Volume 22 Number 1
Leiomyosarcoma - A rare neoplasia arising from a chronic venous ulcer. Case report and literature review
Ion A, Giurcaneanu C, Nichita M, Orzan OA, Popa LG, Beiu C, Tudose I, Mihai MM
Keywords chronic wound, chronic ulcer, atypical wound, leiomyosarcoma, skin cancer
DOI 10.35279/jowm202104.06
Abstract
Background Long-standing wounds are at risk of developing malignant degeneration, including leiomyosarcomas. These exceedingly rare malignant tumours originate from smooth muscle cells and are considered superficial if they affect the dermis and/or subcutis.
Hypothesis To exclude malignancy, a biopsy should be performed on chronic skin ulcers with atypical features or evolutions.
Methods We report the case of a 62-year-old male with a history of a two-year old chronic venous ulcer who presented with a tumour developing over three months, progressively increasing in size on the surface of the wound. As the clinical aspect strongly suggested a malignant tumour arising within a chronic venous ulcer (the initial suspicion was of squamous cell carcinoma), a biopsy from the edge of the lesion was performed.
Results Histopathological and immunohistochemical examinations revealed a diagnosis of leiomyosarcoma. The patient refused further medical care. The tumour arose in the context of severe cutaneous changes of venous insufficiency and had aggressive clinical behaviour. Histopathological and immunohistochemical analysis of the tissue sample is essential for establishing the final diagnosis, as no pathognomonic clinical features of leiomyosarcoma exist.
Conclusions Our case emphasises the importance of considering malignancy in chronic ulcers with atypical features or evolution. Biopsy of atypical skin ulcers is crucial for establishing a diagnosis and initiating therapy at the rightful moment.
Implications for clinical practice The findings from this case highlight the importance of an early diagnosis of atypical, non-healing wounds, based on tissue samples, to decide the optimal approach. Patient denial may interfere with the proper management of this particular clinical context.
INTRODUCTION
Leiomyosarcoma (LMS) is an exceedingly rare malignant tumour that originates from smooth muscle cells, common locations being the dermis, subcutaneous tissue, retroperitoneum, and gastrointestinal tract.1 Leiomyosarcomas found in the dermis and subcutis are considered superficial, while those developed in the gastrointestinal tract and retroperitoneum are considered deep variants.2 The dermal subtype may arise from the arrector pili muscle or areola complex, while the subcutaneous type originates from the smooth muscle of the vessels in the subcutaneous tissue.3 Leiomyosarcomas are usually diagnosed in middle-aged and elderly men.4 Table 1 lists the case reports of leiomyosarcomas of the extremities published in the scientific literature between 1982 and 2018.
Long-standing wounds are at risk of developing malignant degeneration, including leiomyosarcoma. We hypothesis: To exclude malignancy, a biopsy should be performed in chronic skin ulcers with atypical features or evolution.
METHODS
We report the case of a 62-year-old male with a history of chronic venous ulcer (the last two years), obese, heavy smoker (42 pack/year), who presented with a tumour developing over three months, progressively increasing in size on the surface of a lower limb wound associated with cutaneous signs of severe stasis dermatitis. Clinical examination revealed an ulcerated exophytic tumour of 4/7 cm, with areas of active bleeding and covered with purulent secretions on the left lower limb, pretibially (Figure 1A and 1B). Severe stasis signs, such as bilateral leg oedema, induration, and pigmentary changes, were also present. Laboratory findings showed abnormal values of inflammatory markers. Bacteriology tests revealed an infection with Staphylococcus aureus susceptible to antibiotics. Doppler venous ultrasound showed incompetent venous perforators and excluded deep venous obstruction. Radiologic examination of the lower left limb excluded bone involvement. As this clinical presentation strongly suggested a malignant tumour developing on a chronic venous ulcer, a biopsy from the edge of the lesion was performed.
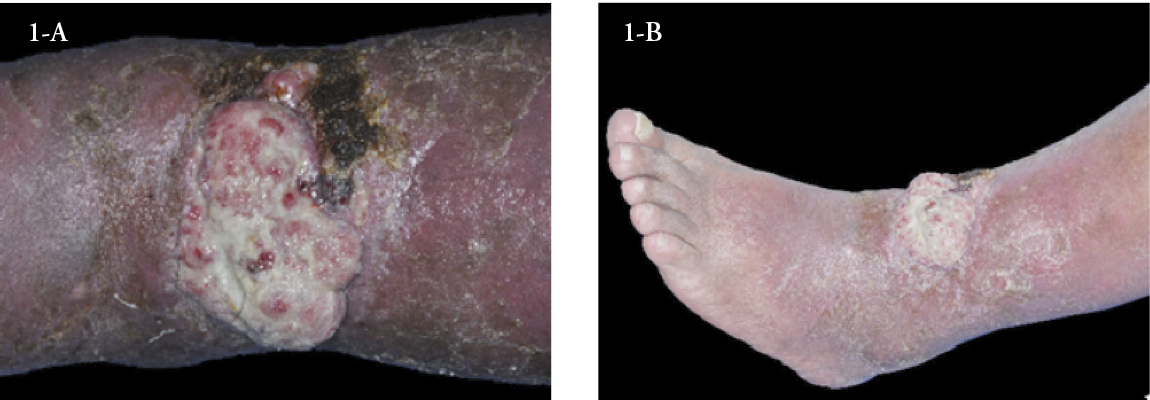
Figure 1- A) and B) Ulcerated exophytic tumour, 4/7 cm, with areas of active bleeding and covered with purulent secretions on the left lower limb, pretibially.
RESULTS
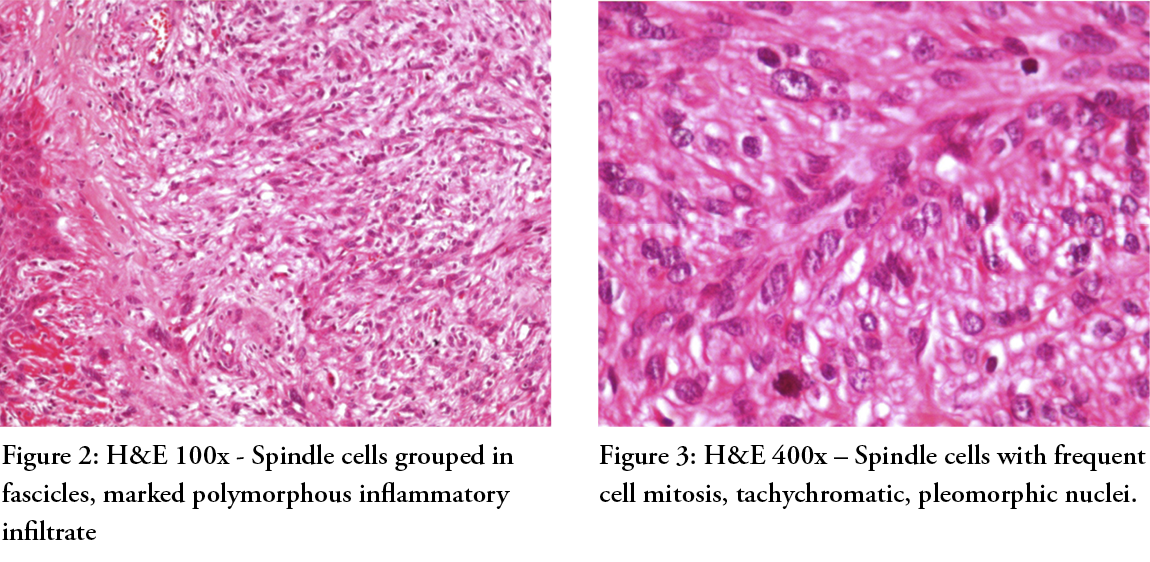
The histopathologic examination revealed a tumoural proliferation infiltrating deep below in the connective tissue; spindle cells grouped in fascicles, marked polymorphous inflammatory infiltrate (Figure 2, H&E 100x), frequent cell mitosis, tachychromatic, pleomorphic nuclei (Figure 3, H&E 400x), the diagnosis being that of a poorly differentiated squamous cell carcinoma, acromic melanoma or sarcoma. Immunohistochemistry revealed the positivation of actin, calciponin, and desmin — all three markers of leiomyosarcoma.
Consequently, the patient underwent additional imaging examination methods: the abdominal ultrasound revealed multiple transonic intrahepatic masses, while the contrast-enhanced thoraco-abdominopelvic CT scan showed localised intrahepatic masses (max. diameter of 14 mm), as well as supra- and infradiaphragmatic adenopathies (10–19 mm). Histopathological examination was required to confirm systemic tumoural spread.
Regarding the management of this case, the patient refused further sampling by biopsy, surgical treatment, or oncologic care. Consequently, the seven-month follow-up revealed an enlarged skin tumour (6/10 cm) and no clinical signs of systemic tumoural spread.
The tumour arose in the context of severe trophic cutaneous changes of venous insufficiency with aggressive clinical behaviour (rapid evolution in 10 months). Our case highlights the importance of considering the possibility of malignancy in chronic ulcerations. No pathognomonic clinical features of leiomyosarcoma exist. Immunohistochemical analysis of the tissue sample established final diagnosis. Delay in diagnosis results in poor prognosis and a decrease in effective therapeutic alternatives.
DISCUSSION
Long-standing wounds, such as osteomyelitis sinuses and burn scars, are at risk of developing secondary malignant cutaneous tumours.5 A well-known example of such clinical presentation is the Marjolin ulcer, an aggressive and rare type of squamous cell carcinoma developed in areas affected by inflammation, scars, and chronic wounds.6,7
The prevalence of malignant wounds that develop within chronic leg ulcers (CLUs) is approximately 2–4%.8 Leiomyosarcomas rarely arise from chronic skin ulcers. A Swedish retrospective study from 1995 by Baldursson et al. that aimed to precisely estimate the relative risk of squamous cell carcinoma in venous CLUs of 10,913 patients showed that only seventeen of them presented with a secondary malignant degeneration.8,9 Senet P et al. (2012) studied the evolution of 154 CLUs in 144 patients and showed a skin cancer frequency of 10.4%, with nine cases of squamous cell carcinomas, five of basal cell carcinomas, one melanoma, and one case of leiomyosarcoma.10
However, a multitude of malignant lower limb skin tumours may have the clinically confusing appearance of non-healing ulcers. Although basal cell carcinomas and squamous cell carcinomas represent frequent aetiologies for primary malignant wounds on the leg, several other tumours may also develop, including melanoma, malignant vascular tumours, Merkel cell carcinoma, cutaneous lymphomas, sarcomas, adnexal tumours, and skin metastases.8
In acute wounds that do not progress towards healing, other scenarios are possible. These include the development of biofilm infections arising from bacterial aggregates and fibrin found at the edge of acute wounds in split stratum corneum11, pyoderma gangrenosum12, and others.
The diagnosis of malignancy in CLUs is often difficult. According to the document of best clinical practice and challenges in the management of atypical wounds of the European Wound Management Association, some common clinical signs suggestive of malignancy are hypertrophic wound edges, excessive granulation tissue of the wound bed and edges, enlarging in the size of an otherwise long-lasting ulcer, or an atypical location.8 Other features of clinically suspicious malignant wounds are increased wound pain, irregular wound borders, wound odour, bleeding, and fragile tissue.8
In a review of neoplastic wounds and degeneration, Meaume et al.13 stated that an ulcer should have been present for at least three years to be considered a malignant transformation, rather than an ulcerated tumour. This information could help increase patient and/or clinician awareness and, therefore, the achievement of an early malignancy diagnosis.
At present, there is no consensus with respect to the ideal moment in which a biopsy from a chronic, non-healing ulcer should be obtained to exclude a malignancy. A previous study indicated that a biopsy should be performed if the ulcer has been present for longer than three months and has not responded to treatment.14 Other studies have suggested that biopsy should be performed only in the presence of suspicious features, such as wounds that have increased in size in spite of appropriate therapy, the presence of granulation tissue that extends beyond the margins, ulcers with irregular bases or margins, and wounds with exophytic growth and changes in drainage, that is, haemorrhage.15,16,17 In a 2018 retrospective study, 143 skin biopsies of CLUs were analysed prompted by atypical clinical signs and/or absence of improvement.18 The results showed a 5% rate of non-vascular causes of ulcers, mainly skin cancers (n = 5, 3.5%).18
Moreover, it is advisable to repeat a biopsy in a patient with previous negative histologic results if the wound maintains its suspicious clinical appearance or inadequate response to appropriate treatment.8
The management of leiomyosarcomas includes diagnostic investigations to establish cancer staging, such as imaging tests, and therapeutic options, such as surgery (including limb amputation in severe cases), radiation therapy, and systemic treatment (chemotherapy, chemoradiation, targeted therapies, and immunotherapy).19 A 2016 review of the presentations and management of these particular neoplasms observed that the first-line treatment of superficial leiomyosarcomas usually included a wide local excision with 2 cm margins or Mohs micrographic surgery.20 In a retrospective review that included 83 patients (64 with dermal leiomyosarcomas and 19 with subcutaneous leiomyosarcomas) highlighted that dermal leiomyosarcomas had an excellent prognosis, whereas subcutaneous leiomyosarcomas had a tendency for local recurrence, metastasis, and a poorer outcome of a lower distant disease-free survival and recurrence-free survival21 (see Table 1).
A common psychological condition found in oncologic patients is denial, which becomes dysfunctional when it prevents the patient from receiving treatment for their illness, as was the case for our patient.22 A proper doctor–patient relationship, different communication practices, or psychiatric help may ease dealing with denial or even anosognosia.22 A 2015 case report described a dramatic situation in which a 59-year-old male patient with leiomyosarcoma of the hand refused the conservative surgical approach to the lesion, accepting only systemic chemotherapy. A year later, local recurrence led to the inevitable amputation of the affected hand after histological confirmation of a high grade leiomyosarcoma. New local recurrences were found two years later at the mid forearm level, and a progression of disease with lung metastasis was observed. In the end, the patient died of the disease.23
CONCLUSION
In the case we presented, a rare tumour, leiomyosarcoma, arose in the context of severe cutaneous changes of venous insufficiency and had aggressive clinical behaviour. Our case emphasises that malignancy should be considered in the differential diagnosis of a chronic ulceration with atypical aspect or evolution. The delay in diagnosis may alter the chances of curative treatment and affect patient survival. This report has valuable implications for clinical practice. Early diagnosis based on tissue samples from a non-healing, clinically suspicious wound is fundamental to determining the correct approach. Patient denial may interfere with proper management and overall outcomes; therefore, effective communication practices or psychiatric help may be effective solutions in such cases.
Key messages
Long-standing wounds are at risk of developing malignant degeneration, including leiomyosar comas.
To exclude malignancy, a biopsy should be performed on chronic skin ulcers with atypical features or evolutions.
Author(s)
Ion A1, Giurcaneanu C1,2, Nichita M1, Orzan OA1,2, Popa LG1,2, Beiu C1,2, Tudose I3, Mihai MM1,2
1. Dermatology and Allergology Department, “Elias” Emergency University Hospital, Bucharest, Romania
2. Oncologic Dermatology Department, “Elias” Emergency University Hospital, „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
3. Pathology Department, “Elias” Emergency University Hospital, Bucharest, Romania
Correspondence: drmaramihai@gmail.com
Conflicts of Interest: None
References
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71(5):919-925.
- Fields JP, Helwig EB. Leiomyosarcoma of the skin and subcutaneous tissue. Cancer. 1981;47(1):156-169.
- Kazlouskaya V, Lai YC, Khachemoune A. Leiomyosarcoma of the skin: review of the literature with an emphasis on prognosis and management. Int J Dermatol. 2020;59(2):165-172.
- Deneve JL, Messina JL, Bui MM, et al. Cutaneous leiomyosarcoma: treatment and outcomes with a standardised margin of resection. Cancer Control. 2013;20(4):307-312.
- Kaplan RP. Cancer complicating chronic ulcerative and scarifying mucocutaneous disorders. Adv Dermatol. 1987;2:19-46.
- Bazaliński D, Przybek-Mita J, Barańska B, Więch P. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21(3):197-202.
- Shah M, Crane JS. Marjolin Ulcer. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 19, 2020.
- Isoherranen K, O’Brien JJ, Barker J, et al. Atypical wounds. Best clinical practice and challenges. J Wound Care. 2019;28(Sup6):S1-S92. doi:10.12968/jowc.2019.28.Sup6.S1
- Baldursson B, Sigurgeirsson B, Lindelöf B. Venous leg ulcers and squamous cell carcinoma: a large-scale epidemiological study. Br J Dermatol. 1995;133(4):571-574.
- Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers: the value of systematic wound biopsies: A prospective, multicenter, cross-sectional study. Arch Dermatol. 2012;148(6):704-708.
- Bay L, Kragh KN, Eickhardt SR, et al. Bacterial Aggregates Establish at the Edges of Acute Epidermal Wounds. Adv Wound Care (New Rochelle). 2018;7(4):105-113.
- Miron A, Giulea C, Tudose I, Petrache D, Giurcaneanu C. Pyoderma gangrenosum, rare parietal complication after colorectal surgery. Chirurgia (Bucharest, Romania : 1990). 2014 Mar-Apr;109(2):248-253.
- Meaume S, Fromantin I, Teot L. Neoplastic wounds and degenerescence. J Tissue Viability. 2013;22(4):122-130.
- Hansson C, Andersson E. Malignant skin lesions on the legs and feet at a dermatological leg ulcer clinic during five years. Acta Derm Venereol. 1998;78(2):147-148.
- Goldman MP. Nonhealing leg ulcers: a manifestation of basal cell carcinoma. J Am Acad Dermatol. 1992;26(5 Pt 1):791-792.
- Phillips TJ, Salman SM, Rogers GS. Nonhealing leg ulcers: a manifestation of basal cell carcinoma. J Am Acad Dermatol. 1991;25(1 Pt 1):47-49.
- Harris B, Eaglstein WH, Falanga V. Basal cell carcinoma arising in venous ulcers and mimicking granulation tissue. J Dermatol Surg Oncol. 1993;19(2):150-152.
- Stansal A, Khayat K, Duchatelle V, et al. Quand poser l’indication d’une biopsie cutanée chez un patient porteur d’ulcère de jambe ? Étude rétrospective sur 143 biopsies consécutives [When to ask for a skin biopsy in a patient with leg ulcer? Retrospective study of 143 consecutive biopsies]. J Med Vasc. 2018;43(1):4-9.
- von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(5):536-563.
- Patt JC, Haines N. Soft tissue sarcomas in skin: Presentations and management. Semin Oncol. 2016;43(3):413-418.
- Wong GN, Webb A, Gyorki D, et al. Cutaneous leiomyosarcoma: dermal and subcutaneous. Australas J Dermatol. 2020;61(3):243-249.
- Rabinowitz T, Peirson R. “Nothing is wrong, doctor”: Understanding and managing denial in patients with cancer. Cancer Invest. 2006;24(1):68-76.
- Angelini A, Barastegui D, Gambarotti M, Ruggieri P. Leiomyosarcoma of the hand. Handchir Mikrochir Plast Chir. 2015;47(2):139-141.
- Ahluwalia VV, Singh PK, Singh H, Samanta S. Advanced magnetic resonance imaging in the evaluation of a chronic non-healing ulcer - A case report of rare primary cutaneous leiomyosarcoma. J Orthop Case Rep. 2018;8(4):7-10.
- Cuenca-Gonzalez C, Berzal-Cantalejo MF, Herranz Torrubiano AM, Lorenzo Vaamonde L. Management of lower limbs leiomyosarcoma: A case report. Radiol Diagn Imaging 2017:2.
- Papageorgiou K, Goodwin P, Travlos J, Dramis A. Leiomyosarcoma of the lower limb presenting as a benign mass: A case report. J Orthop Case Rep. 2018;8(6):13-15.
- R. Rojas Sayol, L. Trullols Tarragó, A. Grau Blanes, J. Martinez Zaragoza, E. Britez Altamirano, A. Peiró Ibañez, et al. Leiomyosarcomas affecting main vessels in the lower extremities, Revista Española de Cirugía Ortopédica y Traumatología. 2018;62(6):401-407
- Sleiwah A, Clinton A, Herbert K. Delayed diagnosis of dermal leiomyosarcoma mimicking keloid scar. BMJ Case Rep. 2018;2018:bcr2017222616.
- Abd Kadir HK, Naik J, Chandrasekar CR. Leiomyosarcoma presenting as ‘idiopathic’ unilateral lower limb lymphoedema. Case Reports 2017:bcr-2017-219898.
- Bonamonte D, Vestita M, Filoni A, Ingravallo G, Sportelli P. Recurrent cutaneous leiomyosarcoma of the inner thigh. Indian J Dermatol Venereol Leprol. 2015;81(3):309-311.
- Bali A, Kangle R, Roy M, Hungund B. Primary cutaneous leiomyosarcoma: A rare malignant neoplasm. Indian Dermatol Online J. 2013;4(3):188-190.
- Abed R, Abudu A, Grimer RJ, Tillman RM, Carter SR, Jeys L. Leiomyosarcomas of vascular origin in the extremity. Sarcoma. Vol. 2009.
- Angeloni M, Muratori F, Magarelli N, et al. Exophytic growth of a neglected giant subcutaneous Leiomyosarcoma of the lower extremity. A case report. Int Semin Surg Oncol. 2008;5:11.
- Killoran TP, Wells WA, Barth RJ, Goodwin DW. Leiomyosarcoma of the popliteal vein. Skeletal Radiol. 2003;32(3):174-178.
- Auroy S, Contesso G, Spatz A, Genin J, Margulis A, Lecesne A, Avril MF. Léiomyosarcomes cutanés primitifs: 32 cas [Primary cutaneous leiomyosarcoma: 32 cases]. Ann Dermatol Venereol. 1999 Mar;126(3):235-42.
- Aranha GV, Molnar ZV, Reyes CV, Skladzien G, Valadka VT. Leiomyosarcoma of the skin: a case report. J Surg Oncol. 1982;19(2):87-89.
