frankfurt am Main. Directive 2001/83/EC — Medicinal products for human use — Article 1(2)(b) — Meaning of ‘medicinal product by function’ — Definition of the term ‘pharmacological action’. 2012. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:62011CJ0308
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–7.
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010 Mar 23;340:c332.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097.
- Price P, Gottrup F, Abel M. EWMA Study Recommendations: For Clinical Investigations in Leg Ulcers and Wound Care. J Wound Care. 2014 May;23 Suppl 5:S1–36.
- Jeffcoate WJ, Bus SA, Game FL, Hinchliffe RJ, Price PE, Schaper NC, et al. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality. Lancet Diabetes Endocrinol. 2016 Sep;4(9):781–8.
- Bardos L, Barankova H. Cold Atmospheric Plasma: Sources, Processes, and Applications. Thin Solid Films. 2010 Sep 1;518:6705–13.
- Fazli M, Bjarnsholt T, Kirketerp-Møller K, Jørgensen B, Andersen AS, Krogfelt KA, et al. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009 Dec;47(12):4084–9.
- Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017 Jan 2;26(1):20–5.
- Dietrich M, Besser M, Debus ES, Smeets R, Stuermer EK. Human skin biofilm model: translational impact on swabbing and debridement. J Wound Care. 2023 Jul 2;32(7):446–55.
- Moelleken M, Jockenhöfer F, Wiegand C, Buer J, Benson S, Dissemond J. Pilot study on the influence of cold atmospheric plasma on bacterial contamination and healing tendency of chronic wounds. J Dtsch Dermatol Ges. 2020 Oct;18(10):1094–101.
- Duchesne C, Frescaline N, Blaise O, Lataillade JJ, Banzet S, Dussurget O, et al. Cold Atmospheric Plasma Promotes Killing of Staphylococcus aureus by Macrophages. mSphere. 2021 Jun 16;6(3):e0021721.
- DIN SPEC 91315:2014-06: Allgemeine Anforderungen an medizinische Plasmaquellen (General requirements for medical plasma sources). 2014 Jun;
- Isoherranen K, Montero E, Atkin L, Collier M, Høgh A, Ivory J, et al. Lower Leg Ulcer Diagnosis & Principles of Treatment. Including Recommendations for Comprehensive Assessment and Referral Pathways. J Wound Management. 2023;24(2 Sup1):1–76.
- Booth JP, Mozetič M, Nikiforov A, Oehr C. Foundations of plasma surface functionalization of polymers for industrial and biological applications. Plasma Sources Science and Technology. 2022;31(10).
- Garscadden A, Kushner MJ, Eden JG. Plasma Physics Issues in GaS Discharge Laser Development. IEEE Transactions on Plasma Science. 1991;19(6).
- Lee CGN, Kanarik KJ, Gottscho RA. The grand challenges of plasma etching: A manufacturing perspective. Journal of Physics D: Applied Physics. 2014;47(27).
- Anders A. Plasma and ion sources in large area coating: A review. Surface and Coatings Technology. 2005;200(5–6).
- Kortshagen UR, Sankaran RM, Pereira RN, Girshick SL, Wu JJ, Aydil ES. Nonthermal plasma synthesis of nanocrystals: Fundamental principles, materials, and applications. Chemical Reviews. 2016;116(18).
- Manoharan D, Stephen J, Radhakrishnan M. Study on low-pressure plasma system for continuous decontamination of milk and its quality evaluation. Journal of Food Processing and Preservation. 2021;45(2).
- Schmidt M, Jõgi I, Hołub M, Brandenburg R. Non-thermal plasma based decomposition of volatile organic compounds in industrial exhaust gases. International Journal of Environmental Science and Technology. 2015;12(12).
- Laroussi M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Frontiers in Physics. 2020;8.
- Guo H, Zhang XN, Chen J, Li HP, Ostrikov K. Non-equilibrium synergistic effects in atmospheric pressure plasmas. Scientific Reports. 2018 Dec;8(1).
- Kuchenbecker M, Bibinov N, Kaemlimg A, Wandke D, Awakowicz P, Viöl W. Characterization of DBD plasma source for biomedical applications. Journal of Physics D: Applied Physics. 2009;42(4).
- Helmke A, Wandke D, Mahmoodzada M, Weltmann KD, Viöl W. Impact of electrode design, supply voltage and interelectrode distance on safety aspects and characteristics of a medical DBD plasma source. Contributions to Plasma Physics. 2013;53(9).
- Graves DB. Mechanisms of Plasma Medicine: Coupling Plasma Physics, Biochemistry, and Biology. IEEE Transactions on Radiation and Plasma Medical Sciences. 2017 Jul;1(4):281–92.
- Helmke A, Gerling T, Weltmann KD. Plasma sources for biomedical applications. 2018. (Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application).
- Sakiyama Y, Graves DB, Chang HW, Shimizu T, Morfill GE. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. Journal of Physics D: Applied Physics. 2012 Oct;45(42).
- Hahn V, Grollmisch D, Bendt H, von Woedtke T, Nestler B, Weltmann KD, et al. Concept for Improved Handling Ensures Effective Contactless Plasma Treatment of Patients with kINPen® MED. Applied Sciences. 2020;10(17).
- Chaerony Siffa I, Gerling T, Masur K, Eschenburg C, Starkowski F, Emmert S. Development of a Mobile Sensory Device to Trace Treatment Conditions for Various Medical Plasma Source Devices. Sensors 2022, Vol 22, Page 7242. 2022 Sep;22(19):7242–7242.
- Sabrin S, Karmokar DK, Karmakar NC, Hong SH, Habibullah H, Szili EJ. Opportunities of Electronic and Optical Sensors in Autonomous Medical Plasma Technologies. ACS Sensors. 2023 Mar;8(3):974–93.
- DIN SPEC 91315 – 2014-06 – Beuth.de. Available from: https://www.beuth.de/de/technische-regel/din-spec-91315/203493369
- prEN IEC 60601-2-91:2023 Particular requirement for basic safety and essential performance of non-thermal plasma wound treatment equipment. Available from: https://genorma.com/en/project/show/cenelec:proj:75583
- Timmermann E, Bansemer R, Gerling T, Hahn V, Weltmann KD, Nettesheim S, et al. Piezoelectric-driven plasma pen with multiple nozzles used as a medical device: risk estimation and antimicrobial efficacy. Journal of Physics D: Applied Physics. 2020;54(2):25201–25201.
- Nastuta AV, Gerling T. Cold Atmospheric Pressure Plasma Jet Operated in Ar and He: From Basic Plasma Properties to Vacuum Ultraviolet, Electric Field and Safety Thresholds Measurements in Plasma Medicine. Applied Sciences 2022, Vol 12, Page 644. 2022 Jan;12(2):644–644.
- Matthes R, Jablonowski L, Pitchika V, Holtfreter B, Eberhard C, Seifert L, et al. Efficiency of biofilm removal by combination of water jet and cold plasma: an in-vitro study. BMC Oral Health. 2022 Dec;22(1):157–157.
- Lehmann A, Pietag F, Arnold Th. Human health risk evaluation of a microwave-driven atmospheric plasma jet as medical device. Clinical Plasma Medicine. 2017;7–8:16–23.
- do Nascimento F, Sampaio A da G, Milhan NVM, Gontijo AVL, Mattern P, Gerling T, et al. A Low Cost, Flexible Atmospheric Pressure Plasma Jet Device With Good Antimicrobial Efficiency. IEEE Transactions on Radiation and Plasma Medical Sciences. 2023;
- do Nascimento F, Gerling T, Kostov KG. On the gas heating effect of helium atmospheric pressure plasma jet. Physica Scripta. 2023 Apr;98(5):055013–055013.
- Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS. MRI Magnetic Field Stimulates Rotational Sensors of the Brain. Current Biology. 2011 Oct;21(19):1635–40.
- Weltmann KD, Kindel E, Brandenburg R, Meyer C, Bussiahn R, Wilke C, et al. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contributions to Plasma Physics. 2009;49(9, Sp. Iss. SI):631–40.
- Metelmann HR, von Woedtke T, Weltmann KD, Emmert S, editors. Textbook of Good Clinical Practice in Cold Plasma Therapy. Cham: Springer International Publishing; 2022.
- Miebach L, Freund E, Clemen R, Weltmann KD, Metelmann HR, von Woedtke T, et al. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radical Biology and Medicine. 2022 Feb;180:210–9.
- Winter J, Tresp H, Hammer MU, Iseni S, Kupsch S, Schmidt-Bleker A, et al. Tracking plasma generated H 2 O 2 from gas into liquid phase and revealing its dominant impact on human skin cells. Journal of Physics D: Applied Physics. 2014;47(28):285401–285401.
- Cimerman R, Mat’Aš E, Sárený M, Hensel K. Electrical and optical characterization of multi-hollow surface dielectric barrier discharge in configuration with the air-exposed electrode. Physics of Plasmas. 2022 Nov;29(11):113510–113510.
- Cimerman R, Hensel K. Multi-hollow Surface Dielectric Barrier Discharge: Production of Gaseous Species Under Various Air Flow Rates and Relative Humidities. Plasma Chemistry and Plasma Processing. 2023 Nov;43(6):1411–33.
- Nishime TMC, Wagner R, Kostov KG. Study of Modified Area of Polymer Samples Exposed to a He Atmospheric Pressure Plasma Jet Using Different Treatment Conditions. Polymers. 2020;12(5).
- Kostov KG, Nishime TMC, Machida M, Borges AC, Prysiazhnyi V, Koga-Ito CY. Study of Cold Atmospheric Plasma Jet at the End of Flexible Plastic Tube for Microbial Decontamination. Plasma Processes and Polymers. 2015;12(12):1383–91.
- Meinke MC, Hasse S, Schleusener J, Hahn V, Gerling T, Rasnani KH, et al. Radical formation in skin by a new cold atmospheric plasma device and preclinical characterization concerning skin integrity and microbicide effects. submitted. 2024;
- Meinke MC, Hasse S, Schleusener J, Hahn V, Gerling T, Bernhardt T, et al. Mikrobiologische Analysen und Heilungserfolg nach kINPen MED Einsatz im Rahmen der Plasma-Therapie chronischer Wunden bei Patienten mit Diabetes mellitus. In: 44 ak-adp Workshop: Von der Forschung zur anwendungsreifen Therapie 8 Workshop Plasmamedizin 27 – 28 September 2023 in Göttingen / Niedersachsen. 2023. Available from: https://www.ak-adp.de/44-ak-adp-workshop/
- ActivCell Group. ActivCell Group AG. 2024 [cited 2024 Jun 28]. Kaltplasma für die medizinische Praxis – Activcellgroup AG. Available from: https://activcellgroup.com/de/
- Kostov KG, Machida M, Prysiazhnyi V, Honda RY. Transfer of a cold atmospheric pressure plasma jet through a long flexible plastic tube. Plasma Sources Science and Technology. 2015 Apr;24(2):025038–025038.
- Bousba HE, Sahli S, Namous WSE, Benterrouche L. On the Stability and Turbulences of Atmospheric-Pressure Plasma Jet Extracted From the Exit of a Long Flexible PVC Tube. IEEE Transactions on Plasma Science. 2022 May;50(5):1218–26.
- Bastin O, Thulliez M, Servais J, Nonclercq A, Delchambre A, Hadefi A, et al. Optical and Electrical Characteristics of an Endoscopic DBD Plasma Jet. Plasma Medicine. 2020;10(2):71–90.
- Decauchy H, Dufour T. Transmission and multiple reflection mechanisms of guided streamers propagating through grounded annular electrode and interacting with grounded surface electrode. Plasma Sources Science and Technology. 2022 Nov;31(11):115017–115017.
- Robert E, Vandamme M, Brull L, Lerondel S, Pape AL, Sarron V, et al. Perspectives of endoscopic plasma applications. Clinical Plasma Medicine. 2013;1(2):8–16.
- Thomson S, Burger H. The Doctor, the Quack, and the Appetite of the Public for Magic in Medicine: (Section of the History of Medicine). Proceedings of the Royal Society of Medicine. 1933 Dec;27(2):171–171.
- Gelker M, Müller-Goymann CC, Viöl W. Plasma Permeabilization of Human Excised Full-Thickness Skin by µs- and ns-pulsed DBD. Skin Pharmacology and Physiology. 2020 Jun;33(2):69–76.
- Weltmann KD, von Woedtke T. Plasma medicine – current state of research and medical application. Plasma Physics and Controlled Fusion. 2017;59(1):14031–14031.
- Weltmann KD, Brandenburg R, von Woedtke T, Ehlbeck J, Foest R, Stieber M, et al. Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs). Journal of Physics D: Applied Physics. 2008;41(19):194008–194008.
- Sun PP, Chen HL, Park SJ, Eden JG, Liu DX, Kong MG. Off-axis chemical crosstalk in an atmospheric pressure microplasma jet array. Journal of Physics D: Applied Physics. 2015;48(42):425203–425203.
- Lee OJ, Ju HW, Khang G, Sun PP, Rivera J, Cho JH, et al. An experimental burn wound-healing study of non-thermal atmospheric pressure microplasma jet arrays. Journal of Tissue Engineering and Regenerative Medicine. 2016 Apr;10(4):348–57.
- Balazinski M, Horn S, Timm M, Hahn V, Wagner R, Lüttjohann P, et al. Safety and efficiency evaluation of a gas switching technology for plasma jet arrays in Argon. in prep. 2024; Available from: https://www.inp-greifswald.de/de/efre/p-array/
- Xiong Z, Robert E, Sarron V, Pouvesle JM, Kushner MJ. Dynamics of ionisation wave splitting and merging of atmospheric-pressure plasmas in branched dielectric tubes and channels. Journal of Physics D: Applied Physics. 2012 Jun;45(27):275201–275201.
- Sun PP, Won J, Choo-Kang G, Li S, Chen W, Monroy GL, et al. Inactivation and sensitization of Pseudomonas aeruginosa by microplasma jet array for treating otitis media. npj Biofilms and Microbiomes 2021 7:1. 2021 Jun;7(1):1–10.
- Jablonowski L, Kocher T, Schindler A, Müller K, Dombrowski F, von Woedtke T, et al. Side effects by oral application of atmospheric pressure plasma on the mucosa in mice. PLOS ONE. 2019;14(4):1–12.
- Schmidt-Bleker A, Winter J, Bösel A, Reuter S, Weltmann KD. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Science and Technology. 2016;25(1):15005–15005.
- Berner J, Miebach L, Herold L, Höft H, Gerling T, Mattern P, et al. Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids. Cancers 2023, Vol 15, Page 1254. 2023 Feb;15(4):1254–1254.
- Schneider S, Lackmann JW, Ellerweg D, Denis B, Narberhaus F, Bandow JE, et al. The Role of VUV Radiation in the Inactivation of Bacteria with an Atmospheric Pressure Plasma Jet. Plasma Processes and Polymers. 2012 Jun;9(6):561–8.
- Koban I, Matthes R, Hübner NO, Welk A, Meisel P, Holtfreter B, et al. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New Journal of Physics. 2010;12(7):73039–73039.
- Matthes R, Jablonowski L, Holtfreter B, Gerling T, von Woedtke T, Kocher T. Fibroblast Growth on Zirconia Ceramic and Titanium DisksAfter Application with Cold Atmospheric Pressure PlasmaDevices or with Antiseptics. The International Journal of Oral & Maxillofacial Implants. 2019;
- Matthes R, Jablonowski L, Miebach L, Pitchika V, Holtfreter B, Eberhard C, et al. In-Vitro Biofilm Removal Efficacy Using Water Jet in Combination with Cold Plasma Technology on Dental Titanium Implants. International Journal of Molecular Sciences 2023, Vol 24, Page 1606. 2023 Jan;24(2):1606–1606.
- Steuer A, Wolff CM, von Woedtke T, Weltmann KD, Kolb JF. Cell stimulation versus cell death induced by sequential treatments with pulsed electric fields and cold atmospheric pressure plasma. PLOS ONE. 2018;13(10):1–28.
- Yang HJ, Lee B, Shin C, You B, Oh HS, Lee J, et al. Improvement in Biocompatibility and Biointegration of Human Acellular Dermal Matrix through Vacuum Plasma Surface Treatment. Bioengineering. 2024 Apr;11(4):359.
