Volume 22 Number 2
Negative pressure wound therapy and patients with severe diabetic foot ulcers: A retrospective cohort study
Rashad A. Bishara, Amr M. Abdel-Mawgoud, Laila Al-Sabbagh, Ihab N. Hanna, Mohammed Ramadan Abdel-Mageed, Nehad A. Fouad, Ramez O. Shehata.
Keywords negative pressure wound therapy, diabetic foot ulcers, diabetic foot, diabetic wound
DOI 10.35279/jowm202107.04
Abstract
Background The annual incidence of diabetic foot ulcers (DFU) is estimated to be 2–5% of the diabetic population, and it is estimated that 15% of diabetic patients will develop a DFU during their lifetime.1 The main contributing factors in the development of DFU are peripheral neuropathy and peripheral vascular disease.2 DFUs constitute a significant burden on the patient, their family, and society and pose an important challenge in the treatment of patients with diabetes.3 Roughly 5–8% of patients with a DFU will require a major amputation within one year of the diagnosis, despite aggressive treatment.1 The WIFI classification, developed by the Society for Vascular Surgery, categorises the risk of major amputation according to the interaction among a wound’s severity (W), degree of foot ischemia (I) and degree of infection (If).4 In this study, we examined the effect of negative pressure wound therapy (NPWT) on the outcome of patients with DFU categorised as W2 and W3 according to the WIFI classification. The treatment of wounds and ulcers by the application of negative pressure was developed in the 1990s. The name “Negative Pressure Wound Therapy” was commonly used to describe this technique5 It aims to improve a patient’s quality of life by improving healing rates and reducing the healing time of DFUs.6 While the effect of NPWT on wound-healing has been investigated in many previous studies,7–12 its effect on DFUs categorised as ‘severe’ has not been described before.
Aim of the study This study aimed to compare the results after using NPWT to standard wound care in severe DFUs, classified in the WIFI classification system as W2 and W3.
METHODS
The study was approved by the Institutional Review Board. The study complies with Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association Declaration of Helsinki. All patients signed an informed consent prior to participating in the study.
This is a retrospective cohort study. The data of all patients presenting to our centre were prospectively entered into a digital database. The information collected included age; sex; risk factors; clinical presentation; description of DFUs according to WIFI classification; laboratory results; clinical progress over time; the use of vacuum therapy; details of revascularisation; and outcome in terms of ulcer healing, foot surgery, major amputation or death.
A search was made of the centre’s digital database to identify patients who presented with DFUs between January and December 2018, and whose DFUs were classified as W2 or W3 according to WIFI criteria.10
Patients presenting with venous ulcers or who showed evidence of malignancy, and patients being treated with immunosuppressive drugs or corticosteroid therapy, were excluded from this study.
Patients presenting with DFUs underwent initial sharp debridement to remove any necrotic tissues and sloughs as much as possible. Subsequently, patients received daily dressings. Patients were followed up every two weeks. Repeated sharp debridement was performed as needed according to the guidelines of the Society for Vascular Surgery.13 All patients initially received broad-spectrum antibiotics with additional treatments according to the results of cultures and sensitivity assessments. Osteomyelitis and infection were treated similarly in both groups according to the guidelines set by the Society for Infectious Diseases.14 Offloading devices were applied for plantar ulcers, according to the guidelines of the International Working Group for Diabetic Foot.15
The degree of foot ischemia was assessed using the Ankle Peak Systolic Velocity (APSV). This parameter was previously compared with the Ankle Brachial Index (ABI) and the Toe Brachial Index (TBI) and showed a good correlation with both of them.16 We did not use ABI or TBI with this cohort of patients because of the potential fallacies of those two tests, which are caused by vessel wall calcification.17 The method for calculating APSV has been described previously. In brief, it is the mean of the peak systolic velocity of the distal anterior tibial artery and posterior tibial artery measured by a duplex scan at the ankle level. The threshold of 35cm/sec was previously identified, below which a DFU is unlikely to heal.18 All patients presenting with DFUs and APSV <35cm/sec were revascularised. The presence of significant PAD was further confirmed by the presence of clinical manifestations of ischemia: the presence of ischemic gangrene; ischemic tissue necrosis; or failure of the wound to reduce in size by 50% within 4 weeks, despite appropriate infection control, wound care and offloading. In such cases, revascularisation was offered via catheter-based techniques or open surgery. The revascularisation treatment offered was similar in both groups and based on the Joint Guidelines of the Society for Vascular Surgery, European Society for Vascular Surgery and the World Federation of Vascular Societies,19 except that we used APSV instead of ABI.16,18 Revascularisation procedures were performed prior to the application of NPWT.
NPWT was applied, according to the discretion of the treating physician, to severe DFUs, those classified as WIFI wound class W2 or W3. NPWT (RENASYS™, Smith & Nephew, Watford, Hertfordshire, UK) was applied after all necrotic tissue was debrided; infection was adequately controlled; offloading devices were used, if necessary; and ischemia corrected. Complete aseptic conditions were used. The edges of the wound were protected with an adhesive barrier, and then the wound surface was covered with polyurethane foam. Deep areas of the wounds were also filled with foam and then covered with adhesive drapes to create an airtight seal. An evacuation tube embedded in the foam was connected to a fluid collection canister within a portable vacuum/suction machine. A standard negative pressure of -120mmHg was applied continuously. NPWT dressings were changed twice weekly.
The primary endpoints were complete ulcer healing (i.e., when the ulcer was completely covered by skin without defect), when a major amputation was completed proximal to the ankle or death.
Statistical analysis
Continuous variables were compared using the student t-test. Categorical variables were compared using chi-square. Multiple regression analysis was used to estimate the influence of treatment group, age, diabetes, hypertension, myocardial impairment, renal impairment, smoking and wound effects on time until complete healing. A multinomial logistic regression was performed to model the relationship between the predictors and membership in different outcome groups (completely healed, amputation and death). The addition of the predictors to a model that contained only the intercept significantly improved the fit between the model and the data: chi-square (320, N=405) = 58.2, Nagelkerke R2 = .17, P<0.001.
RESULTS
In all, 341 patients were identified after presenting to our centre between January and December 2018 with severe DFUs classified according to WIFI classification as W2 or W3. They were divided into two groups: Group A (n=136) received treatment with NPWT, and Group B (n=205) was treated with conventional dressings.
There was no significant difference between the groups in terms of demographic factors or co-morbidities, as shown in Table 1, or wound severity, as shown in Table 2. Surgical debridement and foot surgery were performed before the application of vacuum therapy. No further foot surgery was required after the application of NPWT in either group. More patients presented with severe ischemia and required revascularisation in Group A 60/136 (44%), compared with Group B 64/205 (31%), p<0.05.
Table 1: Demographic factors and co-morbidities in the two groups
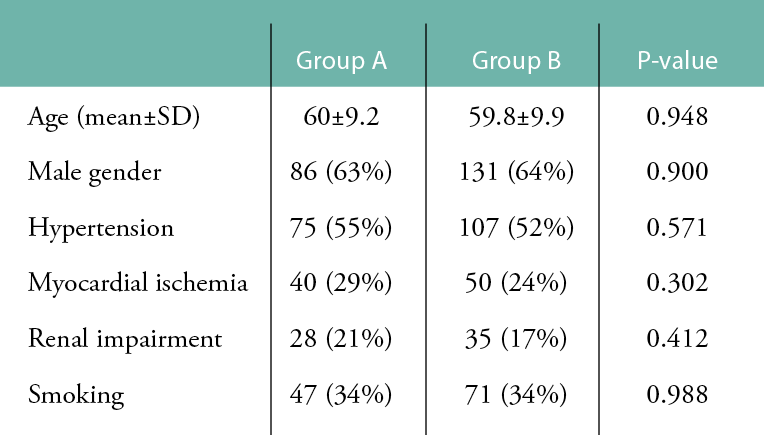
Table 2: Distribution of wounds in the two groups according to WIFI classification.
Complete follow-up was achieved in 131 (96%) patients in Group A, compared to 153 (75%) patients in Group B (P<0.00001). The time of follow-up was calculated from the day of presentation until an endpoint was reached (complete healing, major amputation or death). The follow-up for this study was terminated on 30 June 2019. Patients with DFUs that did not heal prior to this date were considered ‘not healed’. Similarly, mortality was calculated through June 2019. The mean follow-up was 127 (±152.3) days.
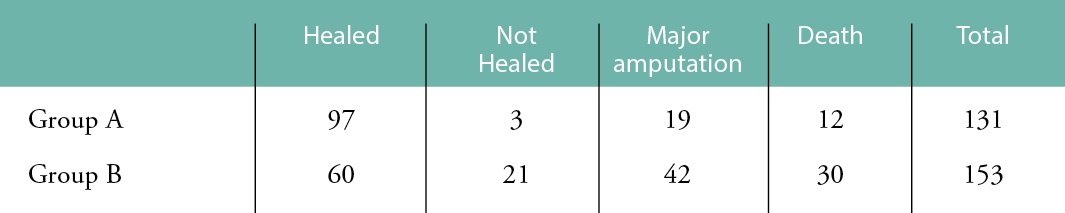
Wound healing was significantly better in Group A, compared to Group B. Complete healing was achieved in 97/131 (74%) in Group A, compared to 60/153 (39%) in Group B (P<0.00001). Three patients (3/131) did not achieve complete healing during the follow-up period in Group A, compared to 21/153 in Group B (P=0.0005) (Fig. 1 and Table 3).
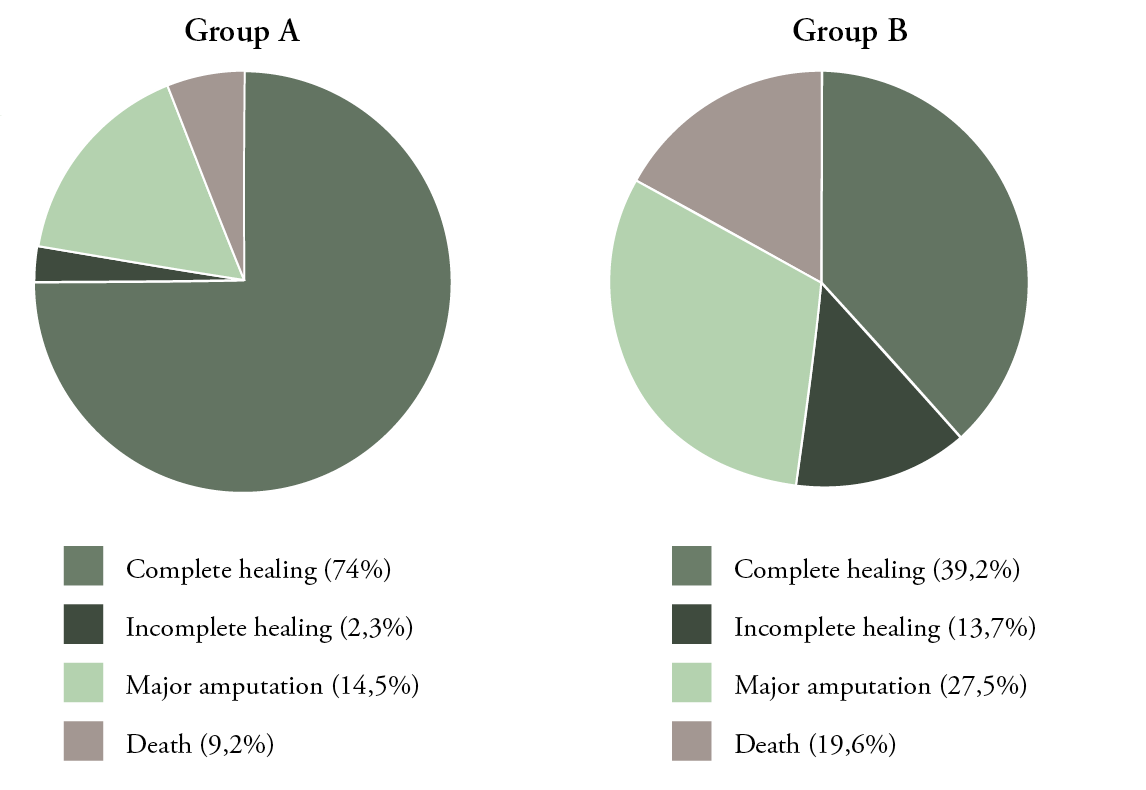
Figure 1: Outcomes in both groups:
Group A: NPWT, Group B: conventional dressing (complete healing; P<0.00001, P=0.0005,
Smajor amputation; P<0.01, death; P=0.01).
Table 3: Healing, major amputations and deaths in Groups A and B.
The mean time to achieve complete healing was 194 (±101) days in Group A, compared to 333.1 (±153.9) days in Group B (P<0.00001) (Fig. 2). Major above-ankle amputation was performed in 19/131 (15%) patients in Group A, compared to 42/153 (27%) patients in Group B (P<0.01) (Fig. 1). There was no 30-day mortality in either group; however, 12 (9%) patients in Group A died in during the follow-up period, compared to 30/153 (20%) patients in Group B (P=0.01) (Fig. 1).
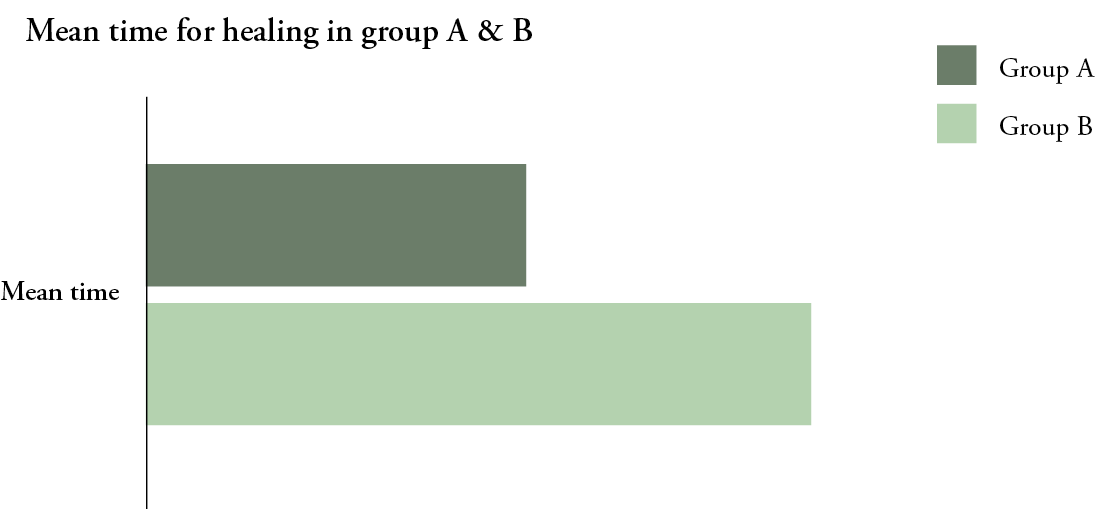
Figure 2: Mean time to complete healing in both groups:
Group A: NPWT, Group B: Conventional dressing (P<0.00001).
A multiple regression analysis showed that there was a significant effect in the treatment groups on time to heal (F(1,135)=21.9, P.<0.001). Patients in Group B took a significantly longer time to heal by 114.6 days than Group A. There was a statistically significant effect of wound degree on healing time (F(1,135)=4.1, P=0.045). Patients with a W3 wound took 60.8 days more to heal than those with a W2 wound. Patients with renal impairment had extended time until healing, an average of 65.5 days longer than those with no such impairment (F(1,135)=5.2, P=0.025).
Multiple logistic regression analysis showed that whether the patients were in treatment Group A or B significantly predicted the odds of amputation over complete healing, b=1.35, Wald x2 (2)=22.6, p=0.001. Patients in Group B were 3.9 times more likely to have major amputation, compared to Group A. The treatment group also significantly predicted the odds of death over complete healing, b=1.74, Wald x2 (2)=22.6, P<0.001. Patients in Group B had 5.7 times greater odds of death compared to Group A.
Wound severity significantly predicted the odds of amputation over complete healing. Patients with severe wounds, classified as W3, were 4.5 times more likely to undergo a major amputation than to heal, compared to patients with less severe wounds, classified as W2, b=1.5, Wald x2 (2) =15, P=<0.001.
DISCUSSION
The management of DFUs is considered a major issue for vascular surgeons, especially for bigger and deeper wounds, as they take a long time to heal and put the patient at risk of recurrent infections and major amputation.20 The principal elements for managing DFUs include the debridement of necrotic and infected tissues; treatment of infection; revascularisation, if needed; offloading, when necessary; and wound dressings.4 NPWT has been shown to be effective and safe in multiple randomised controlled studies,7-11yet none of them have specifically addressed severe DFUs or assessed patients according to the WIFI classification.4
This study aimed to evaluate the results of NPWT when compared to conventional dressings in patients with severe DFUs, those classified as W2 or W3 in the WIFI classification by the Society for Vascular Surgery.4
We identified 341 patients with severe DFUs and classified them into two groups, Group A (NPWT=136) and Group B (conventional dressing=205). In our study, complete wound healing was achieved in 74% of patients in Group A and 39% of patients in Group B (P<0.00001). Our results agree with Blume et al., who reported an improved rate of complete ulcer closure with NPWT (43%), compared to advanced wound dressing (28.9%) within the 112-day active treatment phase (P=0.007).7 The higher rate of complete wound healing in our study, compared to Blume et al.’s, can be explained by the longer duration of follow-up.
In our study, the mean time to achieve complete healing was 194 days in Group A, compared to 333.1 days in Group B (P<0.00001). Blume et al. reported a median estimated time for 100% ulcer closure of 96 days for NPWT and an undeterminable time for advanced modern wound dressing (P=0.001).7 In Blume et al.’s study, skin grafts and flaps were used for wound closure.7 Surgical closure techniques were not used in our patients, which may explain the longer duration to achieve complete healing in our patients.
In our study, a major amputation was performed in only 15% of patients in Group A, compared to 27% of patients in Group B (P<0.01). Similarly, Ulusal et al. showed a reduced major amputation rate with the use of NPWT.12 Dalla Paola et al. showed that the use of NPWT in addition to external fixation and skin substitutes reduced the need for subsequent amputations to zero in patients with calcaneal osteomyelitis and heel ulcers.21 Blume et al. also demonstrated that the incidence of secondary amputations was significantly lower for NPWT (4.1%), compared to advanced modern wound therapy (10.2%; P = 0.035).7 Although the exact mechanism of decline in secondary major amputation remains unclear, the treatment of DFUs with NPWT may offer the advantages of faster removal of infectious material, better preparation of the granulated wound bed and more rapid healing, therefore reducing the risk of major amputation.
In our study, there was no incident of 30-day mortality in either group; however, 12 (9%) patients in Group A died during the follow-up period, compared to 30 (20%) patients in Group B (P=0.01). Patients treated with conventional dressing had a longer time to heal, with the risks of recurrent infection, toxic manifestations, major amputation and death. This was previously shown by Karatepe et al., who reported a shorter time to heal for patients treated with NPWT. Further, by using the SF-36 questionnaire to assess quality of life in patients with DFUs treated with NPWT versus conventional dressing, they showed that the effect of NPWT was significantly positive for both mental (P=0.0287) and physical (P=0.004) health, compared to treatment with conventional therapy.22
We noted that more patients presented with severe ischemia and required revascularisation in Group A (60/136; 44%), compared with Group B (64/205; 31%) p<0.05. The outcomes of patients suffering from severe ischemia were expected to be worse than the outcomes in non-ischemic patients.4 Group A contained more patients suffering from severe ischemia; therefore, this would have negatively affected the results of Group A. This study showed improved results in Group A.
There are some limitations in this study, including nonrandomisation and its retrospective design. No cost analysis was calculated for each group to evaluate the cost-effectiveness of using NPWT compared to conventional dressing for the management of DFUs.
CONCLUSION
Our results support previous findings that the use of NPWT enabled a significantly higher proportion of patients to reach complete ulcer healing in a shorter time and led to a significant reduction in amputations and deaths. These were confirmed in our study of patients with severe DFUs, which were classified as W2 and W3 in the WIFI classification. However, since this is a retrospective cohort study, the results should be viewed with caution.
IMPLICATIONS FOR CLINICAL PRACTICE
The use of NPWT is beneficial in patients presenting with severe diabetic foot ulcers.
Further research
Further research could be directed towards the assessment of the optimal duration of NPWT and the optimal negative pressure in the presence of foot ischemia.
Acknowledgment
We acknowledge the work of Salma N. Mowafi, who performed all the statistical analyses.
Key messages
This study compared the healing of severe diabetic foot ulcers (DFU) in patients treated with conventional dressings, compared to those treated with negative pressure wound therapy (NPWT).
Our study confirmed that NPWT increased the number of ulcers that reached complete healing, reduced the time of healing, reduced major amputations and reduced deaths in patients suffering from DFUs classified as severe.
Author(s)
Rashad A. Bishara, Amr M. Abdel-Mawgoud, Laila Al-Sabbagh, Ihab N. Hanna, Mohammed Ramadan Abdel-Mageed, Nehad A. Fouad, Ramez O. Shehata.
Diabetic Foot Center DFC-Egypt, 15 Sarayat Street, Abbasseya, Cairo 11381, Egypt
Correspondence: rashadbishara@gmail.com
Conflicts of Interest: None
References
- Kim SY, Kim TH, Choi JY, Kwon YJ, Choi DH, Kim KC, et al. Predictors for amputation in patients with diabetic foot wound. Vasc Specialist Int. 2018 Dec; 34(4):109–16. doi: 10.5758/vsi.2018.34.4.109. Epub 2018 Dec 31. PMID: 30671420; PMCID: PMC6340693.
- Boulton AJM, Whitehouse RW. The diabetic foot. [Updated 2020 Mar 15]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. Available at: https://www.ncbi.nlm.nih.gov/books/NBK409609/.
- Rathur HM, Boulton AJ. The diabetic foot. Clin Dermatol. 2007 Jan–Feb; 25(1):109–20. doi: 10.1016/j.clindermatol.2006.09.015. PMID: 17276208.
- Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIFI). J Vasc Surg. 2014 Jan; 59(1):220–34.e1-2. doi: 10.1016/j.jvs.2013.08.003. Epub 2013 Oct 12. PMID: 24126108.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg. 1997; 38(6):563–76; discussion 577.
- Karatepe O, Eken I, Acet E, Unal O, Mert M, Koc B, et al. Vacuum assisted closure improves the quality of life in patients with diabetic foot. Acta Chir Belg. 2011 Sep–Oct; 111(5):298–302. PMID: 22191131.
- Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care. 2008 Apr; 31(4):631–6. doi: 10.2337/dc07-2196. Epub 2007 Dec 27. PMID: 18162494.
- Armstrong DG, Lavery LA, Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet. 2005 Nov 12; 366(9498):1704–10. doi: 10.1016/S0140-6736(05)67695-7. PMID: 16291063.
- Etoz A, Kahveci R. Negative pressure wound therapy on diabetic foot ulcer. Wounds. 2007 Sep; 19(9):250–4. PMID: 25942747.
- Sajid MT, Mustafa Q, Shaheen N, Hussain SM, Shukr I, Ahmed M. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers. J Coll Physicians Surg Pak. 2015 Nov; 25(11):789–93. PMID: 26577962.
- James SMD, Sureshkumar S, Elamurugan TP, Debasis N, Vijayakumar C, Palanivel C. Comparison of vacuum-assisted closure therapy and conventional dressing on wound healing in patients with diabetic foot ulcer: A randomized controlled trial. Niger J Surg. 2019 Jan–Jun; 25(1):14–20. doi: 10.4103/njs.NJS_14_18. PMID: 31007506; PMCID: PMC6452767.
- Ulusal AE, Sahin MS, Ulusal B, Cakmak G, Tuncay C. Negative pressure wound therapy in patients with diabetic foot. Acta Orthop Traumatol Turc. 2011; 45(4):254–60. doi: 10.3944/AOTT.2011.2283. PMID: 21908965.
- Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016 Feb; 63(2 Suppl):3S–21S. doi: 10.1016/j.jvs.2015.10.003. PMID: 26804367.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012 Jun; 54(12):e132–73. doi: 10.1093/cid/cis346. PMID: 22619242.
- Bus SA, Armstrong DG, Gooday C, Jarl G, Caravaggi C, Viswanathan V, et al. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020 Mar; 36 Suppl 1:e3274. doi: 10.1002/dmrr.3274. PMID: 32176441.
- Bishara RA, Taha W, Alfarouk MO, Abdel Aal K, Wasfy S. Duplex detected ankle peak systolic velocity: A new parameter for the assessment of degree of peripheral ischemia. Int Angiol. 2004 Dec; 23(4):368–72. PMID: 15767982.
- Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993 Jul; 36(7):615–21. doi: 10.1007/BF00404070. PMID: 8359578.
- Bishara RA, Taha W, Akladious I, Allam MA. Ankle peak systolic velocity: New parameter to predict nonhealing in diabetic foot lesions. Vascular. 2009 Sep–Oct; 17(5):264–8. doi: 10.2310/6670.2009.00032. PMID: 19769805.
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019 Jul; 58(1S):S1–S109.e33. doi: 10.1016/j.ejvs.2019.05.006. Epub 2019 Jun 8. Erratum in: Eur J Vasc Endovasc Surg. 2020 Mar; 59(3):492–3. Erratum in: Eur J Vasc Endovasc Surg. 2020 Jul; 60(1):158–9. PMID: 31182334.
- Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012 Nov; 3(1):4. doi: 10.1007/s13300-012-0004-9. Epub 2012 Apr 20. PMID: 22529027; PMCID: PMC3508111.
- Dalla Paola L, Carone A, Boscarino G, Scavone G, Vasilache L. Combination of open subtotal calcanectomy and stabilization with external fixation as limb salvage procedure in hindfoot-infected diabetic foot ulcers. Int J Low Extrem Wounds. 2016 Dec; 15(4):332–7. doi: 10.1177/1534734616667865. Epub 2016 Sep 30. PMID: 27694302.
- Karatepe O, Eken I, Acet E, Unal O, Mert M, Koc B, et al. Vacuum assisted closure improves the quality of life in patients with diabetic foot. Acta Chir Belg. 2011 Sep–Oct; 111(5):298–302. PMID: 22191131.
